Viscometric Study of n-Hexylammonium and n-Octylammonium perchlorates in Binary Non-Aqueous Solvents Probed by a Physicochemical Approach in the Temperature Ranging 298 - 328K at Atmospheric Pressure
Vivek Pathania1, Shrutila Sharma1*, S.K. Vermani2, B.K.Vermani1, Navya Grover1 and Manpreet Kaur1
1Department of Chemistry, D. A.V. College, Chandigarh-160011, India
2Higher Education, Haryana, Panchkula-134109, India.
Corresponding Author E-mail: shrutilasharma@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370319
Article Received on : 01 Apr 21
Article Accepted on : 02 May 21
Article Published : 11 May 2021
Reviewed by: Dr. Agastyaraju Hariharan
Second Review by: Dr. kishor Arora
Final Approval by: Dr. Saksit Chantai
Viscosity data were measured for n-hexyl ammonium perchlorate (C6H13NH3ClO4)(HAP) and n-octylammonium perchlorate (C8H17NH3ClO4) (OAP) in acetonitrile (AN), dimethylsulfoxide (DMSO) and their binary mixtures containing 0, 20, 40, 60, 80 and 100 mol%DMSO at 298, 308, 318 and 328 K in the concentration range 60-350×10-4 mol.dm-3. The data was further examined to evaluate ion-ion and ion-solvent interactions in terms of the Aand B coefficients of the Jones-Dole equation respectively. A and B coefficients for the studied electrolytes came out be to be positive throughout the whole composition range. However, smaller values of A as compare to B reveal the dominance of ion-solvent interaction over ion-ion interactions especially at 60 mol% DMSO in AN-DMSO mixtures. Thermodynamic parameters like free energy, enthalpy and entropy change of activation of the viscous flow have also been evaluated by using Erying transition state theory.
KEYWORDS:Ion-solvent interaction; Ion-Ion Interaction; Jones-Dole equation; Thermodynamic parameters; Viscosity
Download this article as:| Copy the following to cite this article: Pathania V, Sharma S, Vermani S. K, Vermani B. K, Grover N, Kaur M. Viscometric Study of n-Hexylammonium and n-Octylammonium perchlorates in Binary Non-Aqueous Solvents Probed by a Physicochemical Approach in the Temperature Ranging 298 - 328K at Atmospheric Pressure. Orient J Chem 2021;37(3). |
| Copy the following to cite this URL: Pathania V, Sharma S, Vermani S. K, Vermani B. K, Grover N, Kaur M. Viscometric Study of n-Hexylammonium and n-Octylammonium perchlorates in Binary Non-Aqueous Solvents Probed by a Physicochemical Approach in the Temperature Ranging 298 - 328K at Atmospheric Pressure. Orient J Chem 2021;37(3). Available from: https://bit.ly/2R9PgUn |
Introduction
Viscosity studies provide adequate information about solvation behavior of ions in pure and binary mixtures of solvents.1-7 From the research data, it is clear that viscosity has proven to be an outstanding tool for predicting various type of ionic interactions taking place within the solution.8-12 Viscometric methods are most important for understanding the behavior of various components in terms of their physicochemical interactions that may be ion-dipole, solvophobic and charge transfer ionic interactions.13-15Information about the viscosity of electrolytic solution is very essential for designing various industrial processes and also furnish important insight into the structure of solution. Adding solute into the solvent can affect the flow process of solvent, which can be described by Jones Dole coefficients present in the Jones Dole Equation16-17 (Eq.1) Jones Dole B coefficient is a very important parameter for measuring the interaction of electrolyte which is added in the solvent and also for determination of behavior of electrolytes in solvents which shows growing change in binary mixture of studied solvents. Ionic solutes are solvated because of electrostatic interactions between the charge on the ions and dipoles of solvent molecules. These interactions break down the solvent structure in the vicinity of ion resulting in the formation of spherically symmetric solvation shell.18 In our previous studies we have reported the solvation studies of monoalkylammonium perchlorates in (acetonitrile) AN and DMA (Dimethylacetamide) mixtures by compressibility measurements. Perchlorates are the high melting inorganic salts which have very high solubility in water and protic solvents. Alkyl substituted ammonium perchlorates are near perfect monopropellants from an energy view point, however, these may also catalyze the combustion reactions of other propellant ingredients by bringing exothermic reactions almost close to the solid propellant surface. These perchlorates are also used in supercapacitors as electrolytes, the solution of these electrolytes in non aqueous solvents in supercapacitors has superior voltage stability, high operation voltage and greater storage density as compare to the conventional aqueous electrolytic solutions.19 In our previous studies we have reported the solvation studies of monoalkylammonium perchlorates in (acetonitrile) AN and DMA (Dimethylacetamide) mixtures by compressibility measurements.
