Efficacy of Herbal Essential Oils against Cowpea Weevil, Callosobruchus Maculatus Fabricus, and Wheat Weevil, Sitophilus Granarius L.
Farnaz Shafaie1, Shahram Aramideh*2, Oruj Valizadegan2, Mohammad Hassan Safaralizadeh2 and Ali Hosseini-Gharalari3
1Department of Plant Protection, Faculty of Agriculture, Pardis of Urmia University, Urmia, Iran.
2Department of Plant Protection, Faculty of Agriculture, Urmia University, Urmia, Iran.
3Agricultural Entomology Research Department, Iranian Research Institute of Plant Protection, Agricultural Research, Education and Extension Organization (AREEO), Tehran, Iran.
Corresponding Author E-mail: shahramaramideh@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350337
Article Received on : 08-04-2019
Article Accepted on : 13-06-2019
Article Published : 21 Jun 2019
Susceptibility of adult Cowpea Weevil, Callosobruchus maculatus F., and Wheat Weevil, Sitophilus granarius L. to essential oils of Cupressus arizonica Greene, Juniperus communis L. and Mentha longifolia L. were studied in the laboratory. The chemical composition of the essential oils were identified by GC/MS. Eicosane (27.42%), Umbellulone (12.92%) and α-pinene (10.51%) were major components of C. arizonica oil. Sabinene (31.93%), Limonene (25.62%) and Bornyl acetate (7.41%) were major components of J. communis oil. Pulegone (25.66%), L-menthone (13.43%) and Cis-para-Menthan-3, 8-diol (10.22%) were major components of M. longifolia oil. Based on the LC50 values, M. longifolia (44.06 µl/L) had the highest toxicity against C. maculatus adults, and J. communis (109.60 µl/L) had the highest toxicity against S. granarius adults. The highest mortality of C. maculatus (96.23%) happened when treated with C. arizonica (LC25) + J. communis (LC25). The highest mortality of S. granarius (63.85%) happened when treated with J. communis (LC25) + M. longifolia (LC25). There is a promising potential for controlling the cowpea weevil and the wheat weevil by using the essential oils of the studied plants.
KEYWORDS:Cowpea Weevil; Essential Oil; GC/MS; Insecticidal Effect; Wheat Weevil
Download this article as:| Copy the following to cite this article: Shafaie F, Aramideh S, Valizadegan O, Safaralizadeh M. H, Hosseini-Gharalari A. Efficacy of Herbal Essential Oils Against Cowpea Weevil, Callosobruchus Maculatus Fabricus, and Wheat Weevil, Sitophilus Granarius L. Orient J Chem 2019;35(3). |
| Copy the following to cite this URL: Shafaie F, Aramideh S, Valizadegan O, Safaralizadeh M. H, Hosseini-Gharalari A. Efficacy of Herbal Essential Oils Against Cowpea Weevil, Callosobruchus Maculatus Fabricus, and Wheat Weevil, Sitophilus Granarius L. Orient J Chem 2019;35(3). Available from: https://bit.ly/2ZxOWx6 |
Introduction
The world population will increase about 70% until 2050. Malnutrition, specifically in the developing countries, is one of the most crucial problems.1 As grains and cereal are important food sources, there have been extensive research and actions to protect them from pests and diseases.2 Damage caused by Cowpea Weevil, Callosobruchus maculatus F. (Col.: Chrysomelidae), in Nigeria is estimated to be as much as 24% to 90% (up to 2900 tons of cowpea).3 Cowpea Weevil is a cosmopolitan field-to-store pest ranked as the principal post-harvest pest of cowpea in the tropics. It causes quantitative and qualitative yield loss by seed perforation and reductions in weight, market value and germination potential of seeds.4 It causes substantial quantitative and qualitative losses manifested by seed perforation and reductions in weight, market value and germination ability of seeds.5 The Wheat Weevil, Sitophilus granarius L. (Col.: Curculionidae) is a key pest of cereals in the world. Its damage decreases yield, nutritional value, germination potential and market value.
