Reaction of cis–[RuCl2 (DMSO)4 with some Aromatic Thioamides (RCSNHCOR') in Presence Nitric Oxide and Pyridine
Department of Chemistry, K.S. SaketP.G. College Ayodhya, Faizabad, India.
Corresponding Author E-mail: singhsantosh429@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340350
Article Received on : April 21, 2018
Article Accepted on : June 10, 2018
Article Published : 23 Jun 2018
Reactions of RCSNHCOR'(R and R¢ are various types of group) with Cis - [RuCl2 (DMSO)4 ] in presence of nitric oxide and pyridine leads to the formation of new complexes of the type cis-[RuCl2(NO)(RCSNHCOR')(py)]+. These cationic complexes were isolated as cis [RuCl2(NO) (RCSNHCOR') (py)] PF6 on reacting with NH4PF6. The complexes were characterised by elemental analysis, spectroscopic (Infrared, UV-Vis), conductometric and magnetic studies. An intence band at 1840-1850 cm–1 appeared in all the complexes, suggesting a {Ru-NO}6 type configuration of all these ruthenium nitrosyls presence of chloride ligand trans to NO and pyridine in plane have considerable change in properties of complexes.
KEYWORDS:Carbothioamides; Charge Transfer Band; Ruthenium Complexes
Download this article as:| Copy the following to cite this article: Singh S. K, Singh P. Reaction of cis–[RuCl2 (DMSO)4 with some Aromatic Thioamides (RCSNHCOR') in Presence Nitric Oxide and Pyridine. Orient J Chem 2018;34(3). |
| Copy the following to cite this URL: Singh S. K, Singh P. Reaction of cis–[RuCl2 (DMSO)4 with some Aromatic Thioamides (RCSNHCOR') in Presence Nitric Oxide and Pyridine. Orient J Chem 2018;34(3). Available from: http://www.orientjchem.org/?p=46911 |
Introduction
Activation of small molecules like CO, N2, NO, CS etc. has gain considerable research interest during the past several years. Although the purpose of such investigation was mainly to get a clear understanding of the principal of coordination chemistry, the understanding of several natural processes could also be possible by such a study. NO is well known to play significant role in certain biological & physiological processes in human body as for example cardiovascular control, regulation of blood pressure and neurotransmission1 etc. Ruthenium nitrosyl compound are known to release NO in aqueous medium under controlled process and administration of the complexes reduces blood pressure more effectively than sodium nitropruside.2, 3 The electronic properties of Ru–NO bond can further be modified by changing the coligands in the coordination sphere of ruthenium ion.4,5 In order to investigate such an effect, we propose to synthesize ruthenium nitrosyls in presence of aromatic thioamides (RCSNHCOR’) and explore their electronic and structural properties. The object of choosing thioamides as ligands lies in the fact that changing R and R’ will generally have more pronounced effect on stereochemistry, electronic structures and other properties of complexes. The studies may lead in a semiquantitative way to understand the electron charge distribution in Ru–NO fragment based on the sensitivity of aromatic carbothioamides and other ligands in the coordination sphere of ruthenium.