Till now, no attempt, however, has been made to measure the viscosities of these electrolytes in AN and DMSO systems. There are hardly any data in the literature in which the viscosity studies of these electrolytes (HAP and OAP) in mixed non-aqueous solvents at variable temperatures have been investigated. Keeping this in mind here we report the viscosity measurements of HAP and OAP in pure AN, DMSO and their binary mixtures at 298, 308, 318 and 328K. Present study is mainly concerned with measuring viscosity as a function of concentration of HAP and OAP, mol percent composition of solvents and temperature. For better understanding of solvation behavior of these electrolytes in AN and DMSO binary mixtures various thermodynamic parameters have also been evaluated which were not available in the literature. The AN-DMSO solvent system was a good choice since both AN and DMSO have similar dielectric constant and it would be interesting to know which of the two solvents studied was preferred as regard solvation of ions of the electrolytes
Experimental Procedure
Materials used
Pyrex A grade quality glassware were used for the experimental procedures. Preparation of the salts, hexylammonium perchlorate (HAP) and octyl ammonium perchlorate (OAP) was done as reported earlier.19-20 AN and DMSO with percentage purity of 99.8% each having CAS numbers75-05-8 and67-68-5 respectively, were obtained from E. Merck. Also, mass fraction purity for AN as well as DMSO was estimated to be around 0.999a (Method used – Gas Chromatography). Water content in both the solvents, AN and DMSO was estimated to be less than 0.00030b and 0.00020b respectively (Method used – Karl Fischer Titration). Silver perchlorate monohydrate (AgClO4.H2O) with CAS No.14242-05-8, was bought from Alfa Aesar and was used as such. (No further purification was done).
Apparatus and Methods Used
HAP and OAP solutions, concentration ranging from 60-350×10-4 mol dm-3 containing 0, 20, 40, 60, 80 and 100 mol%DMSO in AN and DMSO mixtures were prepared. Viscosities were measured with an Ubbelohde type viscometer or suspended level viscometer. For maintaining constant temperature, viscometer was placed in high precision thermostatic water bath (Narang Scientific works, New Delhi, India) having uniform circulation of water throughout the experiment. A known volume of the solution was placed in the Ubbelohde viscometer for at least 20 minutes to attain the desired temperature. Stop watch correct to (±0.01s) was used for noting 20 minutes to attain the desired temperature. Stop watch correct to (±0.01s) was used for noting the efflux flow times of solutions. Precision in these measurements was found to be (±0.01%). Densities of pure solvents and solutions were measured by using density and sound velocity meter.20 (DSA 5000M, instrumentation discussed in previously published paper)
Results And Discussion
The measured values of viscosities and densities of different concentrations of OAP and HAP solutions in the range 60-350×10-4 mol.dm-3 containing 0, 20, 40, 60, 80 and 100 mol% DMSO in AN-DMSO mixtures at different temperatures (298-328 K) are given in Tables 1-2 and Table 4 respectively.From Tables 1-2, it can be seen that viscosity increases with increase in concentration of the solution at a given temperature. The possible explanation for the observed trend may be due to the fact that as concentration of salt increases in the solution number of molecular collisions within the solution increases leading to the loss of kinetic energy due to which molecule comes closer to each other resulting in increase of viscosity. Similar viscosity molecular collisions within the solution increases leading to the loss of kinetic energy due to which molecule comes closer to each other resulting in increase of viscosity.