Environmentally-friendly, cheap and effective methods are needed to control the store-grain pests.6 Control of insect pests relies heavily on the use of synthetic insecticides such as methyl bromide or phosphine. However, their intensive use has led to development of genetic resistance by insect species, residual toxicity, environmental hazards, and serious problems arising from factors such as direct toxicity to predators, pollinators, fish, and man.7 Moreover, the application of methyl bromide is being restricted due to its potential damage to ozone layer.8,9 Therefore, one of the substituted for chemical control is application of herbal essential oils, which can kill, repel, deter or sterilize the insect pest.10
Recently, efficacy of active ingredients of 2000 species, from 2500 known herbs, against different pests was evaluated. The active ingredients of essential oils are alkaloids, glycosides, steroids, volatile oils, and terpens. The insecticidal components of many plant essential oils are monoterpenoids, such as α- Pinene, Limonene, Cineol, Thymol, Menthone and Menthol, which have high volatility, and can be applied as fumigants against stored-product insects.11,12,13 Limonene is a neurotoxin that inhibits reproduction and has growth regulatory effects against insects.14 The most important components of essential oil of M. longifolia are Menthol (32.51%), Menthone (20.71%) and Pulegone (17.76%).15 Main component of essential oils of C. arizonica, J. communis and M. longifolia, cultivated in Iran, Italy and Argentina, is α-pinene.16,17 The α-Pinene is a main component of essential oil extracted from leaves of J. communis in Estonia,18 China19 and Bulgaria.20
There are several studies showing the effective control of insect pests by essential oils of plants, e.g. essential oil of C. arizonica against stored product pests,21,22 essential oil of M. longifolia against C. maculatus23,24 and essential oil of J. communis against Rhyzopertha dominica F. and Tribolium castaneum Herbst.24,25
The goals of this study were to: 1) study the insecticidal effect of essential oils of C. arizonica, J. communis and M. longifolia, against C. maculatus and S. granarius; 2) analyze the chemical composition of essential oils by Gas Chromatography and Mass Spectrometry (GC/MS).
Materials and Methods
Plants material
Aerial parts including leaves of Cupressus arizonica Greene (Cupressaceae), Juniperus communis L. (Cupressaceae) and Mentha longifolia L. (Lamiaceae) were collected from campus of Urmia University (West Azerbayjan Province, Iran) in May 2017. The samples were dried in a shadow (away from sunlight) and ventilated area (32±2°C) for 2-10 days. Each plant sample was cut into small pieces using a blade and a chopping board, and was stored in a refrigerator.
Insects rearing
Colonies of Cowpea Weevil, C. maculatus Fabricus, and Wheat Weevil, S. granarius L. adults were obtained from Toxicology laboratory of Department of Entomology (University of urmia, Iran). The weevils were reared in plastic containers (20 cm length, 14 cm width, and 8 cm height), covered by a fine mesh cloth for ventilation, containing cowpea beans and wheat grains. The culture was maintained in dark, in a growth chamber (27±1°C, 65±5% RH). Adult insects (2-3 days old) were used in fumigant toxicity tests. All experiments were carried out under the same environmental conditions.
Essential oil extraction
Plant materials were grinded into fine powder using a milling machine. Next, 650 ml of distilled water was added to 100 grams of each plant samples. The mixture was subjected to hydrodistillation using a modified Clevenger-type apparatus for 3 hours. Anhydrous sodium sulphate was used to dry out the essential oils dried. The final mixture was transferred into amber-colored vials at were kept at 4°C for further tests.26
GC/MS studies
The analyses were carried out in Jahad-e-Daneshgahi of Urmia, (West Azerbayjan Province, Iran). The chemical composition of essential oils of C. arizonica, J. communis and M. longifolia were analyzed by GC/MS using a gas chromatograph equipped with a flame ionization detector (FID) and with PH-5 capillary column (30 m × 0.1 mm; 0.25 µm film thickness). The oven temperature was held at 60°C for 3 min, programmed at 20°C/ min to 240°C and then held at this temperature for 8.5 minute. The carrier gas was Helium (99.99 %) at a flow rate of 1 ml/min. Mass spectra were taken at 70 eV. The injector temperature was 280°C. Identification of the constituents of the oil was made by comparison of their mass spectra and Retention Indices (RI) with those given in the literature and those authentic samples.27 Chemical compounds of essential oils include esters, aldehydes, alcohols, phenols, chetones and terpens. Computer matching identified compounds used as references (Wiley and Mass Finder 3.1).28,29 The relative concentrations of the separated compounds based on percentage were estimated based on chromatograms obtained from GC/FID/MS system.