The coordinating ability of RCSNHCOR’ is varied by changing R and R’,6,7 so the various ligands used in present work are named and abbreviated as follows:
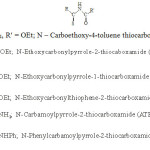 |
Scheme 1 |
Material and Measurements
The chemicals used were either chemically pure or AR grade. All the experiments were performed under pure and dry N2 atmosphere. RCSNHCOR’ and cis-[RuCl2 (DMSO)4] were prepared by the known method.8-11 The starting material RuCl3.3H2O was obtained by treating commercially available RuCl3.xH2O several times with concentrated HCl. DMSO, acetone, chloroform dichloromethane and diethyl ether were purified dried by standard techniques and freshly distilled before their use. Elemental analysis (C,H and N) were carried out by micro analytical section of B.H.U., Varanasi (U.P), India. Standard metods12,13 of analysis were performed to estimate sulphur, chloride, fluoride and phosphorous. Infrared spectra (4000–250cm–1) of all the ligands and complexes were recorded in KBr pellets by Perkin-Elmer FT IR spectrophotometer. Electronic spectra (200-900nm) of ligands and complexes were scanned on cystronic 108 UV model spectrophotometer. Magnetic properties were studied by the help of a Gouy balance at room temperature. Melting points of complexes were checked on a Fisher-John melting point apparatus. Conductivity measurements were performed on CM-82 Elico conductivity bridge. NO was prepared by adding concentrated H2SO4 on NaNO2 and then passing it through a saturated solution of NaOH. The chemical identification of coordinated NO in resultant complexes was made by the reported methods.14,15
Syntheses of Compounds
Cis-[RuCl2 (NO)(LH)(py)]PF6
1mmol ethanolic solution (10 ml) of ligand LH (CTH, 0.24g; or EPH, 0.22g; or ESH, 0.22g) was added slowly to an ethanolic solution (25ml) of Cis-[RuCl2 (DMSO)4] (0.25g~1 mmol) under N2 followed by addition of pyridine (5ml). The resultant solution was refluxed for 1.5h, reaching an orange-red colour of solution. This solution was cooled and reduced to 10 ml at low pressure a dry nitric oxide gas was bubbled for about 0.5h where by the colour of solution changed to orange colour. A solution (10ml) of NH4PF6 (0.2g~1mmol) in CH2Cl2/MeOH mixture was added shaked well, and resultant solution was further reduced to 5ml. Diethyl ether was added for the precipitation of orange solid product. It was filtered off, washed thoroughly with small quantity of water, diethyl ether and dried in vacuo.
Cis-[RuCl2 (NO) (LH)(py)] PF6
1mmol (0.25g) of cis-[RuCl2(DMSO)4] in 10 ml ethanol and a 10 ml ethanolic solution of LH (ATH, 0.18g, or ETH 0.20g) was refluxed for 2h under N2 and cooled [RuCl2(DMSO)4] under N2. Next pyridine (5ml) was added and resultant solution was stirred for about 30 minutes and nitric oxide was bubbled for 30 minutes while stirring was continued on addition of 10 ml NH4PF6 (0.2g~/mmol in CH2Cl2/MeOH mixture) solution the orange-red solid that precipitated was filtered off, washed thoroughly with small quantity of water, diethyl ether and dried in vacuo.
Cis-[RuCl2 (NO) (PTH) (py)] PF6
1mmol of ligand (~0.25g) was taken in 50 ml warm DMF and stirred for 30 minutes whereas an orange turbid solution was obtained. It was filtered, cooled and solvent was removed at reduced pressure to give a 10 ml of ligand solution. Next, this solution was slowly added to continuously stirred solution (10ml) of [RuCl2 (DMSO)4] (0.25g~1mmol) in ethanol followed by addition of pyridine (5 ml). Nitric oxide gas was bubbled for 30 minutes and addition of a solution (5ml) of NH4PF6 (0.2g~1mmol in MeOH/CH2Cl2 mixture) a red brown solid was precipitated. It was filtered, washed with small quantity of H2O, Et2O and dried in vacuo.
Results and Discussion
Starting from Cis-[RuCl2(DMSO)4] precursor, addition of ligand RCS NHCOR’ followed by adding few drops of pyridine and passing NO gas in the resultant solution yielded a set of new nitrosyl complexes containing RCSNHCOR’ as a subsidiary ligand.
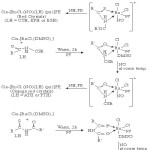 |
Scheme 2 Click here to View scheme |
All the compounds are diamagnetic and soluble in CH2Cl2, (CH3)CO and H2O. The analytical data (Table-1) suggest that RCSNHCOR’ acts as bidentate in all the complexes. The molar conductance of the prepared complexes was carried out in dichloromethane and results are 1:1 type compounds. The spectroscopic features (IR, UV and visible) and magnetic measurements are in good agreement with proposed formulations of complexes.