Table 1: Viscosity (ƞ) and Ψ parameter values for C8H17NH3ClO4 in AN, DMSO and their binary mixtures at different temperatures and at an experimental pressure of 0.1MPa
|
104 c/mol.dm-3 |
ƞ |
102.Ψ
|
||||||||
|
298 K |
308 K |
318 K |
328 K |
298 K |
308 K |
318 K |
328 K |
|||
|
Pure AN |
||||||||||
|
70 |
0.3469 |
0.3248 |
0.2933 |
0.2628 |
20.68 |
16.04 |
10.28 |
7.32 |
||
|
128 |
0.3493 |
0.3266 |
0.2943 |
0.2634 |
21.51 |
16.82 |
10.64 |
7.44 |
||
|
178 |
0.3511 |
0.3279 |
0.2951 |
0.2638 |
22.20 |
17.31 |
11.08 |
7.52 |
||
|
220 |
0.3525 |
0.3289 |
0.2956 |
0.2641 |
22.74 |
17.67 |
11.13 |
7.56 |
||
|
257 |
0.3536 |
0.3297 |
0.296 |
0.2644 |
23.05 |
17.91 |
11.15 |
7.62 |
||
|
289 |
0.3545 |
0.3304 |
0.2965 |
0.2646 |
23.29 |
18.17 |
11.53 |
7.66 |
||
|
20 mol% DMSO |
||||||||||
|
66.99 |
0.5325 |
0.4693 |
0.3886 |
0.3171 |
27.93 |
24.43 |
23.06 |
19.97 |
||
|
123 |
0.5382 |
0.4734 |
0.3917 |
0.3193 |
30.48 |
26.06 |
24.35 |
21.1 |
||
|
171 |
0.5421 |
0.4764 |
0.3939 |
0.3208 |
31.58 |
27.09 |
25.06 |
21.57 |
||
|
212 |
0.5451 |
0.4786 |
0.3956 |
0.322 |
32.32 |
27.62 |
25.57 |
22.01 |
||
|
247 |
0.5476 |
0.4805 |
0.3970 |
0.323 |
33.00 |
28.21 |
26.03 |
22.43 |
||
|
277.99 |
0.5497 |
0.4821 |
0.3982 |
0.3238 |
33.53 |
28.68 |
26.42 |
22.68 |
||
|
40 mol% DMSO |
||||||||||
|
65 |
0.8652 |
0.6461 |
0.4615 |
0.3991 |
35.84 |
31.29 |
28.03 |
26.34 |
||
|
119 |
0.8762 |
0.6528 |
0.4656 |
0.4025 |
38.48 |
32.87 |
29.05 |
27.44 |
||
|
165 |
0.8845 |
0.6577 |
0.4688 |
0.4049 |
40.36 |
33.97 |
30.19 |
28.09 |
||
|
204 |
0.8906 |
0.6615 |
0.4712 |
0.4067 |
41.38 |
34.77 |
30.87 |
28.49 |
||
|
238 |
0.8964 |
0.6648 |
0.4731 |
0.4083 |
42.78 |
35.59 |
31.31 |
29.03 |
||
|
268 |
0.9010 |
0.6673 |
0.4746 |
0.4096 |
43.66 |
35.96 |
31.54 |
29.39 |
||
|
60 mol% DMSO |
||||||||||
|
63 |
1.2021 |
0.867 |
0.5865 |
0.4513 |
45.73 |
40.19 |
36.47 |
29.43 |
||
|
115 |
1.2226 |
0.8795 |
0.5929 |
0.4562 |
50.32 |
43.62 |
37.46 |
32.14 |
||
|
160 |
1.2379 |
0.8896 |
0.5993 |
0.4598 |
53.09 |
46.48 |
40.64 |
33.70 |
||
|
198 |
1.2530 |
0.8969 |
0.6040 |
0.4626 |
56.98 |
47.96 |
42.39 |
34.81 |
||
|
231 |
1.2629 |
0.9038 |
0.6073 |
0.4650 |
58.37 |
49.80 |
43.06 |
35.81 |
||
|
260 |
1.2730 |
0.9084 |
0.6104 |
0.4668 |
60.41 |
50.34 |
43.96 |
36.28 |
||
|
80 mol% DMSO |
||||||||||
|
61 |
1.4565 |
1.1865 |
0.8358 |
0.61 |
32.91 |
28.12 |
24.19 |
20.26 |
||
|
112 |
1.4728 |
1.1977 |
0.8425 |
0.614 |
35.13 |
29.87 |
25.57 |
21.24 |
||
|
154.98 |
1.4846 |
1.2057 |
0.8474 |
0.617 |
36.54 |
30.93 |
26.54 |
22.07 |
||
|
191.99 |
1.4944 |
1.2124 |
0.8517 |
0.6194 |
37.81 |
31.95 |
27.63 |
22.71 |
||
|
223.98 |
1.5022 |
1.2179 |
0.8546 |
0.6212 |
38.68 |
32.75 |
27.94 |
23.03 |
||
|
251.98 |
1.5087 |
1.2231 |
0.857 |
0.6228 |
39.35 |
33.7 |
28.18 |
23.39 |
||
|
Pure DMSO |
||||||||||
|
60 |
2.0422 |
1.6077 |
1.4192 |
1.1514 |
33.86 |
31.00 |
27.12 |
22.13 |
||
|
110 |
2.0638 |
1.623 |
1.4306 |
1.1589 |
35.36 |
32.19 |
27.85 |
22.66 |
||
|
153.46 |
2.0794 |
1.6337 |
1.4393 |
1.1643 |
36.26 |
32.75 |
28.63 |
23.15 |
||
|
188.98 |
2.0913 |
1.6424 |
1.4451 |
1.1683 |
37.03 |
33.55 |
28.84 |
23.33 |
||
|
219.99 |
2.1009 |
1.6493 |
1.4499 |
1.1716 |
37.57 |
34.05 |
29.05 |
23.59 |
||
|
247 |
2.1093 |
1.6546 |
1.4546 |
1.1743 |
38.15 |
34.29 |
29.57 |
23.78 |
||
|
c is the molarity of HAP and OAP solutions in pure AN, DMSO and their binary mixtures. Standard uncertainties are: u (T)=0.01K, u(c)=2.0×10-4 mol dm-3, u(p)=0.01MPa
|
||||||||||
Table 2: Viscosity (ƞ) and Ψ parameter values for C6H13NH3ClO4 in AN, DMSO and their binary mixtures at different temperatures and at an experimental pressure of 0.1MPa.
