Grafting of Oleic Acid on Cyclic Natural Rubber (Resiprene-35) using Dicumyl Peroxide Initiator and Divinylbenzene Compatibilizers for Paint Binder in Polyamide Thermoplastics
Barita Aritonang*1,2 , Tamrin2, Basuki Wirjosentono2 and Eddiyanto3
, Tamrin2, Basuki Wirjosentono2 and Eddiyanto3
1Department of Chemistry, University of Sari Mutiara Indonesia, Medan-20123, Indonesia.
2Doctoral Program Department of Chemistry, University of Sumatera Utara, Medan-20155, Indonesia.
3Department of Chemistry, University of State Medan, Medan-20221, Indonesia.
Corresponding Author E-mail: baritaaritonang11@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350119
Article Received on : 23-11-2018
Article Accepted on : 24-01-2018
Article Published : 18 Feb 2019
In this paper, we reported about modifications of Cyclic Natural Rubber (CNR) with Oleic Acid (OA) and divinylbenzene (DVB) and dicumyl peroxide (DCP). The aim of this research isto improve their compatibility as a paint binder in polyamide thermoplastic substrates. The method of this research is grafting method in order to know grafting process of CNR to generate CNR-g-OA, and its compatibility after being blended with Polyamide (PA). Analysis of CNR-g-OA was carried out by using FTIR and SEM. The results showed that the best compositions ratio of CNR-g-OA are CNR (100 phr), OA (6 phr), DCP (0.5 phr); and DVB (0.5 phr). The FTIR data of CNR-g-OA show that there is a sharp and weak peak at 1700 to 1800 cm-1, indicating that is carbonyl (C=O). It is oleic acid. The SEM data of CNR-g-OA is totally difference compare to CNR-g-OA with polyamide after the addition of DVB. It is caused the grafting was succeed to generate CNR-g-AO and compatible with PA.
KEYWORDS:Cyclic Natural Rubber; Dicumyl Peroxide; Divinylbenzena; Grafting; Oleic Acid; Polyamide
Download this article as:| Copy the following to cite this article: Aritonang B, Tamrin T, Wirjosentono B Eddiyanto E. Grafting of Oleic Acid on Cyclic Natural Rubber (Resiprene-35) using Dicumyl Peroxide Initiator and Divinylbenzene Compatibilizers for Paint Binder in Polyamide Thermoplastics. Orient J Chem 2019;35(1). |
| Copy the following to cite this URL: Aritonang B, Tamrin T, Wirjosentono B Eddiyanto E. Grafting of Oleic Acid on Cyclic Natural Rubber (Resiprene-35) using Dicumyl Peroxide Initiator and Divinylbenzene Compatibilizers for Paint Binder in Polyamide Thermoplastics. Orient J Chem 2019;35(1). Available from: https://bit.ly/2TU67XB |
Introduction
Chemical modification of natural rubber is one of the efforts made to improve the properties of natural rubber so that a new product is obtained, such as modification of natural rubber through cyclizaton reaction is cyclic natural rubber / resiprene-35. Cyclic natural rubber (CNR) is currently widely used as a resin for the paint, adhesive and printing ink industries because it has the advantages that are resistant to abrasion (friction strength), resistant to water (corrosion resistant), and has good adhesion to metals, wood, glass and paper.1,2,3
Cyclic natural rubber also still has the disadvantages of physical chemical properties in terms of the compatibility of polyamide thermoplastic surfaces. The weak adhesive properties can be caused by low compatibility between CNR as a paint binder with the surface of a polyamide thermoplastic.4,5
Blending of cyclic natural rubber with the polyamide thermoplastics is incompatible caused by the properties of polymer materials which have different polarity components. If the two ingredients are blended, a non-polar system is obtained without any chemical bonds between the two polymer materials. Incompatible polymer blends cause the adhesion of cyclic natural rubber are reduced. Besides that, cyclic natural rubber is also susceptible to free radical attacks such as inorganic acid and ozone, due to the presence of carbon double bonds (> C = C <) in cyclic carbon chains.5,6,7
