Characterization and TiO2- Catalyzed Degradation of Polyurethane Biopolymer through Medium Pro Compost
Andi Budirohmi1,2*, Ahyar Ahmad2, Firdaus2and Paulina Taba2
1Sekolah Tinggi Ilmu Kesehatan Gema Insan Akademik Makassar, 90134, Makassar, Indonesia.
2Department of Chemistry, Faculty of Mathematics and Natural Sciences, Hasanuddin University, Makassar 90245, Indonesia.
Corresponding Author E-mail: andibudirohmi@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360121
Article Received on : 07-01-2020
Article Accepted on :
Article Published : 04 Feb 2020
Fungal Trichoderma harzianum and Pleurotus ostreatus contained in the compost pro shows the ability to produce enzymes. which can overhaul the structure of polyurethane so that it breaks down easily in the environment. Polyurethane degradation was carried out by using the blackout method through the compost pro medium. Some characterization techniques carried out include techniques to determine mass loss and polyurethane degradability. Analysis of functional groups with Fourier Transform Infra-Red (FTIR), and surface observation with the Scanning Electron Microscopy technique (SEM). Based on the results of quantitative analysis during biodegradation, there is a mass loss of 87.5% in polyurethanes synthesized from PEG, MDI, KBH starch and degradability 0.1987 g / day. FTIR test showed that before degradation there was a urethane functional group C = O and an N-H functional group after the degradation of urethane functional group C = O and the N-H group is absent in the samples.
KEYWORDS:Biopolymer; Degradation; Polyurethane; Pro compost
Download this article as:| Copy the following to cite this article: Budirohmi A, Ahmad, Firdaus, Taba P. Characterization and TiO2- Catalyzed Degradation of Polyurethane Biopolymer through Medium Pro Compost. Orient J Chem 2020;36(1). |
| Copy the following to cite this URL: Budirohmi A, Ahmad, Firdaus, Taba P. Characterization and TiO2- Catalyzed Degradation of Polyurethane Biopolymer through Medium Pro Compost. Orient J Chem 2020;36(1). Available from: https://bit.ly/2GROSkP |
Introduction
The development of the polymer industry, individually polyurethane (Pu) grows so fast, and every year its production continues to increase, given its many advantages and quite practical in its use 1. Along with the use of polymers, quite a lot also has an impact on an increase in polymer waste that is difficult to degrade in the environment2. Several studies have been conducted to make polymer materials that are resistant to degradation in the environment such as; polyurethane synthesized from Iron (III) acetylacetonate (Fe(acac)3), silver nitrate, oleic acid, oleylamine, 1-octadecanoic, 1-octadecene ethanol, hexane acrylic polyol and polyisocyanate 3.
Polymers synthesized from synthetic chemicals pose new problems, namely polymer waste that is difficult to decompose in nature and if left unchecked will endanger the environment 4. The right way to solve polymer waste and does not cause new problems in the environment is the process of biodegradation. Most polymers on the market are polymers from chemicals that are difficult to degrade in the environment. For the sake of the realization of an environment free of waste polymer, the right step is to synthesize the polymer from organic matter that is easily degraded 5. Several studies of polyurethanes synthesized from organic materials have been developed, for example, Izopianol, purocyn B, polyester polyols, potassium hydroxide, N, N-dimethyl cyclohexylamine, Tris (2- chloro-1-methyl ethyl) phosphate, diphenylmethane-4,4′-diisocyanate (MDI) and potato protein 6. Besides that, polyurethane from cellulose acetate, Isophorone diisocyanate (IPDI), polyethyleneglycol, dibutyltin dilaurate (DBTL), ethylene glycol 7 was synthesized.
Based on previous research shows that if polyurethane is synthesized from natural materials, it will be easily degraded. Biodegradation of organic materials, especially natural polymers, such as cellulose, lignin, or rubber, can occur due to microbiological attacks on these materials. Microorganisms can produce various enzymes that can react with natural polymers 8. Enzymatic reactions to polymers can occur if microorganisms obtain food sources from the polymer,9 through a medium such as pro compost.