|
104 c/mol. dm-3 |
ƞ |
102.Ψ
|
||||||||
|
298 K |
308 K |
318 K |
328 K |
298 K |
308 K |
318 K |
328 K |
|||
|
Pure AN |
||||||||||
|
71 |
0.346 |
0.32335 |
0.293 |
0.2628 |
17.4 |
10.55 |
8.98 |
7.27 |
||
|
129.98 |
0.3482 |
0.32471 |
0.294 |
0.26342 |
18.52 |
11.52 |
9.65 |
7.45 |
||
|
179.99 |
0.3498 |
0.3257 |
0.29467 |
0.26386 |
19.24 |
12.09 |
9.92 |
7.59 |
||
|
222.99 |
0.3511 |
0.3264 |
0.29523 |
0.26422 |
19.83 |
12.33 |
10.2 |
7.74 |
||
|
259.98 |
0.3522 |
0.3271 |
0.29566 |
0.2645 |
20.37 |
12.77 |
10.36 |
7.84 |
||
|
292 |
0.353 |
0.329 |
0.2961 |
0.26473 |
20.59 |
13 |
10.67 |
7.98 |
||
|
20 mol% DMSO |
||||||||||
|
68.99 |
0.5316 |
0.4682 |
0.3875 |
0.3162 |
25.44 |
21.2 |
19.26 |
16.21 |
||
|
126.99 |
0.5368 |
0.4721 |
0.3903 |
0.3181 |
27.61 |
23.14 |
20.71 |
17.35 |
||
|
174.98 |
0.5406 |
0.4749 |
0.3923 |
0.3194 |
29.04 |
24.32 |
21.6 |
17.93 |
||
|
217 |
0.5437 |
0.4773 |
0.394 |
0.3205 |
30.12 |
25.38 |
22.43 |
18.49 |
||
|
252.97 |
0.5463 |
0.4792 |
0.3953 |
0.3214 |
31.04 |
26.1 |
22.91 |
18.94 |
||
|
285 |
0.5486 |
0.4809 |
0.3964 |
0.3221 |
31.86 |
26.78 |
23.3 |
19.18 |
||
|
40 mol% DMSO |
||||||||||
|
68 |
0.8633 |
0.6455 |
0.4611 |
0.3985 |
32.3 |
29.44 |
26.33 |
23.89 |
||
|
124.99 |
0.8747 |
0.6532 |
0.4660 |
0.4025 |
35.95 |
32.64 |
29.13 |
26.78 |
||
|
172.98 |
0.884 |
0.6591 |
0.4696 |
0.4052 |
38.97 |
34.87 |
30.83 |
28.02 |
||
|
213.98 |
0.8912 |
0.6638 |
0.4726 |
0.4075 |
40.89 |
36.45 |
32.26 |
29.21 |
||
|
248.98 |
0.8974 |
0.6678 |
0.4749 |
0.4094 |
42.58 |
37.81 |
33.14 |
30.16 |
||
|
281 |
0.9034 |
0.6714 |
0.4771 |
0.4111 |
44.34 |
39 |
34.1 |
30.99 |
||
|
60 mol% DMSO |
||||||||||
|
66.99 |
1.1999 |
0.8666 |
0.5853 |
0.4512 |
42.02 |
38.39 |
32.79 |
28.26 |
||
|
122.99 |
1.2236 |
0.8815 |
0.5937 |
0.4568 |
49.44 |
44.32 |
37.49 |
32.31 |
||
|
169.99 |
1.2429 |
0.893 |
0.6008 |
0.4612 |
54.81 |
48.2 |
41.44 |
35.13 |
||
|
210.98 |
1.2581 |
0.9031 |
0.6064 |
0.4653 |
58.22 |
51.54 |
43.97 |
37.94 |
||
|
245.99 |
1.2705 |
0.9112 |
0.6112 |
0.4684 |
60.74 |
53.88 |
46.09 |
39.61 |
||
|
275.99 |
1.2819 |
0.9182 |
0.6155 |
0.471 |
63.26 |
55.88 |
48.05 |
40.95 |
||
|
80 mol% DMSO |
||||||||||
|
66 |
1.4718 |
1.1996 |
0.8429 |
0.6154 |
44.9 |
40.92 |
33.91 |
30.54 |
||
|
121 |
1.4959 |
1.2175 |
0.8532 |
0.6222 |
48.59 |
44.24 |
36.46 |
32.85 |
||
|
167.99 |
1.5143 |
1.2304 |
0.861 |
0.6271 |
51.24 |
46.12 |
38.28 |
34.18 |
||
|
207.01 |
1.5279 |
1.2409 |
0.8668 |
0.6309 |
52.81 |
47.83 |
39.4 |
35.19 |
||
|
241.99 |
1.5396 |
1.2493 |
0.872 |
0.6342 |
54.14 |
48.89 |
40.52 |
36.08 |
||
|
271.99 |
1.5492 |
1.2564 |
0.8762 |
0.6369 |
55.17 |
49.82 |
41.32 |
36.75 |
||
|
Pure DMSO |
||||||||||
|
65 |
2.0687 |
1.6224 |
1.4326 |
1.1648 |
49.05 |
41.4 |
38.01 |
35.94 |
||
|
119 |
2.0996 |
1.6431 |
1.4490 |
1.1769 |
50.49 |
42.68 |
38.91 |
36.36 |
||
|
164.99 |
2.1222 |
1.6577 |
1.4606 |
1.1856 |
51.72 |
43.49 |
39.54 |
36.86 |
||
|
204 |
2.1400 |
1.6692 |
1.4694 |
1.1921 |
52.77 |
44.24 |
39.99 |
37.17 |
||
|
237.99 |
2.1548 |
1.6784 |
1.4767 |
1.1975 |
53.68 |
44.76 |
40.43 |
37.51 |
||
|
268 |
2.1668 |
1.6861 |
1.4827 |
1.2018 |
54.27 |
45.17 |
40.74 |
37.67 |
||
|
c is the molarity of HAP and OAP solutions in pure AN, DMSO and their binary mixtures. Standard uncertainties are: u (T)=0.01K, u(c)=2.0×10-4 mol dm-3, u(p)=0.01MPa
|
||||||||||