Bioassay tests
Estimating LC50 value of essential oils:
Bioassay tests with essential oils were performed based on Negahban et al.,26. Five concentrations of essential oils of the three plant species (8.88, 11.74, 29.99, 129.94, 200.70 µl/L) were applied on C. maculatus adults. Concentrations applied against S. granarius adults were 13.33, 77.76, 144.04, 203.66, 471.58 µl/L. Control treatment was distilled water. Each concentration was applied into a glass container by a micropipette on a filter paper. Twenty insects (2 to 3 days old) were placed in each glass, containing 20 grams of diet (cowpea beans and wheat grains). The glass containers were sealed with a para-film strip. Treatments and control were kept at 27±1 °C and 65±5 % RH, in the dark. The number of dead insects in the treated and control dishes was counted after 24 hours and the mortality rate was estimated. Insects that did not move the legs or two posterior segments of the abdomen were considered dead.
Toxicity Effects of Essential Oils
After estimating the LC25 and LC50 values for essential oils of C. arizonica, J. communis and M. longifolia against adults, the following treatments were applied against adults of C. maculatus and S. granarius: (LC50 C. arizonica), (LC50 J. communis), (LC50 M. longifolia), (LC25 C. arizonica + LC25 J. communis), (LC25 C. arizonica + LC25 M. longifolia), (LC25 J. communis + LC25 M. longifolia) and control. The mortality rate was estimated after 24 hours.30
Data Analysis
The LC25 and LC50 values (with 95% confidence limits) were estimated using Probit Analysis. Mean mortality rates were subjected to analysis of variance (One-way ANOVA) followed by Tukey test (α=5%) using SPSS statistical analysis software.
Results
Gas Chromatography-Mass Spectrometry of Three Essential Oils
The essential oils obtained from C. arizonica had 23 compounds, the most abundant ones were Eicosane (C20H42): (27.42%), Umbellulone (C10H14): (12.92%) and α-Pinene (C10H16): (10.51%) (Table 1).
Table 1: Essential oil composition (%w/w) of leaves of Cupressus arizonica Greene cultivated in Iran.
| Component | RI | RT | % | Component | RI | RT | % |
| α-Pinene | 934 | 5.28 | 10.51 | Elemicin | 1560 | 18.9 | 0.47 |
| Sabinene | 970 | 6.05 | 1.6 | Cedrol | 1608 | 19.84 | 1.27 |
| β-Myrcene | 990 | 6.38 | 0.71 | 1-Cubenol | 1635 | 20.35 | 4.18 |
| O-Cymene | 1014 | 7.11 | 2.95 | a-acorenol | 1638 | 20.42 | 0.64 |
| Limonene | 1030 | 7.24 | 5.87 | α -Cadinol | 1657 | 20.78 | 0.78 |
| Camphor | 1148 | 9.92 | 1.41 | Eudesm-7(11)-en-4-ol | 1694 | 21.47 | 3.9 |
| Umbellulone | 1176 | 10.57 | 12.92 | Eicosane | 2158 | 28.96 | 27.42 |
| Terpeinene-4-ol | 1179 | 10.64 | 4.08 | Tetratriacontane | 2390 | 31.24 | 0.66 |
| α-Terpineol | 1193 | 10.97 | 1.38 | Nonacosane | 2606 | 33.54 | 4.82 |
| Alloaromadedrene | 1467 | 17.03 | 3.34 | Dotriacontane | 3046 | 39.92 | 1.45 |
| 1s,cis-calamenene | 1525 | 18.22 | 3.47 | 17-Pentatriacontene | 3644 | 48.6 | 0.98 |
| Candina-1(2),4-diene,cis | 1538 | 18.46 | 0.86 |
The essential oils obtained from J. communis had 25 compounds, the most abundant ones were Sabinene (C10H16): (31.93%), Limonene (C10H16): (25.62%) and Bornyl acetate (C12H20): (7.41%) (Table 2).
Table 2: Essential oil composition (%w/w) of leaves of Juniperus communis L. cultivated in Iran.