Table 1: Analytical Data, Melting Point and Colour of Complexe
|
SN. |
Compounds |
Colour |
MP (OC) |
Molar Conduct ance Ohm-1 mo-1 cm2 |
Yield (%) |
Analyses Found (Caled) |
|||||||
|
C |
H |
N |
S |
P |
Cl |
F |
Ru |
||||||
|
1. |
Cis-[RuCl2 (NO) (CTH)(py)]PF6 |
Orange Red |
116 |
110 |
26 |
29.40 (29.55) |
2.96 (2.78) |
6.32 (6.4) |
5.08 (4.96) |
5.12 (4.93) |
10.84 (10.94) |
17.6 (17.5) |
15.3 (15.5) |
|
2. |
Cis-[Ru Cl2 (NO) (ETH)(py)]PF6 |
Orange |
108 |
115 |
24 |
25.68 (25.77) |
2.46 (2.33) |
8.86 (8.7) |
5.06 (4.94) |
4.92 (4.8) |
11.16 (11.03) |
17.12 (17.7) |
15.32 (15.68) |
|
3. |
Cis-[Ru Cl2 (NO) (EPH)(py)]PF6 |
Red |
120 |
112 |
25 |
25.62 (25.77) |
2.64 (2.33) |
8.52 (8.7) |
5.08 (4.94) |
4.96 (4.8) |
10.92 (11.03) |
17.5 (17.7) |
15.46 (15.68) |
|
4. |
Cis-[Ru Cl2 (NO) (ESH)(py)]PF6 |
Red |
95 |
100 |
35 |
25.68 (25.89) |
2.32 (2.18) |
6.76 (6.52) |
10.12 (9.98) |
5.86 (4.84) |
11.60 (11.8) |
17.58 (17.78) |
15.62 (15.79) |
|
5. |
Cis-[Ru Cl2 (NO) (ATH)(py)]PF6 |
Orange Red |
115 |
110 |
40 |
22.6 (22.2) |
2.28 (2.02) |
11.88 (11.76) |
5.52 (5.40) |
5.12 (5.21) |
12.08 (11.92) |
19.22 (19.1) |
16.88 (17.00) |
|
6. |
Cis-[Ru Cl2 (NO) (PTH)(py)]PF6 |
Red Brown |
105 |
115 |
40 |
30.04 (30.4) |
2.56 (2.38) |
10.12 (10.43) |
4.88 (4.77) |
4.76 (4.60) |
10.46 (10.58) |
16.88 (17.00) |
15.2 (15.05) |
Spectroscopic Characterization
In the spectra of complexes the n(NO) bands are very close to 1840-1850 cm suggesting that NO group in all the complexes are indeed the {RuII– NO+} type.16-18 This idea in brought out from molecular orbital approach, which emphasizes the extremely important role of p* MO of NO in charge transfer from dp orbital of metal. Further, the dp charge transfer from metal to p* MO of NO is enhanced when chloro ligand is present trans to NO. The observed stretching frequencies of NO in complexes are in good agreement with the structural trans effect of chloro ligand.
RCSNHCOR’ may exhibit three resonance structures as follows:
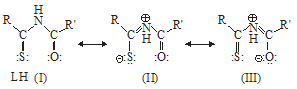
Although I in the preferential structure of the ligand but II and III may also exist under certain condition irrespective of fact that ligand acts as a bidentate or monodentate, it can coordinate with metal ion through any two or one of the three potential donor sites viz, imido N, thiocarbonylsulphur or carbonyl oxygen atom. The possible mode of coordination of ligands (LH) has been arrived at on the basis of following IR spectral studies. The characteristic IR bands of pyrrole, thiophene and aromatic ring do not undergo any shift in the spectra of complexes (maximum shift ±5 cm-1), indicating as expected, non involvement of pyrrole ring nitrogen, thiophene ring sulphur and aromatic ring in bond formation with metal. In case of bonding through -C(S)NHC(O)- moiety of ligand, shift in positions of thioamide and amide bands are expected. The major shifts in these band positions are discussed below.
Cis-[RuCl2 (NO) (LH) (py)]PF6
(LH=CTH, EPH, ESH)
Free ligand band of v(C=S) and those of thaioamide band IV19 (contribution from v(C=S)) undergoes downward shift with a decrease in intensity suggesting the bonding of ligand to metal ion through thio carbonyl sulphur atom.
Thioamide I (δNH + v(C=N)), II (v(C=N) + v (C=S) +δ (C-H)) and III (v (C-N)+ n(C-S)) band20 (1000-1600 cm-1) shifted to lower wave number (Table-2), suggesting the possiblity of nitrogen atom of -C(S)NHC(O)- moiety in bond formation with metal ion.
The free ligand band due to n (CO) shifted to higher wave number, suggesting the non involvement of CO group in coordination with metal ion.
Complexes exhibit an intense absoroption band at 1840 cm-1, assignable to v(NO).21
The new bands around 360-480 cm-1 in the spectra of complexes are assigned to coupled vibrations of v(M–S) with other bonding modes of ligand molecule.