Similar viscosity trends were obtained as DMSO mol% was increased in the solution. With increase in temperature viscosities were found to be decreasing. This may be explained on the basis that with increase in temperature fluidity of the solution is enhanced because of increase in thermal motion of the molecules that leads to lowering of viscosity. The viscosity data for HAP and OAP in AN and DMSO and in their binary mixtures have been further analyzed by using Jones-Dole equation (Eq. 1) which may be rearranged further in the form of Eq. 2
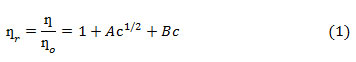
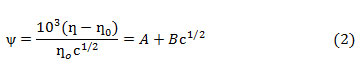
where ƞ is viscosity of solution, ƞ˳ is viscosity of pure solvent, ƞr is relative viscosity, A and B are Jones Dole coefficientsand c is the molar concentration of the solution. A and B coefficients provide information regarding ion-ion and ion-solvent interactions occurring in the solution respectively. A and B coefficients are obtained from the intercept and slope of the linear plots of ψ versus √c respectively (ψ values are tabulated in Tables 1-2). Representative plots of ψ versus √c for HAP and OAP in pure AN at 308 K are shown in Fig.1. A and B viscosity coefficients so obtained are shown in Table 3.
 |
Figure 1: lots of ψ versus √c for HAP and OAP in pure AN at 308 K. |
Table 3: B (10-3m3.mol-1) coefficients and A (10-3/2m3/2.mol-1/2) coefficients of the Jones-Dole equation for HAP (C6H13NH3ClO4)and OAP (C8H17NH3ClO4)in AN+DMSO mixtures at different temperatures and at an experimental pressure of 0.1MPa
|
mole fraction DMSO |
A-Coefficients |
B-Coefficients |
||||||
|
|
298 K |
308 K |
318 K |
328 K |
298 K |
308 K |
318 K |
328 K |
|
C6H13NH3ClO4 |
||||||||
|
0 |
0.14 |
0.08 |
0.07 |
0.06 |
0.37 |
0.28 |
0.18 |
0.08 |
|
20 |
0.19 |
0.16 |
0.15 |
0.13 |
0.74 |
0.65 |
0.48 |
0.35 |
|
40 |
0.20 |
0.20 |
0.19 |
0.17 |
1.41 |
1.12 |
0.91 |
0.81 |
|
60 |
0.22 |
0.21 |
0.18 |
0.16 |
2.51 |
2.08 |
1.81 |
1.53 |
|
80 |
0.35 |
0.32 |
0.27 |
0.25 |
1.23 |
1.06 |
0.89 |
0.74 |
|
100 |
0.44 |
0.38 |
0.35 |
0.34 |
0.64 |
0.46 |
0.33 |
0.22 |
|
C8H17NH3ClO4 |
||||||||
|
0 |
0.18 |
0.14 |
0.09 |
0.07 |
0.31 |
0.24 |
0.13 |
0.04 |
|
20 |
0.23 |
0.21 |
0.20 |
0.17 |
0.64 |
0.49 |
0.39 |
0.31 |
|
40 |
0.28 |
0.27 |
0.24 |
0.23 |
0.93 |
0.57 |
0.44 |
0.36 |
|
60 |
0.31 |
0.30 |
0.28 |
0.23 |
1.80 |
1.28 |
0.99 |
0.84 |
|
80 |
0.27 |
0.23 |
0.20 |
0.17 |
0.80 |
0.67 |
0.52 |
0.39 |
|
100 |
0.30 |
0.28 |
0.25 |
0.20 |
0.53 |
0.42 |
0.30 |
0.20 |
|
Standard uncertainties are: u (T)=0.01K, u(p)=0.01MPa, u(B)= 0.02×10-3m3.mol-1 and u(A)= 0.06×10-3/2m3/2.mol-1/2 |
||||||||
Lower values of A coefficients as compare to corresponding B-coefficients in all binary mixtures and at all studied temperatures except in pure AN and DMSO (with smaller difference between A and B values) indicates greater degree of solvation than ion-ion interactions in studied binary mixtures. Also, both A and B values were found to be decreasing with rise in temperature that may be because of increased kinetic energy of the ions in the solution.22 The B-coefficients of the Jones Dole viscosity equation for the electrolytes if comes out to be negative or very low represents structure breaking behavior of ions and positive B-coefficients indicates structure making behavior of ions.23 A perusal of Table 3 shows that B values are positive throughout the whole composition range at all studied temperatures showing better ion-solvent interactions. However, most positive values are obtained at 60 mol% DMSO therefore suggesting presence of strong ion-solvent interactions at this composition. It was also observed that more positive B may be