Pro compost is an effective formulation in decomposing or decomposing organic matter and can be biocontrol so that it is very useful to break down various types of wastes that contain the fungus Trichoderma sp, Pleurotus estrous, termites and microbes. Application of Trichoderma sp; such as Trichoderma harzianum, into the soil can accelerate the breakdown of organic matter 10,11, because this fungus can produce three enzymes namely; enzymes cellobiohydrolase (CBH), which actively destroys natural cellulose; an endoglucanase enzyme that actively destroy dissolved cellulose; and the glucosidase enzyme that actively hydrolyzes the cellobiose unit into molecule glucose. This enzyme works synergistically so that decomposition can take place more quickly and intensively12 that, siderophore also has the potential to control phytopathogenic fungi and bacteria 11 through antagonist mechanism by inhibiting pathogenic enzyme activities 7 and mechanism of nutrient competition 12.
Material and Methode
Material
Kepok banana hump (KBH) was obtained from Sengkang, South Sulawesi, Indonesia, Methylendifenyl diisocyanate (MDI), polyethylene glycol (PEG) 400, nanoparticles titanium dioxide (TiO2) and aqua dest, obtained from Merck and Sigma Aldrich. Pro Compost from the Hasanuddin University Faculty of Agriculture Biotechnology.
Procedure
The polyurethane synthesis process is carried out by a one-step process method. Where KBH starch was weighed in a glass of 2.597 g, then 10 g of PEG and 1,342 g of TiO2 were added, then stirred with a magnetic stirrer for 3 hours, to a temperature of 100 ° C. After that MDI was added then stirred together for 15 minutes. Preprinted polyurethanes are printed in 4 cm x 5 cm. The ready-made polyurethane is cut to a size of 2cm x 2 cm, and then it is demolished in a compost pro medium every seven days, 14 days to 35 days.
Results and Discussion
Quantitative methods are the simplest way to characterize the biodegradation of a polymer by determining the mass loss and degradability of the polymer material. Mass loss is determined by weighing the mass of the polymer before and after the biodegradation process over a specified time interval, as shown in Egs.(1)
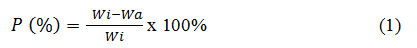
P = loss mass (%)
Wi = sample mass before the biodegradation process
Wa = sample mass after the biodegradation process
Degradability can indicate the ease of degradation of a polymeric material, can be determined by dividing the mass loss by biodegradation time, as shown in the Egs (2).

The results of the calculation of Pu mass loss synthesized from KBH Starch, PEG400-MD and TIO2 (Figure 1a), and Figure 1b is the calculation of the result of Pu mass loss synthesized from PEG, MDI, and amilyum materials.
 |
Figure 1A: PU+Starch+KBH Baru biodegradation |
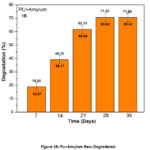 |
Figure 1B: Pu+Amylum Baru Degradarasi |
Figure 1a. Percentage of Polyurethane (Pu) loss synthesized from KBH, starch, and TiO2 after degradation and Figure 1b, the results of the FTIR polyurethane test were synthesized from amylum after degradation.