A weak band22 at around 600 cm-1 in IR spectra of all the comlexes is assigned to v(Ru-NO) vibrations.
The characteristic band of pyridine23 due to C=C and C=N stretching (in ring) (1430-1600 cm-1) shifted to lower wave number (± 15 cm-1), suggesting the coordination of pyridine molecule to metal ion.
Based on the above IR spectral observation of complexes and its comparison with free ligand, the bonding of LH occurs, possibly through N and S donor atom.
Cis-[RuCl2 (NO) (LH) (py)] PF6
(LH = ATH, PTH)
The amide band I (1750 – 1680 cm-1) which arises among to the normal coordinate having major24 contribution from v(CO) Shifted to lower wave number on complexation with metal ion, indicating coordination through oxygen of the CO group.
Thioamide band24 II and III shift to lower wave number amide bands II and III shifted to higher wave number (+ 15cm-1). This suggests the coordination through N and O atom of the ligands.
v(C=S) and thiomide band25 IV shifted to higher wave number, excluding the involvement of CS group in bond formation with metal ion.
Presence of an intense band at 1850cm-1 in spectra of complexes, in assigned to stretching vibrate of coordinated NO molecule.
The broad bands around 430-520cm-1 in spectra of complexex may be assigned to coupled vibrations of v(Ru-N) with other bonding mode of legand.
A weak band at around 5.10 cm-1 in IR spectra of complexes may be assigned to v(Ru-NO) mode of vibration.26
The characteristic bands of pyridine appeared at lower wave numbers (+ 15 cm-1) indicating the coordination of pyridine molecule to the metal ion.
Based on the above observations of IR spectral data, the bonding of ligand is concluded through nitrogen of imido group and oxygen of sulphur group (Table-2).
Cis-[RuCl2 (NO)(LH) (py)] PF6
(LH = ETH)
The amide band I at 1775 cm-1 which arises owing to v(CO) shifted to lower wave number (~40cm), indicating the bonding of ligand through carbonyl oxygen bonding of metal ion (electron withdrawing group) with oxygen atom of carbonyl group of ligand will shift the position of thioamide bands I and II, amide band II and III and band due to v(CO) of the (COOEt) group towards higher wave number. The broad band at 1500 cm-1 (thioamide band I and amide band II, 1310 cm-1, 1260 cm-1 (thioamide band II and amide band III) and at 1190 cm-1 v(CO) of (C-OEt) shifted to higher wave numbers (~30cm-1) present in the spectrum of ligand. This further confirms the bonding of metal ion with the carbonyl group and most probably with the sulphur atom of thiocarbonyl group.
A donwward shift (20 cm–1) in the position thioamide IV band (875 cm-1) of free ligand, suggesting the bonding of metal through thiocarbonylsulphur atom.
This complexes exhibit an intense absorption and at 1850 cm-1 is assigned to v(NO) of bonded NO.
Weak bands around 470-400 cm-1 in the spectrum of complex may be assigned to the coupled vibrations27,28 of v(Ru-Cl) + v(Ru-O) or v(Ru-Cl) + v(Ru-S).
A band of medium intensity at around 590cm-1 may be attributed the v(Ru-NO) vibration.
The characteristic bands of pyridine ligand were present at slightly shifted positions (-10 cm-1).
Based on the above observations, the bonding of ligand is concluded suphur atom of thiocarbonyl and oxygen atom of carbonyl group (Table-2).
Electronic Spectra
Electronic Spectra of ligands (CTH, ETH, PTH) exahibited absorption band in EtOH at 415 – 450nm and 365 – 390nm. These absorption bands are assigned as n→π* and π→π* respectively. The electronic absorption bands of ligands (EPH, ESH and ATH) in the same solvent appeared at 300-350nm and 262-292 nm assignable to n→π* and π→π* respectively (Table-3). The intense absorption bands characteristic of substituted pyrrole ring (235 and 345 nm), substituted thiophene ring (295, 270 nm) and substituted benzene ring (260 nm) were present in the electronic spectra of (ETH, EPH, ATH, PTH), ESH and (CTH, PTH) ligands respectively, these absorption bands assigned as π-π* transitions did not shift on complexation, suggesting that the metal ion did not interact with the p system of ligands consistant with IR spectral data. The free ligand band characteristic of π→π* transition shows a red shift (~ 15 nm) while that of π→π* transition shows a blue shift (~10 -15 nm) on coordination of ligand with metal ion (electron withdrawing system). In practice the bonding of hetroatom with metal ion would stabilize the orbitals that possess27 lone pair of electron while destabilize the p molecular orbitals. These effect could be seen in the electronic spectrum of ligand after coordination with metal ion. The comparison of electronic spectra of ligands and corresponding complexes suggested that n→π* transition move towards longer wave length while that π→π* towards shorter wave length constant with the IR spectral interpretations.