because of increased kinetic energy of the ions in the solution.22 The B-coefficients of the Jones Dole viscosity equation for the electrolytes if comes out to be negative or very low represents structure breaking behavior of ions and positive B-coefficients indicates structure making behavior of ions.23 A perusal of Table 3 shows that B values are positive throughout the whole composition range at all studied temperatures showing better ion-solvent interactions. However, most positive values are obtained at 60 mol% DMSO therefore suggesting presence of strong ion-solvent interactions at this composition. It was also observed that more positive B values are obtained for HAP as compare to OAP. This may be attributed to the smaller size of cation. Smaller the size of an ion greater is the electrostatic force of attraction hence more is the Solvation.22 It can be seen from Table 3 that B values decreases with increase in temperature hence present structure promoting effect of the electrolytes which means both OAP and HAP are behaving as structure maker in non aqueous solutions. This is because of ordered arrangement of solvent molecules around the solute, at high temperature, the neighboring solvent molecules around the ions is destroyed which weakens the interactions between ion and solvent molecules which in turn increases the chances of ions to interact with each other hence decreases solvation.
Thermodynamic parameters like enthalpy change of activation (ΔH*), entropy change of activation (ΔS*) and free energy change of activation (ΔG*) in pure AN, DMSO and their binary mixtures containing 0, 20, 40, 60, 80 and 100 mol% DMSO are tabulated in Table 4. The enthalpy change of activation and entropy change of activation were obtained from the linear plots of
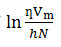
versus 1/T (as per the Erying transition state theory) 24 shown in Fig.2. Slope and intercept of the straight line gives the value of enthalpy of activation ΔH* and entropy of activation ΔS* respectively.
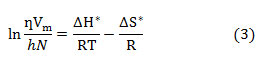
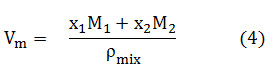
where Vm is the molar volume, M1, M2 and x1, x2 are the molar masses and mass fractions of solute and solvent respectively, Pmix is the density of mixture which is tabulated in Table S1.
 |
Figure 2: Plot of ln(ƞVm/hNA) versus 1/T for OAP at concentration 71×10-4mol.dm-3. |
Table 4: Entropy change of activation (ΔS#/ kJ.mol-1.K-1), enthalpy change of activation (ΔH#/kJ.mol-1) and free energy change of activation at 298K (ΔG#/kJ.mol-1) of C6H13NH3ClO4 (HAP) and C8H17NH3ClO4 (OAP) in AN, DMSO and binary mixtures at different temperatures and at an experimental pressure of 0.1MPa
|
C6H13NH3ClO4 |
C8H17NH3ClO4 |
|||||||
|
104.c /mol. dm-3 |
102.ΔS# (kJ.mol-1.K-1) |
(ΔH#) (kJ.mol-1) |
(ΔG#) (kJ.mol-1) |
104.c /mol.dm-3 |
102.ΔS# (kJ.mol-1) |
(ΔH#) (kJ.mol-1) |
(ΔG#) (kJ.mol-1) |
|
|
Pure AN |
||||||||
|
71 |
-18.33 |
7.07 |
61.71 |
70 |
-18.35 |
6.19 |
60.87 |
|
|
129.98 |
-18.31 |
6.41 |
60.97 |
128 |
-18.32 |
6.31 |
60.89 |
|
|
180 |
-18.29 |
6.47 |
60.97 |
178 |
-18.30 |
6.38 |
60.90 |
|
|
223 |
-18.29 |
6.55 |
61.05 |
220 |
-18.29 |
6.39 |
60.91 |
|
|
259.98 |
-18.27 |
6.60 |
61.03 |
257 |
-18.28 |
6.43 |
60.91 |
|
|
292 |
-18.26 |
6.66 |
61.06 |
289 |
-18.26 |
6.49 |
60.92 |
|
|
20mol%DMSO |
||||||||
|
69 |
-16.53 |
12.86 |
62.11 |
67 |
-16.53 |
12.80 |
62.12 |
|
|
127 |
-16.52 |
12.94 |
62.13 |
123 |
-16.51 |
12.89 |
62.14 |
|
|
174.98 |
-16.48 |
13.05 |
62.15 |
171 |