| Component | RI | RT | % | Component | RI | RT | % |
| Thujene | 928 | 5.1 | 0.07 | Terpinene-4-ol | 1179 | 10.64 | 3.37 |
| α-Pinene | 934 | 5.28 | 1.29 | α-Terpineol | 1193 | 10.97 | 0.51 |
| Sabinene | 970 | 6.05 | 31.93 | Trans-carveol | 1219 | 11.57 | 0.3 |
| β- Myrcene | 989 | 6.35 | 2.52 | Isobronyl formate | 1226 | 11.73 | 0.34 |
| 1,4-Cineole | 1014 | 6.89 | 1.86 | Caryone | 1245 | 12.17 | 0.61 |
| O-Cymene | 1014 | 7.11 | 3.36 | Bronyl acetate | 1287 | 13.13 | 7.41 |
| Limonene | 1030 | 7.24 | 25.62 | Laranduly acetate | 1293 | 13.26 | 3.33 |
| C-Terpinene | 1057 | 7.84 | 1.87 | A-Selinene,7-epi- | 1517 | 18.05 | 0.67 |
| Linolool | 1098 | 8.78 | 2.13 | Germacrene B | 1551 | 18.73 | 3.42 |
| Limonene oxide,trans- | 1122 | 9.32 | 0.51 | Cubenol,1,10-di-epi- | 1614 | 19.95 | 0.51 |
| Terpen-1-ol | 1134 | 9.61 | 0.49 | A-Muurolol | 1645 | 20.54 | 1.2 |
| Camphor | 1148 | 9.92 | 0.65 | A-Cadinolii | 1658 | 20.79 | 2.39 |
| 3-Hexenyl butanoate,(z)- | 1168 | 10.4 | 0.41 |
The essential oils obtained from M. longifolia had 29 compounds, the most abundant ones were Pulegone (C10H16O) (25.66%), L-Menthone (C10H18O): (13.43%) and Cis-para-Menthan-3, 8-diol (C10H20O): (10.22%) (Table 3).
Table 3: Essential oil composition (%w/w) of leaves of Mentha longifolia L. cultivated in Iran.
| Component | RI | RT | % | Component | RI | RT | % |
| α-Pinene | 934 | 5.28 | 5.49 | Cis-piperitone oxide | 1257 | 12.45 | 3.61 |
| Sabinene | 970 | 6.05 | 0.97 | Thymol | 1280 | 13.19 | 0.52 |
| β-Myrcene | 990 | 6.38 | 0.51 | P-Cymen-7-ol | 1294 | 13.28 | 0.66 |
| O-Cymene | 1014 | 7.11 | 1.19 | Piperitenone | 1343 | 14.37 | 0.79 |
| Limonene | 1030 | 7.24 | 2.91 | α-Terpinyl acetate | 1350 | 14.53 | 9.47 |
| 1,8-Cineole | 1032 | 7.3 | 0.99 | Piperitenone oxide | 1368 | 14.92 | 2.17 |
| Linalool 1 | 1099 | 8.8 | 0.33 | Trans-Caryophyllene | 1425 | 16.15 | 0.66 |
| Camphor | 1148 | 9.92 | 0.54 | Alloaromadendrene | 1467 | 17.03 | 1.36 |
| L- Menthone | 1157 | 10.15 | 13.43 | 1s,cis-Calamene oxide | 1525 | 18.22 | 2.19 |
| Isomenthone | 1166 | 10.35 | 1.56 | Caryophyllene oxide | 1589 | 19.49 | 0.74 |
| Cis-para-Menthan-3,8-diol | 1175 | 10.56 | 10.22 | Cedrol | 1608 | 19.84 | 0.79 |
| Terpinene-4-ol | 1179 | 10.64 | 2.87 | 1-Cubenol,epi- | 1635 | 30.35 | 2.42 |
| α-Terpinneol | 1193 | 10.97 | 0.96 | α-Cadinol | 1657 | 20.78 | 0.77 |
| Cis-dihydrocaryone | 1202 | 11.11 | 1 | Eudesm-7(11)-en-4-ol | 1694 | 21.47 | 2.74 |
| Pulegone | 1240 | 12.15 | 25.66 |
Bioassay Tests
Fumigant Toxicity
The LC50 values of C. arizonica, J. communis and M. longifolia essential oils against adult Cowpea Weevil after 24 hours were 106.64, 96.83 and 44.06 µl/L air, respectively (Table 4).
The results of the analysis of variance of mean mortality of C. maculatus treated with the seven treatments showed that there was significant difference among treatments which included M. longifolia essential oils (F6,14=20.059; P≤ 0.001).; however, no significant differences was observed between treatments of C. arizonica and J. communis.