UV-Visble spectra of Ru(II) complexes (low spin) generally exhibit29 two low energy transitions of very weak intensity corresponding to 1A1g→1T2g and 1A1g→3T2g and at higher energies of comparatively more intensity (Σ>10) corresponding to 1A1g→1T1g and 1A1g→1T2g. The intensity of two bands around 830-860 nm and 580-620 nm suggested that the bands in all the complexes are due to 1A1g→1T1g and 1A1g→1T2g respectively consistent with analogous complexes of Ru(II).
The region above 500nm is dominated by d-d transition and 400-500 nm is dominated by d→π* transition while 400-300 nm and below 300 nm are dominated by LMCT and IL respectively. Spectra off all the complexes show an intense band in each at 400-440 nm which would be assigned to dπ(Ru)→π*(NO).30-32 The energy of transition decreases in complex of CTH, EPH, ESH ligands, than ATH, PTH, ETH, this observation suggested that ligands as N and S donor sites binds metal ion more effectively as compared to N and O or S and O donor sites. This effect would further lead to stronger M-N bond and weaker N-O bond in complexes, consistent to IR spectral data of M-N and NO stretching frequencies (Table-2).
Table 2: Major IR Bands and Comparision of IR Spectra of Ligand and Complexes (cm-1)
|
SN. |
Compounds |
v(NO) |
v(NH) |
v(C=O) |
v(C=S) |
Thioamide Bands |
|||
|
I |
II |
III |
IV |
||||||
|
1. |
CTH Cis-[RuCl2 (NO) (CTH)(py)]PF6 |
– 1840s |
3220 – |
1765s 1775s |
1130s 1120m |
1540s 1500s |
1360s 1330m |
1075s 1070s |
850m 840m |
|
2. |
ETH Cis-[Ru Cl2 (NO) (ETH)(py)]PF6 |
– 1850s |
3350m 3325m – |
1765s 1745s |
1120s 1100m |
1540s 1550s |
1340s 1380s |
1070s 1070s |
870m 865m |
|
3. |
EPH Cis-[Ru Cl2 (NO) (EPH)(py)]PF6 |
– 1840s |
3210m – |
1730s 1780d |
1125s 1110s |
1500s 1480s |
1320s 1310s |
1015s 1010m |
880s 860s |
|
4. |
ESH Cis-[Ru Cl2 (NO) (ESH)(py)]PF6 |
– 1840s |
3240s 3380 3100br |
1730s 1740m |
1180s 1160s |
1510s 1500s
|
1360s 1350s |
1020s 1000m
|
770s 740s |
|
5. |
ATH Cis-[Ru Cl2 (NO) (ATH)(py)]PF6 |
– 1850s |
3400 3370 3250 – |
1730s 1700s |
1120s 1130m |
1580s 1560s |
1330s 1310s |
1060s 1050s |
845s 860m |
|
6. |
PTH Cis-[Ru Cl2 (NO) (PTH)(py)]PF6 |
– 1850s |
3410m 3260m 3160m – |
1720s 1690s |
1120s 1140m |
1525s 1510s |
1350s 1320s |
1000s 990m |
860m 870m |
Intensity of absorption bands at 460-425 nm and 360-350 nm (ε max~6 x 103 m-1 cm2) in complexes suggested that these bands appear as a result of LMCT (Ligand to metal charge transfer). Further, the intensity of absorption bands at 360-350 and 280-350 nm (ε max~8×103 m-1 cm2) in complexes suggested that they arise due to IL (Infra-ligand) transitions.33