-16.48 |
13.04 |
62.16 |
|
|
217 |
-16.46 |
13.12 |
62.16 |
212 |
-16.47 |
13.09 |
62.17 |
|
|
252.97 |
-16.44 |
13.20 |
62.18 |
247 |
-16.46 |
13.12 |
62.19 |
|
|
285 |
-16.42 |
13.26 |
62.18 |
278 |
-16.45 |
13.17 |
62.20 |
|
|
40mol%DMSO |
||||||||
|
68 |
-14.31 |
20.76 |
63.39 |
65 |
-14.35 |
20.63 |
63.39 |
|
|
125 |
-14.27 |
20.89 |
63.41 |
119 |
-14.29 |
20.81 |
63.41 |
|
|
172.98 |
-14.26 |
20.96 |
63.44 |
165 |
-14.26 |
20.96 |
63.45 |
|
|
214 |
-14.25 |
20.99 |
63.47 |
204 |
-14.25 |
20.99 |
63.46 |
|
|
248.98 |
-14.22 |
21.09 |
63.48 |
238 |
-14.23 |
21.07 |
63.49 |
|
|
281 |
-14.20 |
21.18 |
63.50 |
268 |
-14.23 |
21.09 |
63.49 |
|
|
60mol%DMSO |
||||||||
|
67 |
-12.79 |
26.23 |
64.42 |
63 |
-12.81 |
26.21 |
64.53 |
|
|
123 |
-12.80 |
26.30 |
64.06 |
115 |
-12.80 |
26.29 |
64.57 |
|
|
170 |
-12.78 |
26.39 |
64.75 |
160 |
-12.78 |
26.34 |
64.73 |
|
|
210.98 |
-12.77 |
26.47 |
64.81 |
198 |
-12.77 |
26.46 |
64.82 |
|
|
245.99 |
-12.75 |
26.53 |
64.84 |
231 |
-12.75 |
26.50 |
64.86 |
|
|
275.99 |
-12.68 |
26.74 |
64.73 |
260 |
-12.72 |
26.71 |
64.89 |
|
|
80mol%DMSO |
||||||||
|
66 |
-14.09 |
23.16 |
65.14 |
61 |
-14.09 |
23.13 |
65.12 |
|
|
121 |
-14.06 |
23.29 |
65.18 |
112 |
-14.05 |
23.21 |
65.15 |
|
|
167.99 |
-14.03 |
23.39 |
65.21 |
154.98 |
-14.03 |
23.33 |
65.17 |
|
|
207.01 |
-14.02 |
23.47 |
65.24 |
192 |
-14.02 |
23.39 |
65.18 |
|
|
242 |
-14.01 |
23.51 |
65.25 |
223.98 |
-14.00 |
23.45 |
65.20 |
|
|
272 |
-14.00 |
23.54 |
65.27 |
252 |
-14.01 |
23.49 |
65.24 |
|
|
PURE DMSO |
||||||||
|
65 |
-15.86 |
18.72 |
65.98 |
60 |
-15.87 |
18.66 |
65.96 |
|
|
119 |
-15.83 |
18.85 |
66.02 |
110 |
-15.84 |
18.79 |
65.98 |
|
|
165 |
-15.78 |
18.09 |
66.05 |
153.46 |
-15.79 |
18.95 |
66.00 |
|
|
204 |
-15.76 |
19.01 |
66.07 |
188.98 |
-15.86 |
18.74 |
66.01 |
|
|
238 |
-15.75 |
19.06 |
66.08 |
220 |
-15.75 |
19.02 |
66.03 |
|
|
268 |
-15.73 |
19.11 |
66.10 |
247 |
-15.74 |
19.10 |
66.04 |
|
|
Standard uncertainties are: u(T)=0.01K and u(p)=0.01MPa, u(ΔG)*=0.04kJ.mol-1, u(ΔH*)=0.5kJ.mol-1and u(ΔS*)=0.002kJ.mol-1.K-1
|
||||||||
From the results of Table 4 it is clear that enthalpy change of activation increases with increase in DMSO mol% till 60 mol% DMSO and then starts decreasing. With increase in concentration of both the salts (HAP and OAP) in the solution, the enthalpy change of activation (∆H*) was also found to be increasing throughout the whole composition range. This may be due to the fact that at high concentration number of ions in the solution will be more as a result there will be hindrance in mobility of ions which will cause difficulty in producing vacant sites in solvation matrix hence greater amount of energy will be required for ion-solvent interactions to take place. 25 Also, the negative values of entropy change of activation (∆S*) for both the salts throughout the whole composition range means more ordered arrangement of molecules in the studied system which indicate that transition state is associated with bond making hence both salts here behave as structure makers. Also, change in activation entropy increases as DMSO mol% is increased till 60 mol% DMSO and then starts decreasing again which shows that solvation effects are playing definite role in 60 mol% DMSO in AN-DMSO mixtures. ΔG* values were obtained by using following equation
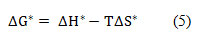
From the results of Table 4 it can be seen that ΔG* values are positive in all cases which suggests the non-spontaneity of the process.