To overcome the disadvantages of the cyclic natural rubber as well as to improve the performance of the adhesive properties and compatibility with the surface of the polyamide thermoplastic, it is necessary to modify the surface of cyclic natural rubber in order to obtain high adhesive properties so that it can interact with polyamide. The effective method for modifying the surface of cyclic natural rubber is by grafting.8,9,10
Previous studies on chemical modification with grafting techniques have been carried out to produce a product as expected. Siregar, et.al11 conducted research on maleic anhydride grafted on cyclic natural rubber in internal mixers, physical properties and compatibility with polyamide. Based on the results of research, CNR-g-AM products were obtained, where increasing maleic anhydride concentration causes the maleic group grafted on the CNR polymer chain also increases. Grafting products have improved compatibility with polyamide. Nasution et.al12 conducted research of cyclic natural rubber grafting with methyl methacrylate using dicumyl peroxide initiator. Based on the results of research, CNR-g-MMA products were obtained, which are characterized by the appearance of wave number absorption peaks in the area of 1731 cm-1 (absorption of carbonyl groups) typical for carbonyl (C=O) from methyl methacrylate.
Oleic acid is a polar un saturated fatty acid and has a double bond in the carboxylic group. Oleic acid is expected to improve physical and mechanical properties, and compatibility of cyclic natural rubber because oleic acid can be converted into epoxide oil which will be used as a monomer, so that the process of grafting oleic acid monomers in the molecular chain of cyclic natural rubber can occur. This has been proven by Zhou13 that the oleic acid monomers was successfully grafted into the main chain of acrylonitrile-butadiene-styrene through grafting process using benzoyl peroxide initiator in 1,2-dichloroethane solution. The results obtained that oleic acid can increase the flexibility, elasticity and stability of the polymer against heat and ultraviolet radiation. Liu14 explained that oleic acid monomers have been successfully grafted on low density polyethylene in the internal mixer to form copolymers by using dicumyl peroxide initiator. In order to obtain good morphological results for incompatible polymeric materials, a compatibilizers are needed, such as divinylbenzene (DVB).15,16
In this research, it is expected that the addition of DVB compatibility can increase reactivity in CNR interactions with polyamide thermoplastics. Characterization of CNR-g-OA includes morphology test by Scanning Electron Microscopy (SEM), and chemical-physics interaction test by Fourier Transform Infrared (FTIR) spectroscopy.
Experimental
Materials
The chemicals used are from E-Merck, namely Acetone, Ethanol, Xylene, Dicumyl Peroxide (DCP), Divinylbenzene (DVB), Oleic Acid, Hydrochloric Acid (HCl), and Polyamide (PA).
Modified material is Cyclic Natural Rubber (CNR)/ Resiprene-35) sourced from PT. Nusantara Rubber Industry (PT. IKN) in the North Sumatera Province which is engaged in processing natural rubber into resin.
Oleic Acid Grafting Process Onto CNR Using Initiator Dicumyl Peroxide
100 phr (33 g) cyclic natural rubber (CNR) is weighed, then CNR is slowly inserted into the internal mixer, after that the temperature is set to 160°C on the internal mixer, and left for 4 minutes until the CNR melts perfectly, then added with oleic acid 6 phr (2 g) followed by the addition of DCP initiators using several variations 0.25 phr (0.1 g); 0.5 phr (0.2 g); and 1.0 phr (0.3 g) until homogeneous. The blending process is carried out for 8 minutes to experience a grafting reaction. After 8 minutes, the process is stopped. In hot conditions quickly the reaction product is removed from the internal mixer. The results obtained are then cooled and made into granules/pellets. The same was also carried out for variations of oleic acid 3 phr (1 g), 6 phr (2 g), and 9 phr (3 g) with DCP initiators 0.5 phr (0.2 g). The variations of blends are listed, as in Table 1 and Table 2.