Table 3: Electronic Spectra of Ligands and 0.25m Solution of Complexes in CH2Cl2
|
SN. |
Compound |
Band Position |
Assignment |
| CTH | 450 | n→π* | |
| 310 | π→π* | ||
| 270 | π→π* | ||
|
1. |
Cis-[RuCl2 (NO) (CTH)(py)]PF6 | 850 (125) | 1A1g→1T1g |
| 600 (150) | 1A1g→1T2g | ||
| 460 (6 x 103) | LMCT | ||
| 440 (2.5×102) | dπ(Ru)→π*(NO) | ||
| 360 (8 x 103) | IL | ||
| 260 (10 x 103) | π→π* | ||
| ETH | 440 | n→π* | |
| 365 | π→π* | ||
|
2. |
Cis-[RuCl2(NO) (ETH)(py)]PF6 | 840 (125) | 1A1g→1T1g |
| 500 (150) | 1A1g→1T2g | ||
| 450 (6 x103) | LMCT | ||
| 410 (2.5 x 102) | dπ(Ru)→π*(NO) | ||
| 350 (8 x 103) | IL | ||
| 345 (9.6 x 103) | π→π* (pyrrole) | ||
| 235 (10.4 x 103) | π→π* | ||
| 260 (9.6 x 103) | π→π* pyridine | ||
| ATH | 350 | π→π* | |
| 290 | π→π* | ||
| 345 | π→π* (pyrrole) | ||
| 235 | π→π* | ||
|
3. |
Cis-[RuCl2 (NO)(ATH)(py)]PF6 | 830 (125) | 1A1g→1T1g |
| 580 (150) | 1A1g→1T2g | ||
| 400 (2.5 x 102) | dπ(Ru)→π*(NO) | ||
| 360 (6 x 103) | LMCT | ||
| 280 (8 x 103) | IL | ||
| 345 (10 x 103) | π→π* (pyrrole) | ||
| 235 (8 x 103) | π→π* | ||
| 260 (7 x 103) | π→π* pyridine | ||
| 345 | π→π* | ||
| 235 | π→π* pyrrole |
|
4. |
Cis-[RuCl2 (NO) (EPH)(py)]PF6 | 850 (120) | 1A1g→1T1g |
| 600 (130) | 1A1g→1T2g | ||
| 440 (3 x102) | dπ(Ru)→π*(NO) | ||
| 330 (5 x 103) | LMCT | ||
| 260 (8 x 103) | IL | ||
| 345 (9 x 103) | π→π* Pyrrole | ||
| 235 (6 x 103) | π→π* | ||
| 268 (7 x 103) | π→π* pyridine | ||
| ESH | 350 | n→π* | |
| 292 | π→π* | ||
| 295 | π→π* | ||
| 270 | π→π* Thiophene | ||
|
5. |
Cis-[RuCl2 (NO) (ESH)(py)]PF6 | 560 (130) | 1A1g→1T1g |
| 620 (140) | 1A1g→1T2g | ||
| 440 (2 x102) | dp(Ru)→π*(NO) | ||
| 360 (5 x103) | LMCT | ||
| 280 (8 x 102) | IL | ||
| 295 (8 x 103) | π→π* Thiophene | ||
| 270 (6 x 103) | |||
| 260 (5 x 103) | π→π* Pyridine | ||
| PTH | 415 | n→π* | |
| 390 | π→π* | ||
| 270 | π→π* Benzene | ||
|
6. |
Cis-[RuCl2(NO) (PTH)(py)]PF6 | 820 (110) | 1A1g→1T1g |
| 580 (130) | 1A1g→1T2g | ||
| 425 (103) | LMCT | ||
| 410 (2 x 102) | dπ(Ru)→π*(NO) | ||
| 360 (9 x 103) | IL | ||
| 270 (8 x 103) | π→π* benzene | ||
| 260 (7 x 103) | π→π* Pyridine |
Acknowledgement
The authors are highly greatful to Dr. Paritosh Bhatacharya, Agrasen Autonomous P.G. College Varanasi, for his kind and needful support in scaning UV-Visible spectra of complexes. We also express deep gratitude to Dr. T. Singh, Associate Professor of Chemistry Department, S. G. R. P. College, Dhobhi, Jaunpur, for providing thoughtful suggestions in our research work.
References
- Ignarro I.J.; Murad P.; Nitric oxide : Biochemistry, Molecular Biology and Therapeutic implications; Acadmic Press; California, 1995.
