Removal of Cr(VI) from the Chrome Electroplating Effluent by Reduction and Adsorption using Powdered Activated Charcoal
Dnyaneshwar R. Shinde1, Ramdas A. Pawar2 and Manohar G. Chaskar1
1PDEA’s Prof. Ramkrishna More College, Akurdi, Pune-44, affiliated to Pune University.
2PDEA’s Baburaoji Gholap College, Sangavi, Pune-27, affiliated to Pune University.
Corresponding AuthorE-mail: drshinde1970@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/340154
A powdered activated charcoal (PAC) based process was evaluated at a bench and pilot scale to determine its effectiveness towards the removal of Cr(VI) from the electroplating effluent. In the first step, reduction of Cr(VI) to Cr(III) was achieved while in a second step Cr(III) was removed by adsorption. In both steps PAC is utilized. Different experimental parameters affecting the reduction of Cr(VI) by PAC were investigated. The rate of reduction reaction was found to be dependent on the pH of effluent and the dose of PAC. Removal of Cr(III) from initially treated effluent was achieved under optimum condition of pH and dose of PAC. After a bench scale experiments, the reduction and removal of chromium from the effluent was achieved at a pilot scale successfully. The experimental result suggests that PAC is a suitable material for the reduction and removal of chromium from the electroplating effluent.
KEYWORDS:Chromium; Reduction; Adsorption; Activated Charcoal; Removal
Download this article as:| Copy the following to cite this article: Shinde D. R, Pawar R. A, Chaskar M. G. Removal of Cr(VI) from the Chrome Electroplating Effluent by Reduction and Adsorption using Powdered Activated Charcoal. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Shinde D. R, Pawar R. A, Chaskar M. G. Removal of Cr(VI) from the Chrome Electroplating Effluent by Reduction and Adsorption using Powdered Activated Charcoal. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=42773 |
Introduction
Pollution due to the toxic metal ions is a worldwide problem and received widespread attention of researchers, environmentalists and pollution controlling boards of different countries. Among the number of toxic metal ions, ionic chromium is of great environmental concern. Chromium in the ionic form is released into the environment through effluents of the industries like electroplating, chrome ore processing, chromium metal and chromium salt production, tanneries, pigment production and processing, etc. (Xiaoli and Xiali, 2013; Gottipati and Mishra, 2010; Xiaaoshu et al., 2012). The effluents of these industries mostly consists of chromium in the two ionic forms; trivalent Cr(III) and hexavalent Cr(VI). Both of these ionic forms are toxic in nature, but the toxicity of Cr (VI) is higher than Cr (III) (Tangujank et al., 2009). In acidic solution, Cr (VI) exists in the dichromate form (Cr2O72-), while in alkaline solution, it mostly exists in the chromate form (CrO42-) (Malkoc et al., 2006). A long term exposure to Cr(III) can cause allergic skin reaction and cancer (Tangujank et al., 2009) while Cr(VI) in the form of dichromate or chromate is a well known carcinogen (Gupta and Babu, 2009). Chromium in ionic form can also cause skin disorders and liver damage (Selvraj et al., 2003). Hence, the Agency for Toxic Substances and Diseases Registry (ATSDR) classified Cr(VI) in the top sixteenth hazardous materials (ATSDR, 2000). The World Health Organization also paid attention towards the toxicity of Cr (VI) and passed the strict regulation regarding the maximum allowable level of ionic chromium in drinking water. It is 0.05 ppm for Cr(VI) and 5 ppm for Cr(III) (Gupta and Babu 2009; Alfa-Sika, et al., 2010; Levankumar et al., 2009). The methods like ion exchange, coagulation and filtration, reverse osmosis, adsorption, electrocoagulation, evaporative recovery are in use to remove chromium from effluents (Malkoc et al., 2006; Verga et al., 2013). Solubility of Cr(VI) compounds in acidic as well as in alkaline medium is high. Hence, Cr(VI) is mostly removed as Cr(III) (Khezami and Capart, 2005; Selvi et al., 2001). Various reducing agents were reported in literature to convert Cr(VI) to Cr(III) (Vogel, 1963; Beukes et al., 1999; Chulsung et al., 2001; Niekerk et al., 2007; Gang et al., 2005). The use of reducing agents releases many unwanted chemical species into the effluent which increases load on the treatment process as well as makes the recovery of chromium in pure form difficult. Iron metal is the most preferred reducing agent for Cr(VI), however in the process equivalent quantity of Fe(III) is released in the effluent which do not allows recovery of Cr(III). hence, in the present investigation, we are reporting the reduction of Cr(VI) to Cr(III) by the powdered activated charcoal (PAC) which is a clean process. This is in contrast to many other reports where PAC is reported as adsorbent to Cr(VI) (Xiaoli and Xiali, 2013; Gottipati and Mishra, 2010; Tangujank et al., 2009; Gupta and Babu 2009; Alfa-Sika et al., 2010; Verga et al., 2013; Selvi et al., 2001; Shaochen et al., 2013). There are no reports on the reduction of Cr(VI) to Cr(III) by PAC, which makes this investigation significant. The use of PAC as a reducing agent was found to be adventitious as it does not release other chemical species in the effluent and helps the recovery of Cr(III) without contamination. The recovered Cr(III) is in concentrated solution form and can be reused in the electroplating process. In the proposed method, sludge is not formed which is the most important advantage of this method. In many reported methods, sludge is formed which contains chromium compounds and the disposal of such a sludge is a big environmental problem as it creates the pollution at the disposal site.