Conclusion
The results show that the value of viscosity B-coefficients for HAP and OAP in the studied solvent systems is positive, therefore suggesting the existence of strong ion-dipole interactions and these interactions are found to be decreasing with increase in the size of alkyl chain length and temperature in the studied mixtures. More positive B-coefficient values obtained at 60 mol% DMSO for both the electrolytes studied and the derived thermodynamic parameters of viscous flow show preferential solvation of HAP and OAP ions by DMSO in AN rich region and by AN in DMSO rich region in AN-DMSO mixtures at all the investigated temperatures.
Acknowledgement
The authors wish to thank D.A.V. College, Sector-10, Chandigarh (India) for financial assistance.
Conflict 0f Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
References
- Ayachithula, N.; Shaik, B.; Konkyana, G.R.; Thummaluru, K., Asian J. Chem., 2018, 30, 2008-2015.
CrossRef - Zamir, T.; Quickenden, T.I., J. Solution Chem., 2003,32, 463-472.
CrossRef - Parker, A.J.; Diggle, J.W.; Avramides, J., Aust. J. Chem., 1974, 27, 721-725.
CrossRef - Das, B.; Hazara, D.K., J. Chem. Engg. Data. 2000,45,353-.357
CrossRef - Gill, D.S.; Sharma, A.N.; J. Chem. Soc. Faraday Trans. 1, 1982,178,475-478.
CrossRef - Roy, M.N.; Banerjee, A.; Das, R.K., J. Chem. Therm., 2009, 41,1187-1192.
CrossRef - Kratochvil, B.; Yeager, H.L., Can. J. Chem., 1972, 53, 3448-3455.
- Gill, D.S.; Pathania, V.; Vermani, B.K.; Sharma, R.P., Z. Phys. Chem., 2003, 217, 739-749.
CrossRef - Das, B.; Hazra, D.K., J. Indian Chem. Soc., 1997, 74, 108-114.
- Nikam, P.S.; Sawant, A.B., Bull. Chem. Soc. Jpn., 1998, 71, 2055-2061.
CrossRef - R. Qadeer, R.; Khalid, N., J. Chem. Soc. Pak., 2005, 27, 462-465.
- Rattan, V.K.; Kapoor, S.; Tochigi, K., J. Chem. Eng. Data., 2002,47, 1182-1184.
CrossRef - Fahim, U.; Saeed, R.; Fazal, A., J. Chem. Eng. Data., 2002,47, 1359-1362.
CrossRef - Uddin, F.; Seed, R., Pak. J. Sci. Ind. Res., 2000, 43, 7-12.
- Roy, M.N.; Jha, A., J. Chem. Eng. Data., 2001,46, 1327-1329.
CrossRef - Jones, G.; Dole, M., J. Am. Chem. Soc., 1929, 51, 2950-2964.
CrossRef - Madhurambal, G., Asian J. Chem., 2002, 14, 583-589.
CrossRef - Uddin, F.; Seed, R.; Nisar, F., J.Bangladesh Acad. Sci., 2000, 24, 207- 212.
- Pathania, V.; Sharma, S.; Vermani, S.K.; Vermani, B.K.; Grover, N., J Solution Chem., 2020, 49, 798-813.
CrossRef - Pathania, V.; Sharma, S.; Vermani, S.K.; Vermani, B.K.; Grover, N., Phys. Chem. Res., 2020, 8, 737-753.
- Anand, H.; Verma,R., Z.Phys.Chem., 2019, 233, 737-753.
CrossRef - Seed, R.; Uddin, F.; Masood, S.; Asif, N., J.Mol.Liq. 2009, 146, 112-118.
CrossRef - Sarmad,S.; Zafarani-Moattar, M.T.; Nikjoo, D.; Mikkola, J.P., Front. Chem., https://doi.org/10.3389/fchem.2020.593786
CrossRef - Eyring, H.; John, M.S., “Significant Liquid Structure”, Wiley, New York, USA (1969).
- Ohtaki, H., Monatshefte fuer Chemie. 2001, 132, 1237-1245.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