The loss of Pu mass synthesized from PEG, MDI, TiO2, and KBH starch increased with increasing biodegradation time until it did not change (fixed) on the 28th day and 35th day. The loss of Pu mass synthesized from KBH starch is 87.52% on the 28th day and 35th day. Likewise, what occurs to the polyurethane synthesized from PEG, MDI and amilyum materials, losing 70.59% of mass on days 28 and 35. This data shows that the longer the degradation time, the more part of the bio polyurethane molecule has been damaged by the attack of microorganisms. Whereas the Pu degradability synthesized from KBH starch on the 28th day was 0.1987 g / day and the 35th day was 0.1587 g / day. While the Pu degradability synthesized from amilyum on the 28th day was 0.1715 g / day and on the 35th day was 0.1372 g / day. Degradability data show degradability decreases with increasing biodegradation time. These conditions show that the decrease in the rate of biodegradation can be caused by the smaller portion of Pu molecules that can be attacked by microorganisms because the surface of Pu has been saturated covered by biodegradation products. Also, it can be caused by functional groups that can be attacked or hydrolyzed by fewer and fewer enzymes from microorganisms.13
Polymer Function Group Analysis with FTIR (Fourier Transform Infrared)
 |
Figure 2: Polyurethane (PU) FTIR test results were synthesized from KBH |
Polyurethanes synthesized from PEG 400, MDI, KBH starch and TiO2 from FTIR test results in Figure 2, before degradation showed urethane group C = O in the range of wave number 1700 cm-1 and N-H functional groups in the range of numbers 3250 cm-1 after degradation the two functional groups no longer exist. Whereas polyurethane synthesized by PEG 400, MDI and amylium material before degradation showed urethane functional group C = O which is in the range of 1700 cm-1 and NH functional groups that are in the range of 3250 cm-1 after degradation of the two functional groups are no longer there is, which is in the range of 3250 cm-1 after the degradation of the two functional groups is gone. Also shown in this figure after the degradation of the OH group to increase in intensity, this gives an indication. An indication that in the degradation process, the involvement of hydroxyl groups facilitates access to polyurethane biodegradation.
Polymer surface observation with SEM (Scanning Electron Microscopy). SEM testing aims to show the morphology of a sample. In this case, the test is carried out to see the morphology of polyurethanes before degradation and after degradation. From this data, it appears that the polyurethane biodegradation process is going well.
Polymer Surface Observation with SEM (Scanning Electron Microscopy)
 |
Figure 3a: SEM Pu test results synthesized from PEG, MDI, TiO2 and KBH Click here to View Figure |
Polyurethanes synthesized from PEG 400, MDI, KBH starch and TiO2 from FTIR test results in Figure 2, before degradation showed urethane group C = O in the range of wave number 1700 cm-1 and N-H functional groups in the range of numbers 3250 cm-1 after degradation the two functional groups no longer exist. Whereas polyurethane synthesized by PEG 400, MDI and amylium material before degradation showed urethane functional group C = O which is in the range of 1700 cm-1 and N-H functional groups that are in the range of 3250 cm-1 after degradation of the two functional groups are no longer there is, which is in the range of 3250 cm-1 after the degradation of the two functional groups is gone. Also shown in this figure after the degradation of the OH group to increase in intensity, this gives an indication. An indication that in the degradation process, the involvement of hydroxyl groups facilitates access to polyurethane biodegradation.
Polymer surface observation with SEM (Scanning Electron Microscopy). SEM testing aims to show the morphology of a sample. In this case, the test is carried out to see the morphology of polyurethanes before degradation and after degradation. From this data, it appears that the polyurethane biodegradation process is going well.
Polyurethanes before degradation and after degradation can be seen in Figure 2. Polyurethanes synthesized from PEG, MDI, TiO2 and KBH starch before degradation showed that there were many uneven pores and after degradation, the pores appeared to have been closed, this is because polyurethanes had experienced the cracks and fragments cover the pores of polyurethane. In the picture also visible polyurethane before degradation the colour is slightly brownish-white, and after degradation, the colour changes to fade or turn pale white. In Figure 2b, the polyurethane synthesized from PEG, MDI and amylum material shows the existence of uneven pores, and after degradation, the pore has been closed, this occurs because the polyurethane has been brittle, and cracked, due to this fracture these particle fragments enter the pore and cover the polyurethane pores. Polyurethanes before degradation appear slightly brownish white colour, but after degradation, the colour fades to turn pale white. From this test shows the polyurethane has degraded well
Conclusion
Polyurethane (Pu) synthesized from KBH PEG, MDI and TiO2 starches experience a mass loss with increasing biodegradation time until there is no change (constant) on the 28th day and 35th day. The loss of Pu mass synthesized from KBH starch is 87.52% on Day 28 and Day 35. Likewise, polyurethane synthesized from PEG, MDI and amilyum experienced a mass loss of 70.59% on days 28 and 35. Whereas Pu was synthesized from KBH starch material on day 28 losing mass 0, 1987 g / day and day 35 lost mass 0, 1587 g / day. Whereas the degradability of Pu synthesized from amilyum on the 28th day was 0.1715 g / day and on the 35th day was 0.1372 g / day. FTIR test showed that before degradation there was a functional group of urethane C = O and N-H functional group after the degradation of the two groups disappeared. This also occurs in Pu synthesized from amilyum compounds before degradation, there appears to be C = O and N-H functional groups but after degradation, both of these functional groups have disappeared.