Table 1: Variation of dicumyl peroxide initiator.
|
Sample |
Cyclic Natural Rubber (CNR) |
Oleic Acid (OA) |
Dicumyl Peroxide (DCP) |
|||
| DCP-0 |
100 phr |
33 g |
6 phr |
2 g |
0.00 phr |
0.0 g |
| DCP-0,25 |
100 phr |
33 g |
6 phr |
2 g |
0.25 phr |
0.1 g |
| DCP-0,5 |
100 phr |
33 g |
6 phr |
2 g |
0.50 phr |
0.2 g |
| DCP-1 |
100 phr |
33 g |
6 phr |
2 g |
1.00 phr |
0.3 g |
*phr (Parts per Hudred Rubber)
Table 2: Variation of oleic acid.
|
Sample |
Cyclic Natural Rubber (CNR) |
Oleic Acid (OA) |
Dicumyl Peroxide (DCP) |
|||
| OA-3 |
100 phr |
33 g |
3 phr |
1 g |
0.50 phr |
0.2 g |
| OA-6 |
100 phr |
33 g |
6 phr |
2 g |
0.50 phr |
0.2 g |
| OA-9 |
100 phr |
33 g |
9 phr |
3 g |
0.50 phr |
0.2 g |
* phr (Parts per Hudred Rubber)
Then the result of CNR that has been grafted with oleic acid (CNR-g-OA) are refluxed with 100 mL of xylene with a series of reflux devices which are heating, boiling pumpkin and liebig condenser at 110°C until dissolved. After dissolving, 100 mL of ethanol is added until the precipitate is formed. The precipitate formed is filtered using filter paper which has been connected to a vacuum pump and washed with ethanol repeatedly in order to dissolve the remaining acidic reactions. The precipitate formed was dried in oven at a temperature of 120°C for 24 hours. Specific results were characterized by FTIR.
Oleic Acid Grafting Process Onto CNR using Initiator Dicumyl Peroxide and Compatibilizers Divinylbenzene
100 phr (33 g) Cyclic natural rubber (CNR) is weighed, then CNR is slowly inserted into the internal mixer, after that the temperature is set to 160°C on the internal mixer, and left for 4 minutes until the CNR melts perfectly, then added with oleic acid as much as 6 phr (2 g), followed by the addition divinylbenzene 0.25 phr (0.1 g); 0.5 phr (0,2 g); and 1.0 phr (0,3 g) and DCP initiators of 0.5 phr (0.2 g) until homogeneous. The blending process is carried out for 8 minutes to experience a grafting reaction. After 8 minutes, the process is stopped. In hot conditions quickly the reaction product is removed from the internal mixer. The results obtained are then cooled and made into granules / pellets. The variations of blends are listed, as in Table 3.
Table 3: Variation of Divenylbenzene.
|
Sample |
Cyclic Natural Rubber (CNR) |
Oleic Acid (OA) |
Dicumyl Peroxide (DCP) |
Divenyl Benzene (DVB) |
||||
| DVB-0,25 |
100 phr |
33 g |
6 phr |
2 g |
0.50 phr |
0.2 g |
0.25 phr |
0.1 g |
| DVB-0,5 |
100 phr |
33 g |
6 phr |
2 g |
0.50 phr |
0.2 g |
0.50 phr |
0.2 g |
| DVB-1 |
100 phr |
33 g |
6 phr |
2 g |
0.50 phr |
0.2 g |
1.00 phr |
0.3 g |
*phr (Parts per Hudred Rubber)
Then the result of cyclic natural rubber that has been grafted with oleic acid (CNR-g-OA) are refluxed with 100 mL of Xylene with a series of reflux devices which are heating, boiling pumpkin and Liebig condenser at 110°C until dissolved. After dissolving, 100 mL of ethanol is added until the precipitate is formed. The precipitate formed is filtered using filter paper which has been connected to a vacuum pump and washed with ethanol repeatedly in order to dissolve the remaining acidic reactions. The precipitate formed was dried in oven at a temperature of 120°C for 24 hours. Specific results were characterized by FTIR.
