Characterizing Crystalline Chromium Oxide Thin Film Growth by Sol-gel Method on Glass Substrates
Khadher AL-Rashedi1,2, Mazahar Farooqui3 and Gulam Rabbani1,2
1Dr. Rafiq Zakaria Center for Higher Learning and Advanced Research Aurangabad, Maharashtra, India.
2Research Center, Maulana Azad College, Aurangabad, 431001 Maharashtra, India.
3Dr. Rafiq Zakaria College for women, Aurangabad, Maharashtra, India.
Corresponding Author E-mail: khadheraskar20@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/3404064
Article Received on : 12-11-2017
Article Accepted on : 23-06-2018
Article Published : 31 Jul 2018
In this work, nano-crystalline chromium oxide (Cr2O3) thin film was synthesized using the Sol-gel method. The thin film was deposited on a glass substrate at 50°C by using chromium nitrate, methanol, ethanol, polyvinyl alcohol with a magnetic stirrer device and a hot plate. Each deposited thin film annealed in the microwave oven at 200°C. The structure of the prepared thin film was examined by means of X-ray diffraction (XRD). The Shearer formula was used to calculate the size of the film granules. The scanning electron microscopy (SEM) was used to study the surface morphology. UV-VIS measurement was also carried out to study optical properties of a sample by recording transmittance and reflectance data in the range 341-800 nm wavelength. All the tests confirmed the existence of a thin layer of Cr2O3 on the glass substrates.
KEYWORDS:Chromium Oxide; Crystallites; Dip Coating; Optical Properties; Sol-Gel Method; Substrates; SEM; Thin Film; UV; XRD
Download this article as:| Copy the following to cite this article: AL-Rashedi K, Farooqui M, Rabbani G. Characterizing Crystalline Chromium Oxide Thin Film Growth by Sol-gel Method on Glass Substrates. Orient J Chem 2018;34(4). |
| Copy the following to cite this URL: AL-Rashedi K, Farooqui M, Rabbani G. Characterizing Crystalline Chromium Oxide Thin Film Growth by Sol-gel Method on Glass Substrates. Orient J Chem 2018;34(4). Available from: http://www.orientjchem.org/?p=47915 |
Introduction
Alkali transition metal oxides have been attracted great attention due to their scientific and technological importance.1-3 Among these oxides, chromium oxide exhibits an interesting type of magnetic ordering which has been studied by various methods.4,5 Chromium oxides can be deposited by thermal spraying,6 CVD method, Rf and DC reactive sputtering and vacuum evaporation.7 The metal-organic CVD technique allows to produce thin films with uniform surface and thickness at relatively low temperatures.8 Deposition of Chromium oxide has been reported in literature by various techniques,9-11 Chromium nitrate and chromium oxide are suitable candidates for protection of steels and decorative applications due to their distinct colors12-14 and can also be used in optical applications as Electrochromic material.15 High solar absorbance and low thermal remittance properties of Cr2O3 are valuable in solar absorber coatings.16-17 The chromium oxides have been extensively studied as potential cathode material for lithium batteries.18 In this paper, we report on the deposition, structure and optical characterization of chromium oxide films obtained by sol-gel method from chromium nitrate as a precursor and at low substrate temperature of 50°C. The thin films were characterized by XRD measurements, and Scanning Electron Microscopy (SEM). The thin films were optically analyzed by UV–VIS spectroscopy.
Experimental
Initially, the chronic nitrate solution was prepared by dissolving (4.0015g) in pure distilled water (pH ≈ 7.0, Conductivity ≈ 36.00µS/m), then prepared polyvinyl alcohol solution was mixed with chromic nitrate solution in equal volume. The mixed solution was stirred on a hot plate for 3 hours at a room temperature (30°C) under normal atmospheric pressure. After that, the temperature was raised to 50°C with continuous stirring for more than 5 hours. Detailed step by step experimental procedure is illustrated in Fig.1. The glass substrate with dimension (24×40 mm) was dipped in the solution for 60 minutes. Deposited thin film onto glass substrate was dried, and then heated for 10 min in a microwave oven (65 watts, 200°C and at frequency 7 (Hz)).
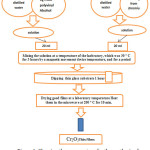 |
Figure 1: Showing the process steps for the synthesis of a thin film of Nickel oxide. |
The thin film was characterized and the morphology of the CrO thin film was examined using (SEM). The structure and the grain size of the CrO thin film were studied using X-Ray diffraction. The UV-visible spectrum of the sample was obtained by using an SL 210 UV VIS Spectrophotometer-1800, in the wavelength range of 341 to 800 nm.