Table 4: Fumigant toxicity of three essential oils on adult Cowpea Weevil, C. maculatus.
| Treatment† | Slope (±SE) | Intercept (±SE) | Chi square§ | LC25 (µl/L air) | 95% confidence limits for dose |
| (lower-upper) | LC50 (µl/L air) | ||||
| 54.8 | (lower-upper) | ||||
| C. arizonica | 2.356 (±0.074) | -0.291 (±0.821) | 0.581 | (31.84-71.83) | 106.64 |
| 39.28 | (81.16-145.27) | ||||
| J. communis | 1.675 (±0.004) | -0.846 (±0.743) | 1.174 | (30.69-48.87) | 96.83 |
| 25.18 | (65.18-124.05) | ||||
| M. longifolia | 2.843 (±0.062) | -1.698 (±0.957) | 1.285 | (19.99-29.62) | 44.06 |
| (29.14-55.99)* |
* 95 % lower and upper fiducial limits are shown in parenthesis.
† There were 60 insects n each treatment.
§ DF= 3
LC50 values of C. arizonica, J. communis and M. longifolia essential oils against adults of wheat weevil after 24 hours were 171.08, 109.60 and 297.35 µl/L air, respectively.
The results of the analysis of variance of mean mortality of S. granarius treated with the seven treatments showed that there was significant difference among treatments (F6,14=116.848; P≤ 0.001) (Table 5).
Table 5: Fumigant toxicity of three essential oils on adult Wheat Weevil, S. granarius.
|
Treatment† |
Slope (±SE) | Intercept (±SE) | Chi square§ | 95% confidence limits for does | |
| LC25 (µl/L air) | LC50 (µl/L air) | ||||
| (lower-upper) | (lower-upper) | ||||
| C. arizonica | 1.163 (±0.054) | -0.978 (±0.561) | 2.638 | 64.47 | 171.08 |
| (55.47-82.31) | (149.53-237.89)* | ||||
| J. communis | 2.201 (±0.028) | -1.942 (±0.795) | 3.374 | 38.36 | 109.6 |
| (15.14-48.13) | (68.46-127.54)* | ||||
| M. longifolia | 1.326 (±0.116) | -0.615 (±0.870) | 2.085 | 137.38 | 297.35 |
| (94.36-176.39) | (261.39-378.64)* |
* 95 % lower and upper fiducial limits are shown in parenthesis.
† There were 60 insects n each treatment.
§ DF= 3
Toxicity effects of essential oils
The results of Tukey test indicated that the highest moratlity rate of C. maculatus was observed when treated with ‘LC25 C. arizonica + LC25 J. communis’ (96.23%) followed by ‘LC25 C. arizonica + LC25 M. longifolia’ (85.06%) (Figure 1).
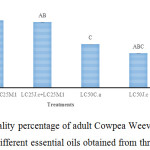 |
Figure 1: Mean (±SE) mortality percentage of adult Cowpea Weevil, C. maculatus, 24 hours after treatment with different essential oils obtained from three plant species. |
Columns which have at least one letter in common were not significantly different based on Tukey test (α=5%).
The results of Tukey test indicated that the highest moratlity rate of S. granarius, was observed when treated with ‘LC25 J. communis + LC25 M. longifolia’ (63.85%) (Figure 2). Therefore, the essential oils resulted in higher mortality rates when applied in combination compared to when applied alone.
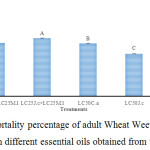 |
Figure 2: Mean (±SE) mortality percentage of adult Wheat Weevil, S. granarius 24 hours after treatment with different essential oils obtained from three plant species. |
Columns which have at least one letter in common were not significantly different based on Tukey test (α=5%).
Discussion
In recent years, due to the excessive application of chemical pesticides which have had harmful effects on environment, much tendency has gone towards the application of less harmful compounds such as herbal extracts. In this study, three essential oils were tested for their fumigant toxicity against adults of C. maculatus and S. granarius. Monoterpenoids have strong toxicity to insects due to high volatility that can penetrate into insects rapidly and interfere in physiological functions.31,32 C. arizonica, J. communis and M. longifolia are composed mainly of monoterpene (18.18, 45.89 and 50.16) % and cyclic monoterpene (5.87, 26.13 and 2.91) %, respectively.