Compatibility of CNR-g-OA and Polyamide Resin
80 g of polyamide are put into erlenmeyer and then dissolved with 20 mL of HCl 25% and 2 mL of acetone after that stirred for ± 20 minutes until all polyamide resins dissolve completely. 20 g of CNR-g-OA is put into erlenmeyer and then dissolved with 100 mL of xylene, and stirred using a magnetic stirrer for ± 20 min until it dissolves and homogeneous. Polyamide solution is added to CNR-g-OA solution, then the mixture is stirred using a magnetic stirrer for ± 20 minutes until it is mixed homogeneously. The blends is then formed thin layer on the glass using a film forming device, with a thickness of 120 micrometers. The thin layer formed was waited until dry after it was characterized by FTIR and Scanning Electron Microscopy to determine the compatibility of CNR-g-OA with Polyamide. The same procedure was also carried out for CNR-g-OA/ PA using DVB compatibilizer.
Result and Discussion
Results of Grafting Oleic Acid on CNR Using Dicumyl Peroxide Initiator
The grafting process of oleic acid on CNR is carried out using the initiator dicumyl peroxide, and the composition refers to Table 1. The results obtained from the dicumyl peroxide variations are shown in Figure 1.
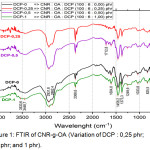 |
Figure 1: FTIR of CNR-g-OA (Variation of DCP : 0,25 phr; 0,5 phr; and 1 phr). |
Of all the variations, it appears that all peaks with wide absorption and low intensity at 3448.7 cm-1 shows the presence of OH from oleicacid. The presence of absorption at 2931.8 cm-1 and low intensity shows the presence of the aliphatic -CH of CNR. At absoption 1604.8 cm-1 it shows the strain vibration -C=C-. In the area 1500 to 500 cm-1, there is a peak at 1458.2 cm-1 indicates a vibration strain CH2, at 1373.3 cm-1 indicates the presence of CH3. At 1033.8 cm-1 indicates the presence of C-O.
Meanwhile, in the variation of DCP-0.5, sharp absorption with very low intensity was seen at 1712.8 cm-1 which indicates the presence of C=O, this means that oleic acid has been grafted on CNR even though the intensity is very low.17 Furthermore, variations are made for oleic acid (3, 6, and 9) phr, with the composition of the DCP initiator is fixed 0.5 phr, so that the FTIR results are obtained as shown in Figure 1.
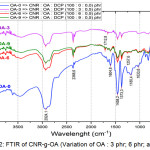 |
Figure 2: FTIR of CNR-g-OA (Variation of OA : 3 phr; 6 phr; and 9 phr). |
Of all variations of OA-3, OA-6, and OA-9 peak appeared on 3448.7 cm-1; 2931.8 cm-1; 1604.8 cm-1; 1458.2 cm-1; 1373.3 cm-1; 1033.8 cm-1. At OA-6 there is a C=O at 1712.8 cm-1 which indicate that oleic acid has been grafted on CNR even though in very small amounts. At OA-0 there is a different spectrum form for CNR without oleic acid with high intensity, as shown at 2924.1 cm-1; 1604.8 cm-1; 1458.2 cm-1; 1373.3 cm-1; 1033.8 cm-1, also in the absorption area of 779.2 cm-1.
Results of Oleic Acid Grafting on CNR Using Dicumyl Peroxide Initiator and Divinyl Benzena
The addition of divinylbenzene in the blends as a compatibilizer, so that the bond between CNR and OA becomes stronger than before the additions of DVB, as shown in Figure 3.