Results and Discussion
Structural Analysis
The crystal structure and the orientation of the Cr2O3 thin films were investigated by X-ray diffraction. Fig. 2 shows XRD patterns of Cr2O3 film annealed at 200oC. Some small and narrow peaks superimposed on the large and broad background indicates the amorphous component of the films and the glass substrate (Fig.2). These peaks were contributed by crystallites in the films, and thus these films consisted of micro crystallites embedded in an amorphous matrix. The peaks occurred at 2θ = 24.6°, 40.9°, 41.5°, 44°, 51°, 53.1°, 57°, 58.9°, 63.4°, 65.1°, 71.8°, 73.4°, 77° and 78.5°, are corrosspond to (012), (006), (113), (202), (024), (116), (211), (122), (214), (300), (1010), (119), (220) and (306) planes (JCPDS card 38–1479).18 These peaks are attributed to a rhombohedral structure with preferred orientations, and the peaks (012), (113), (024), (116), (214), and (300), respectively are similar with the peaks obtained by [19,20], and the peaks (012), (113), (006), (202), (024), (116), (211), (122), (214), (300), (119), 220) and (306) respectively are observed similar with the peaks obtained by.21-23
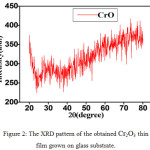 |
Figure 2: The XRD pattern of the obtained Cr2O3 thin film grown on glass substrate. |
In order to obtain detailed information of the structure of the film, the size of grain along the z-axis, and the Scherrer equation was used to calculate the average size of the crystalline based. It was observed about 77.728 nm.
D = 0.9λ/βcosθ (1)
Where λ – wavelength of x-ray (0.154 x 10-9m.)
θ – Bragg angle of peaks.
β – Full width at half maximum value (FWHM).
Morphological Analysis
To obtain insight information about the surface morphology and particle size of the Cr2O3 thin film, SEM was used to get a micrograph of Cr2O3 thin film, which was calcined at 200°C for 10 min as shown in Fig. 3 (A and B).
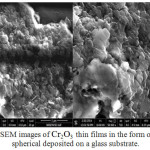 |
Figure 3: SEM images of Cr2O3 thin films in the form of particles spherical deposited on a glass substrate. |
The Cr2O3 micrograph shows a compact structure composed of a single type of spherical granules which are coherent with each other with cavities and of a variable size. The mean crystallite size is found to be about ~77.7nm.
Optical Study
The optical properties of the synthesized chromic oxide nanostructures were examined via Spectrum of UV by falling light on the sample. Optical absorption spectra is shown in Figure 4. It is observed from figure that the sample absorbs the radiation in the ultraviolet region of 341 nm to 800 nm. The UV-light spectrophotometry analysis showed that the absorption peak at 392 nm. The energy gap of the thin layer of the nickel oxide was calculated by curve between (hν) and (α hν)2. The energy band gap obtained from the extrapolation curve is observed 3.16eV as shown in Figure 5. These values are in good agreement with the reported data for CVD chromium oxide films24 and close to the results obtained from those thin films with impurities prepared by the pyrolysis spraying technique and electron-beam evaporation technique.25,26
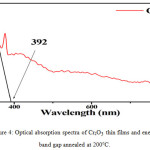 |
Figure 4: Optical absorption spectra of Cr2O3 thin films and energy band gap annealed at 200°C. |
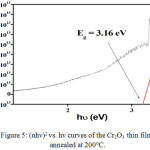 |
Figure 5: (αhν)2 vs. hν curves of the Cr2O3 thin films annealed at 200°C. Click here to View figure |
Conclusion
The chromium oxide thin film was successfully synthesized by Sol-gel method. The XRD results revealed that the Cr2O3 thin film is a nano crystalline with hexagonal structure. SEM results showed that the Cr2O3 micrograph has a compact structure composed of a single type of spherical granules with cavities and variable size. The mean crystallite size 77.728 nm is observed. Uv study showed low absorption of optical absorption in the infrared and visible region with a energy gap of 3.16 eV.
References
- Egharevba, G.O.; Eleruja, M.A.; Osasona, O.; Akinwunmi, O.O.; Olofinjana, B.; Jeynes, C.; Ajayi, E.O.B. Synthesis and Some Properties of Metal Organic Chemical Vapour Deposited Lithium Chromium Oxide Thin Films. Journal of Materials Science Research. 2012, 1 (1), 130.
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. LixCoO2 (0< x<-1): A new cathode material for batteries of high energy density. Materials Research Bulletin, 1980, 15 (6), 783-789.
CrossRef - Schmuki, P. From Bacon to barriers: a review on the passivity of metals and alloys. Journal of Solid State Electrochemistry, 2002, 6 (3), 145-164.
CrossRef - Ela, E.; Abou, A.H.; Mahmoud, S. Some optical properties of thin chromium films. Physica status solidi (a),1981, 66 (1).
CrossRef - Herring, C., Rado, G. T., & Suhl, H. (1966). Magnetism, vol. 4. by GT Rado and H. Suhl, Acad. Press, New York.