- Rathgeb, A.; Bohm, A.; Novak, M.S.; Gavriluta, A.; Domotor, O.; Tommasino, J.B.; Enyedy, E.A.; Shova, S.; Meier, S.; Jakupec, M.A.;Luneau, D. and Arion, V.B. Inorg. Chem.,2014, 53, 2718.
CrossRef - Fogler, E.; Efremenro, I.; Gargir, M.; Leitus, G.; Posner, Y.D.; Ben-David, Y.; Martin Jan, M.L. and Milstein, D. Inorg. Chem.,2015,54, 2253.
CrossRef - Canpolat, E. and Kaya, M.Coord, J. Chem.,2004,57, 1217.
- Ferrari, M.B.; Capacchi, S.; Pelosi, G.; Reffo, G.; Tarasconi, P.; Albertini, R.; Pinelli, S. and Lunghi, P. Inorg. Chim. Acta.,1999, 286, 134.
CrossRef - Chauhan, V.; Gupta, H.K. and Dikshit, S.K. Polyhedron,1988,1, 623.
CrossRef - Chauhan, V. andDikshit, S.K.Trans. Met. Chem.,1988,13, 440.
CrossRef - Papadopolous,E.P. J. Org. Chem., 1976, 41, 962.
CrossRef - Papadopolous, E.P. J. Org. Chem., 1973,38, 962.
- Papadopolous, E.P.J. Org. Chem., 1974,39, 2540.
CrossRef - Evans, I.P.; Spencer, A.; Wilkinson,G.J. Chem. Soc., Dalton Trans.1973, 204.
CrossRef - Vogel, A.I. A Text Book of Quantitative Inorganic Analysis. IV ed., ELBS and Longmans, London1973, 154-155, 433-434, 494-495, 504-505.
- Burton, J.D. and Riley, J.P.; Analyst. 1955, 391.
CrossRef - Maurya, R.C. Synthesis and Characterization of some Novel Nitrosyl Compounds, first Ed., Pioneer Publications, Jabalpur, 2000.
- Svehla, G.Vogel’s qualitative Inorganic Analysis, Sixth Ed., Orient Longman Limited, U.K.,1987, P163.
- Souza, D.H.F.; Oliva, G.; Teixeira, A.; Batista, A.A.Polyhedron,1995, 14, 1031.
CrossRef - Batista, A.A.; Perciva, C.; Wohnvath, K.; Queriroz, S.L.; Santos, R.H.D.; Gambardella, M.T.D. Polyhedron,1999,18, 2079.
CrossRef - Mahesh, V.; Arman, H.D. and Tonzetich, Z.J. Dalton Trans., 2017,46, 1186.
CrossRef - Colthup, N.B.; Dalay, L-H. andWiberly, S.E. Introduction to Infrared and Raman Spectroscopy (Academic Press, New Yark),1975, 304-305.
- Rao, C.N.R.;Venpataraghavan, R. and Kasturi, T. Can. J. Chem., 1964, 42, 36.
CrossRef - RichterAddo, G.B.; Legzdins,P. J. Am. Chem. Soc.,2012,134, 1243.
- Lehnett, J.T. Inorg. Chem.,2010,49, 7197.
CrossRef - Chauhan,V.; Dikshit, S.K.Bull Chem. Soc., Japan,1987,60, 3005.
CrossRef - Nakamoto,K.Infrared Spectra of Coordination Compounds. Wiley, New York,1963.
- Suzuki, I. Bull. Chem. Soc., Japan, 1962,35, 1286, 1456.
- Durig, J.R.; Wertz, D.W.ApplSpectrosc.,1968,22, 627.
CrossRef - Arulsany, K.S.; Ashok, R.F.N. and Agarwala, U.C. Ind. J. Chem.,1984,23A, 122.
- Adams,D.M.Metal ligand and Related Vibrations St.Martins Press, New York, 1968,284, 316.
- Drago, R.S. Physical Methods in ChemistryW.B. Saunders Co. London,1986.
- Borges, S.D. and Daranzo, C.U.Inorg. Chem.,1998,37, 2670.
CrossRef - Lewandowska, H. Structure and Bonding, 2013, 109, 430.
- Berto, T.C. and Praneeth, V.K. J. Am. Chem. Soc.,2009, 131, 17116.
CrossRef - Malecki, J.G.;Jaworska,M. and Kruszynski, R. Polyhedron, 2005, 24, 359.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