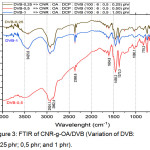 |
Figure 3: FTIR of CNR-g-OA/DVB (Variation of DVB: 0,25 phr; 0,5 phr; and 1 phr). |
Of all variations of DVB-0.25; DVB-0.5; and DVB-1 shows the absorption peak at 3425.6 cm-1; 2924.1 cm-1; 1604.8 cm-1; 1458.2 cm-1; 1373.3 cm-1; 1080.1 cm-1. In the variation of DVB-0.5, there was no peak in absorption of 3425.6 cm-1 which showed the presence of OH bonds. This means that the addition of DVB is able to shift the hydroxyl bonds in blends, so that DVB makes the blends more compatible.
Compatibility of CNR-g-OA in Polyamide Resin
Compatibility of CNR-g-OA in polyamide resin was carried out for CNR-g-OA samples with oleic acid variations as shown in Figure 4, and DCP initiators variations as shown in Figure 5.
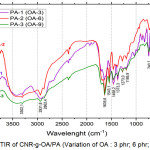 |
Figure 4: FTIR of CNR-g-OA/PA (Variation of OA : 3 phr; 6 phr; and 9 phr). |
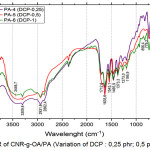 |
Figure 5: FTIR of CNR-g-OA/PA (Variation of DCP : 0,25 phr; 0,5 phr; and 1 phr). |
Based on Figure 5, there is an absorption at 3302.1 cm-1and low intensity which indicating the presence of OH from oleic acid. Absorption at 2931.8 cm-1, it shows the presence of an aliphatic -CH from CNR. At 1604.8 cm-1, it shows the strain vibration -C=C-. In the area of 1500 to 500 cm-1, it shows the absorption at 1458.2 cm-1; 1373.3 cm-1; 1033.8 cm-1. While the variation of PA-2 and PA-5 shows sharp absorption and low intensity at 1712.8 cm-1 which indicates the presence of C=O carbonyl bonds, meaning that the blends has been grafted.
The results of SEM characterization for CNR-g-AO/PA with the composition CNR: OA: DCP (100: 6: 0.5) phr as shown in Figure 6.
 |
Figure 6: SEM of CNR-g-OA/ PA. |
Based on Figure 6 which is the surface structure of CNR-g-OA / PA there is a white surface which is polyamide blended in CNR-g-OA. In figure 6, it appears that the surface structure is a little smoother; the PA material also seems less homogeneous even though it has been dispersed evenly.
Compatibility of CNR-g-OA Using Divinylbenzene in Polyamide Resin
Compatibility of CNR-g-OA using divinylbenzene in polyamide resin, as shown in Figure 7.
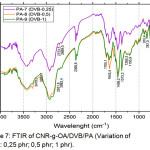 |
Figure 7: FTIR of CNR-g-OA/DVB/PA (Variation of DVB : 0,25 phr; 0,5 phr; 1 phr). |
Of all variations of PA-7, PA-8, and PA-9 in Figure 7, there was an absorption peak at 3410.1 cm-1; 2931.8 cm-1; 1643.4 cm-1; 1458.2 cm-1; 1373.3 cm-1; 1080.1 cm-1. At PA-8, it appears that the absorption peak at 1705.1 cm-1 means that there is a bond of -C=O, and also absorption at 1643.4 cm-1 and medium intensity which shows the presence of C=C.
Based on the comparison of the FTIR spectrum in Figure 5 and Figure 7, there has been a change in FTIR spectrum after the addition of DVB. In Figure 7, shows an increase in intensity at 1458.2 cm-1 and 1373.3 cm-1 which indicates the presence of CH2 and CH3. This is clearly a change in CNR-g-OA after DVB was added. While the decrease in intensity occurs at 1643.4 cm-1 because there is a lot of disconnection in the double bond chain C = C after the grafting process occurs.