- Ichimura, H.; Kawana, A. High temperature oxidation of ion-plated CrN films. Journal of materials research, 1994, 9 (1), 151-155.
CrossRef - Ivanova, T.; Surtchev, M.; Gesheva, K. Characterization of CVD Chromium Oxide thin films. physica status solidi (a). 2001, 184 (2), 507-513.
- Bhushan, B.; Theunissen, G.S.; Li, X. Tribological studies of chromium oxide films for magnetic recording applications. Thin Solid Films. 1997, 311 (1), 67-80.
CrossRef - Abu-Shgair, K.; Abu-Safe, H.H.; Aryasomayajula, A.; Beake, B.; Gordon, M.H. Characterizing crystalline chromium oxide thin film growth parameters. Rev. Adv. Mater. Sci, 2010, 24, 64-68.
- Nörenberg, H.; Neumann, H.G. Electron and X-ray diffraction investigations of thin chromium films. Thin solid films, 1991, 198 (1-2), 241-250.
CrossRef - Jin, P.; Xu, G.; Tazawa, M.; Yoshimura, K.; Music, D.; Alami, J.; Helmersson, U. Low temperature deposition of α-Al2O3 thin films by sputtering using a Cr2O3 template. Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films, 2002, 20 (6), 2134-2136.
CrossRef - Bodart, F.; Terwagne, G. Characterisation of reactive unbalanced magnetron sputtered chromium oxynitride thin films with air. Surface and Coatings Technology, 2004, 180, 164-168.
- Agouram, S.; Bodart, F.; Terwagne, G. LEEIXS and XPS studies of reactive unbalanced magnetron sputtered chromium oxynitride thin films with air. Journal of electron spectroscopy and related phenomena, 2004, 134 (2), 173-181.
CrossRef - Kupfer, H.; Hecht, G.; Hoyer, W.; Moussaoui, H. The reactive magnetron deposition of CrN x O y films: first results of property investigations.Surface and Coatings Technology, 1999, 112 (1), 181-184.
- Hutchins, M.G.Selective thin film coatings for the conversion of solar radiation. Surface technology, 1983, 20(4), 301-320.
CrossRef - Wang, J.; Gupta, A.; Klein, T.M. Plasma enhanced chemical vapor deposition of Cr2O3 thin films using chromium hexacarbonyl (Cr (CO) 6) precursor. Thin Solid Films, 2008, 516 (21), 7366-7372.
CrossRef - Norby,P.; Christensen, A.N.; Fjellvåg, H.; Nielsen, M. The crystal structure of Cr8O21 determined from powder diffraction data: Thermal transformation and magnetic properties of a chromium-chromate-tetrachromate.Journal of Solid State Chemistry, 1991, 94 (2), 281-293.
CrossRef - Hussain Ismail Abdulah & Lamyaa Jabbar Abbas. Photosynthesis of Chromium oxide Nanoparticles from chromium complexes. International Journal of Applied, Physical and Bio-Chemistry Research (IJAPBCR), 2017, 7, 1-8.
- Farzaneh, F. Synthesis and characterization of Cr2O3 nanoparticles with triethanolamine in water under microwave irradiation. Journal of Sciences, Islamic Republic of Iran. 2011, 22 (4), 329-333.
- Abu-Shgair, Khaleel, et al. Characterizing crystalline chromium oxide thin film growth parameters.” Rev. Adv. Mater. Sci. 2010, 24, 64-68.
- Iwaszko, J.Ó.Z.E.F.; Szafarska, M.O.N.I.K.A. Tekstura powłok tlenkowych Cr 2 O 3 natryskiwanych plazmowo i przetopionych za pomocą lasera CO 2.” Materiały Ceramiczne/Ceramic Materials. 2011, 63(4), 702-706.
- Culity, B.D.; and Stock, S. R.; Elements of X-ray Diffraction.” Reading, MA: Addison-Weseley, 1978, 102.
- Aghaie-Khafri, M.; Kakaei Lafdani, M.H. A novel method to synthesize Cr 2 O 3 nanopowders using EDTA as a chelating agent. Powder technology. 2012, 222, 152-159.
CrossRef - Ivanova, T.M.; Surtchev; Gesheva, K. Characterization of CVD Chromium Oxide thin films. Physica Status Solidi (a) 2001, 184 (2), 507-513.
CrossRef - Khodair, Ziad Tariq, Gailan Asad Kazem, and Ammar Ayesh HabeebStudying the optical properties of (Cr2O3: I) thin films prepared by spray pyrolysis technique.Iraqi Journal of Physics. 2012, 10 (17), 83-89.
- Al-Kuhaili, M.F.; Durrani, S.M.A. Optical properties of chromium oxide thin films deposited by electron-beam evaporation. Optical Materials. 2007, 29 (6), 709-713.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