There was difference in the percentage of the ingredients of the essential oils in our study compared to the other similar reports. The chemical constituents of M. longifolia oil collected by Monfared et al.33 from Iran (Tehran province), extracted by the same method as ours, included Carvone (61.8%) and Limonene (19.4%) as the major constituents out of 23 compounds. Salman et al.34 from Saudi Arabia (Taif), reported that M. longifolia oil has 38 compounds with Pulegone (33.21%) and Menthone (28.53%) being the major constituents. Another study showed that the essential oil components of M. longifolia are cis-epoxy piperitone (18.4%), pulegone (15.5%) and piperitenone oxide, (14.7%).35 Another research by Saeidi et al.36, conducted using specimens collected from five different regions in South-West of Iran, showed that the major compounds of M. longifolia are Piperitenone oxide (7.41 to 59.67%) and Pulegone (3.61 to 49.43%). In our research, which was conducted using specimens collected from North-West of Iran, 29 compounds were found, among which, Pulegone (25.66%) and L-Menthone (13.43%) were the major constituents.
Angioni et al.37 reported that the essential oils of leaves of J. communis, from Italy, included 35 components, among which Sabinene was the most abundant component (61.09%). Also Sabinene has been reported as the major component of essential oil in the plant species collected from North of Iran (19.2%).38 The major components of J. communis are monoterpene (83.21%) and sesquiterpene (1.27%). Results of our study showed that the essential oil composition of J. communis included 25 components, among which Sabinene (31.93%), Limonene (25.62%) and Bronyl acetate (31.93%) were the main components. In our study, Sabinene was the dominate component was similar to report from Italy and Iran.37,38 The composition of the essential oil from the leaves of J. communis in our research was different from reports of China, Republic of Macedonia and Albania, as in other studies α-Pinene was the major component. Carroll et al.19 reported that the major component of J. communis oil in China was α-Pinene (26.9%). Sela et al.39 reported that essential oil of junipers in the Republic of Macedonia included mainly α-Pinene (15.59-43.19%), β-Pinene (1.65%-5.35%), β-Myrcene (2.89%-26.50%) and Sabinene (2.80-11.77%). Buci et al.,40 reported that the chemical composition of J. communis essential oil in Albania icluded 56 compounds among which the major components were α-pinene (35.8%), β-myrcene (44.9 %), Sabinene (10.0%) and Germacene D (4.59%). Also J. communis oil is composed mainly of monoterpenes (71.8%).
This study results showed that α-Pinene (10.52%) was one of the main components of the essential oil of C. arizonica, which was similar to other reports.The essential oil of C. arizonica in Tunisia was mainly composed of α-Pinene (20%), Umbellulone (18.4%) and Limonene (5.8%).41 The α-Pinene was one of the main components of the oil obtained from leaves of C. arizonica cultivated in Iran (19.2%)16, Italy (7.8%)42 and Argentina (22.9%).17
In literature there are differences among the major compounds, the number and percentage of compounds in essential oils of plants. These differences can be due to factors such as geographic location, plant organs used, extraction methods, season of sampling and soil composition.43,44,45
Studies of Javadi Elmi et al.46 about the respiratory toxicity of M. longifolia essential oil against adults of C. maculatus indicated that this essential oil, at the highest concentration of 685.42 µl/L air, caused 100 % mortality. They also reported the LC50 value of M. longifolia essential oil as 134.04 µl/L air. In our study, M. longifolia essential oil had a LC50 value of 44.06 µl/L air, which resulted in an acceptable control of C. maculatus. The study of Khani and Asghari47, on insecticide activity of essential oils of M. longifolia, Pulicaria gnaphalodes and Achillea wilhelmsii against C. maculatus and T. castaneum adults, showed that essential oil of M. longifolia (LC50 value= 13.05 µl/L air) and A. wilhelmsii (LC50 value= 10.02 µl/L air) had strong insecticidal activity. Our study showed that J. communis essential oil had a high insecticidal effect against S. granarius (LC50 value=109.60 µl/L air). Hashemi and Roostaefar24 study on the insecticidal effect of J. communis essential oil on Rhizopertha dominica and Tribolium confusum showed that this essential oil was efficient against both pests, while LC50 value was 36.96 µl/L air for R. domonica and 107.96 µl/L for T. confusum. In our study, LC50 value of J. communis against C. maculatus was96.83 µl/L air, while it was109.60 µl/L air against S. granarius. Therefore, C. maculatus was more susceptible than S. granarius.