Similarly, the surface structure as shown in Figure 8 which is CNR-g-AO / DVB / PA with the composition CNR: OA: DCP: DVB (100: 6: 0.5: 0.5) phr. In Figure 8 the surface structure of CNR-g-OA / DVB / PA is smooth and PA material is evenly dispersed, and the surface structure also doesn’t show any PA material, which is caused by the presence of DVB which makes the blends more homogeneous and compatible.
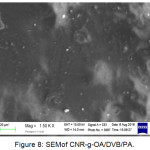 |
Figure 8: SEM of CNR-g-OA/DVB/PA. |
Conclusion
The best compositions ratio of CNR-g-OA products are CNR (100 phr), OA (6 phr), DCP (0.5 phr); and DVB (0.5 phr) with results of grafting formed is CNR-g-OA which indicated by the presence of sharp and weak peaks at 1700 to 1800 cm-1, indicating that is carbonyl (C=O) (typical for carbonyl) from oleic acid. It is caused the grafting was succeed to generate CNR-g-OA. Blending CNR-g-OA with polyamide after the addition of DVB is difference compare to CNR-g-OA. This indicates that CNR-g-OA is compatible (homogeneous) after blending with PA.
Acknowledgements
The authors would like to thank the Directorate of Research and Community Service, Directorate General for Research and Development, Ministry of Research, Technology and Higher Education of the Republic of Indonesia for research funding assistance so as to support the completion of this doctoral dissertation research with research contract number 131/K1.1/LT.1/2018.
References
- Wongthong, P.; Nakason, C.; Pan, Q.; Rempel, G. L.; Kiatkamjornwong, S., Eur. Polym. J. 2013, 49, 4035.
CrossRef - Phinyocheep, P., In Chemistry, Manufacture and Applications of Natural Rubber; Elsevier, 2014; pp. 68–118.
CrossRef - Siregar, M. S.; Thamrin; Basuki; Eddiyanto; Mendez, J. A., Chem. Mater. Res. 2014, 6, 15.
- Carone Jr., E.; Kopcak, U.; Gonc alves, M. C.; Nunes, S. P., Polymer (Guildf). 2000, 41, 5929.
CrossRef - Hamid, F.; Akhbar, S.; Halim, K. H. K., Procedia Eng. 2013, 68, 418.
CrossRef - Narang, J.; Chauhan, N.; Singh, A.; Pundir, C. S., J. Mol. Catal. B Enzym. 2011, 72, 276.
CrossRef - Wang, Q.; Zhu, J.; Wang, P.; Li, L.; Yang, Q.; Huang, Y., J. Appl. Polym. Sci. 2012, 124, 5064.
- Nakason, C.; Pechurai, W.; Sahakaro, K.; Kaesaman, A., Polym. Adv. Technol. 2005, 16, 592.
CrossRef - Song, Y.-W.; Do, H.-S.; Joo, H.-S.; Lim, D.-H.; Kim, S.; Kim, H.-J., J. Adhes. Sci. Technol. 2006, 20, 1357.
CrossRef - Liqun, Z.; Irwan, G. S.; Kondo, T.; Kubota, H., Eur. Polym. J. 2000, 36, 1591.
CrossRef - Siregar, M. S.; Thamrin, M.; Basuki, W. S., Agrium 2015, 17.
- Nasution, A. S.; Said, E.; Siregar, M. S., Agrium 2015, 19.
- Zhou, Z. F.; Huang, H.; Liu, N. C., J. Appl. Polym. Sci. 2002, 85, 726.
CrossRef - Liu, M.; Liu, Z.; Ding, S.; Li, S.; Zhang, L., J. Appl. Polym. Sci. 2003, 90, 3299.
CrossRef - Vigo, F.; Canepa, G.; Munari, S., J. Polym. Sci. Part A‐1 Polym. Chem. 1969, 7, 3015.
CrossRef - Tank, R.; Gupta, D. C., J. Porous Mater. 2009, 16, 387.
CrossRef - Kralevich, M. L.; Koenig, J. L., Rubber Chem. Technol. 1998, 71, 300.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