Acnowledgememts
The author would like to thank the staff of the Integrated Chemistry Laboratory of the Department of Chemistry, Faculty of Mathematics and Natural Sciences, Hasanuddin University, LIPI Bandung, and the Makassar National Police Forensic Laboratory for helping to analyze the sample, and the Indonesian Lecturer Excellence Scholarship – Indonesian Educational Fund Management Institute (BUDI-DN, LPDP) which has provided education funding.
Conflict of interest
The authors declare no conflict of interest.
References
-
- Patrycja, K.;Tamara, C.C.; Arantxa, E.; Janusz,D. European Polymer Journal., 2016, 85, 26-37.
CrossRef - Ravindra,V.G.; Pratiks, K.; Prakash, A.M.; Pradeep T.G. International Journal of Adhesion and adhesive., 2019, 95, 102427.
CrossRef - Thi, N.L.; N, TrucV.D.; Thien, V.N.; Phi, H.D.; Van, T.T.; Van, P.M.; Anh, H.N.; Duc, A.D.; Tuan, A.N.; Thi, K.A.V.; Dai, L.T.; Trong, L.L. Progress in organic Coatings., 2018, 132, 15-20.
- Fengwei, X.; Tianlong, Z.; Peter, B.; Valsala,K.B. J. Progress in Polymer Science., 2019, 90, 211-268.
CrossRef - Lisa,T.; Tim, G.M.; Raju, A.; François, M.; Ranjith, J.; Ian, G.; Pathiraja A.G., Biomaterials., 2007, 36, 5407-5417.
- Sylwia, C.; Massimo, F.B.; Krzysztof,S. Polymer Testing., 2018, 68, 135-145.
CrossRef - Tabinda,R.; Adnan,A.; Sidra,S.; Muhammad, A.; Fahad, J.; Abdul, M.; H,Tahir. Carbohydrate Polymers.. 2016, 153, 582-591.
CrossRef - Khan, S.; Nadir, S.; Shah, Z.U.; Shah, A.A.; Karunarathna, S.C.; Xu, J.; Khan, A.; Munir, S.; Hasan, F.. Environmental Pollution., 2017, 225, 469-480.
CrossRef - Kay, M.J.; Morton, L.H.G.; Prince, E.L. J International Biodeterioration., 1991, 27, 205-222.
CrossRef - Alessandro, B.; Giuliana, B.; Adriano, S.; Salvatore, M.; Donatella, C. Science of The Total Environment., 2016, 557–558,733-739.
CrossRef - Francesco, V.; Krishnapillai, S.; Emilio, L. G.; Roberta, M.; Sheridan, L. W.; Matteo, L. Soil Biology and Biochemistry ., 2008, 40,1-10.
CrossRef - Rajendra, S.; Manoj, K.; Anshumali, M.; Praveen, K. M. 3 Biotech., 2016, 6(2), 17- 26.
CrossRef - Harini, B. G. Recycling of Polyurethane Foams., 2018, 3, 29-44.
CrossRef - Sanita, S,R.; Mikelis, K.; Ugis, C. Polymer Degradation and Stability., 2019, 167, 50-57
.CrossRef
- Patrycja, K.;Tamara, C.C.; Arantxa, E.; Janusz,D. European Polymer Journal., 2016, 85, 26-37.

This work is licensed under a Creative Commons Attribution 4.0 International License.