In our study, C. maculatus adults were more susceptible to M. longifolia essential oil (LC50 = 44.06 μl/L air) than to J. communis (LC50 = 96.83 μl/L air) and C. arizonica (LC50 = 106.64 μl/L air). In addition, C. maculatus was more susceptible than S. granarius. Saeidi and Moharramipour48 reported that T. confusum adults were more susceptible to Artemisia khorassanica (LC50 = 22.45 μl/L air) and R. officinalis essential oils (LC50 = 22.14 μl/L air) than to M. longifolia oil (LC50 = 39.96 μl/L air). Hesami Rad and Aramideh49 studied the effects of essential oils from Juniperus polycarpus L., wood vinegar and acetone on C. maculatus and S. granarius. The LC50 values of J. polycarpus, wood vinegar and acetone on S. granarius and C. maculates, after 24 hour, were 21.28, 93.72, 20.05 and 59.53, 96.23, 35.76 µl/L air, respectively. The results showed that acetone in mixed with J. polycarpus had the highest mortality on S. granarius and C. maculatus. The results of our research indicated that the highest mortality rate of C. maculatus was observed when treated with ‘LC25 C. arizonica + LC25 J. communis’ (96.23% mortality) while the highest mortality rate of S. granarius was observed when treated with ‘LC25 J. commonis + LC25 M. longifolia’ (63.85% mortality). Therefore, the application of three essential oils of C. arizonica, J. communis and M. longifolia against Cowpea Weevil and Wheat Weevil, can be recommended as a suitable and safe control method in pest management programs.
Acknowledgement
We thank the members of the laboratory of Entomology and the Department of Plant Protection of Urmia University and Jahad-e-Daneshgahi of Urmia for supplying the herbal essential oils and the assisting in chemical analysis.
References
- Talukder, F. J. Agr. Mar. Sci. 2009, 14, 9-15.
- Kim, S. I.; Roh, D. H.; Lee, H. S.; and Ahn, Y. J. J. Stored. Prod. Res. 2003, 39, 293-303.
- Tapondjou, A. L.; Adler, C.; Fontem, D. A.; Bouda, H; and Reichmuth, C. J. Stored. Prod. Res. 2001, 41, 91-102.
- Bamphitlhi, T.; Kesegofetse, T.; and Seipati, S. Int. J. Insect. Sci. 2014, 6, 77-84.
- Oluwafemi, A. R. SOAJ. Entomol. 2012, 1, 87–99.
- Stejskal, V.; and Kucerova, Z. J. Applied. Entomol. 1996, 120, 143-146.
- Mahmud, M. K.; Khan, M. K. H.; Husain, M.; Alam, M. I.; and Afrad, S.I. J. Asiat. Soc. Bangladesh. 2002, 28, 11-18.
- Athanassiou, C.G.; Kavallieratos, N. G.; Vayias, B. J.; and Panoussakis, E. C. J. Stored. Prod. Res. 2008, 44, 279-284.
- Lolestani, F. A.; and Shayesteh, N. J. Biologic. Sci. 2009, 9, 92-95.
- Kieta, S. M.; Vincent, C.; Schmit, J.; Arnason, J. T.; and Belanger, A. J. Stored. Prod. Res. 2001, 37, 339-349.
- Coats, J.; Karr, L.; and Drewes, C. ACS. Washington, DC. 1991, 305–316.
- Regnault-Roger, C.; and Hamraoui, A. J. Stored. Prod. Res. 1995, 31, 291-299.
- Ahn, Y.; Lee, B.; Lee, H.; and Kim, H. J. Chem. Ecol. 1998, 24, 1-90.
- Khani, M.; Muhamad, A. R.; and Omar, D. J. Med. Plants. 2012, 11, 97–110.
- Hafedh, H.; Fethi, B. A.; Mejdi, S.; Emira, N.; and Amina, B. Afr. J. Microbial. Res. 2010, 4, 1122-1127.
- Afsharypuor, S.; and Tavakoli, P. J. Essent. Oil. Res. 2005, 17: 225-226.
- Malizia, R. A.; Acardell, D.; Molli, J. S.; Gonzalez, S.; Guerra, P. E. and Grau, R. J. J. Essent. Oil. Res. 2000, 12, 59-63.
- Orav, A.; Kailas, T.; and Murrisepp, M. Nation. Pro. Res. 2010, 24, 1789-99.
- Carroll, J. F.; Tabanca, N.; Kramer, M.; Elejalde, N. M.; Wedge, D. E.; Bernier, U. R.; Coy, M.; Becnel, J. J.; Demirci, B.; Baser, K. H. C.; Zhang, J.; and Zhang, S. J. Vector. Ecol. 2011, 36, 258-268.
- Adams, R. P.; and Tashev, A. N. Phytologya. 2013, 95, 302-307.
- Abad, M. K. R.; Besheli, B. A.; and Sharif, M. M. Int. J. Agron. Plant. Pro. 2013, 4, 2531-2536.
- Kabiri Raiisabad, M.; Mohammadi Sharif, M.; and Kabiri Nasab, M. Agric. Sci. J. 2014, 3, 1-11. (In Persian).
- Almasi, S.; Shakarami, J.; and Fallahzadeh, M. National. Con. Agric. Manage. 2011.
- Hashemi, S. M.; and Roostaefar, A. Ecologia. Balkanica. 2014, 6, 87-93.
- Gordien, A. I.; Gray, A. I.; Franzblau, S. G.; and Seidel, V. J. Ethnopharmacol. 2009, 79, 57-67.
- Negahban, M.; Moharramipour, S.; and Sefidkon, F. J. Stored. Prod. Res. 2007, 43, 123-128.
- Adams, R. P. Allured. Pub. Co. Carol. Stream. IL. USA, 2001, 456 p.
- Koenig, W. A., Joulain, D. and Hochmuth, D. H. 2004, 493 pp.
- Mc Lafferty, F.W.; and Stauffer, D. B. J. Chem. Educ. New York. 1989, 66, 1-7.
- Abbott, W. S. J. Econ. Entomol. 1995, 18, 265-267.
- Negahban, M.; Moharramipour, S.; and Sefidkon, F. J Asia Pac Entomol. 2006, 9, 381 – 8.
- Lee, B. H.; Lee, S. E.; Annis, P. C.; Pratt, S. J.; Park, B. S.; and Tumaalii, F. J Asia Pac Entomol. 2002, 5, 237-40.
- Monfared, A.; Nabid, M. R.; and Rustaeiyan, A. A. H. J. Essent. Oils Res. 2002, 14, 51-5.
- Salman, M.; Abdel Hamed, E. S. S.; Bazaid, A. S.; and Dabi, M. M. Sch. Res. Lib. 2015, 7, 34-40.
- Gulluce, M.; Sahin, F.; Sokmen, M.; Ozer, H.; Daferera, D.; Sokmen, A.; Polissiou, M.; Adiguzel, A.; and Ozkan, H. Food Chem. 2007, 103, 1449-1456.
- Saeidi, Z.; Babaahmadi, H.; Saeidi, K. A.; Salehi, A.; Saleh Jouneghani, S.; Amirshekari, H.; and Taghipour, A. J. Med. Plants. Res. 2011, 6, 4522-4525.
- Angioni, A.; Barras, A.; Russo, M. T.; Coroneo, V.; Dessi, S.; and Cabras, P. J. Agric. Food. Chem. 2003, 51, 3073-3078.
- Shahmir, F.; Ahmadi, L.; Mirza, M.; and Korori, S. A. A. Flavour Fragr J. 2003, 18, 425.
- Sela, F.; Karapandzova, M.; Stefkov, G.; and Kulevanova, S. Maced Pharm Bull. 2011, 57, 43–51.
- Buci, A.; Hodaj Celiku, E.; Manaj, H.; Abazi, S.; Drushku, S.; and Lazari, D. J Int Env App Sci. 2018, 13, 15-19.
- Che´raif, H.; Jannet, B.; Hammami, M.; Khouja, M. L.; and Mighri, Z. Biochem Syst Ecol. 2007, 35, 813-820.
- Flamini, G.; Cioni, P. L.; Morelli, I.; Bighilli, A.; Castola, V.; and Casanora, J. J. Essent. Oil Res. 2003, 15, 302-304.
- Farooqi, A.; Sharama, S.; Naqvi, A.; and Khan, A. J. Essent. oil Res. 1993, 5, 305-309.
- Jaymand, K.; and Rezaie, M. B. Iranian J. Med. Aro. Plants. 2001, 9, 2-145.
- Bakkali, F.; Averbeck, S.; Averbeck, D.; and Idaomar, M. Food. Chem. Toxicol. 2008, 46, 446-75.
- Javadi Elmi, M.; Shakarami, J.; and Bandani, A. New. Agric. Finding. 2007, 2, 75-81. (In Persian).
- Khani, A.; and Asghari, J. J. Insect Sci. 2012, 12, 1536-2442.
- Saeidi, M.; and Moharramipour, S. Crop. Prot. 2013, 2, 23-31.
- Hesami Rad, Sh.; and Aramideh, Sh. Master. Sci. thesis. 2014.

This work is licensed under a Creative Commons Attribution 4.0 International License.