We are also reporting a PAC based pilot scale process for the removal of Cr(VI) from the electroplating effluent. The aim of the pilot scale study is to evaluate the effectiveness of the bench scale method for the large scale removal of the chromium from the electroplating effluents. It was found that the pilot scale process is equally effective as the bench scale process for the removal of chromium. In the pilot scale process, more that 97% removal of chromium was achieved which allows less than 2 ppm discharge of Cr(III) in the treated effluent.
Materials and Methods
Source and Analysis of the Electroplating Effluent
The untreated chrome electroplating effluent was procured from a small scale electroplating unit located in the Bhosari industrial area, Pune (India). The pH of the electroplating effluent was determined by using a pH meter. The analysis of the procured electroplating effluent was performed for Cr(VI) using the prescribed volumetric method (Vogel, 1963). A UV-visible spectrum of the diluted effluent was recorded on double UV-visible spectrophotometer (Systronics, 2202 model).
Preliminary Experiment
In this experiment, 100 mg PAC was added into the 100 mL effluent and stirred for 90 minutes. The PAC was separated from the effluent by centrifugation and the supernatant was analysed by the prescribed spectrophotometric method for Cr(VI) content by using the diphenyl carbazide (DPC) reagent (Vogel, 1963). PAC was separated by the centrifugation and then PAC was qualitatively tested for the presence of Cr(VI) on it with the DPC reagent.
Reduction of Cr(VI) to Cr(III) by PAC at Bench Scale
In a first step, an influence of pH of the effluent was studied by varying the pH of the effluent from 1.62 to 7 (1.62 – original pH of the effluent). In this study, 100 mg PAC was added in the 100 mL effluent and pH was adjusted to specific value by drop wise addition of 0.1 N NaOH. The content in the flask was stirred at a room temperature at the rate of 200 rpm for 45 minutes using the magnetic stirrer. The PAC was then separated by centrifugation and supernatant was analyzed for the residual concentration of Cr(VI) by the spectrophotometric method.
The effect of dose of PAC on the quantity of Cr(VI) converted to Cr(III) in specified time was investigated by varying the dose of PAC from 25 to 150 mg at a interval of 25 mg in the 100 mL effluent. The experiment was performed at the optimized pH of the effluent and at the room temperature. Kinetics of the reduction of Cr(VI) was studied at optimized condition of pH of the effluent and dose of PAC. In the experiment, 100 mg PAC was added to the 100 mL of the effluent and stirred at 200 rpm for 90 minute. At a definite time interval, 2 mL effluent was withdrawn from the reaction mixture, centrifuged and supernatant was analysed for Cr(VI) content.
Per gram reduction capacity of PAC was evaluated in batch experiments. In this experiment, 1 g PAC was stirred with 300 mL effluent at original pH of the effluent till complete reduction of Cr(VI) take place. Then, PAC was recovered by filtration and reused again for next batch of 300 mL effluent. Same experiment was repeated till 100% reduction of Cr(VI) was observed.
Removal or Cr(III) at Bench Scale
The PAC treated and the filtered effluent containing Cr(III) was used in this step. The effective pH to the removal of Cr(III) was obtained experimentally. In this experiment, 100 mg PAC was added to the 100 mL effluent and pH was adjusted to the definite value. Resulted suspension was stirred for 45 minutes and supernatant was analysed for Cr(III) content (Vogel, 1963). The pH at which Cr(III) get precipitated completely was obtained by turbidity measurement. For this study, pH of Cr(III) containing effluent (in absence of PAC) was varied from 2 to 8 and turbidity was determined at a interval of 0.5 unit.
Pilot Scale Removal of Cr(VI)
After the bench scale experiment, the reduction and the removal of Cr(VI) was performed on a pilot scale (100 litre effluent per batch). The pilot scale experiment was performed in a tank of 120 L capacity consisting of stirrer, pump and outlet fitted with the carbon filter. The 100 g PAC was added in the 100 L effluent and stirred until the complete reduction of Cr(VI) takes place to Cr(III). The PAC was allowed to settle and then supernatant was transferred into another tank. To it 100 g fresh PAC was added and the pH was adjusted to 6.5±0.1 (optimized value) by addition of 1 N NaOH. The resulted suspension was stirred for 45 minutes. The PAC was then allowed to settle and the supernatant was taken out through the outlet fitted with carbon filter. The small quantity of thick suspension remained in the tank, which was finally filtered under vacuum. The recovered PAC was then washed with 1 L 2 N H2SO4, dried in an oven at 110 °C for 120 minutes and reused.
Calculation
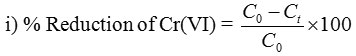
where, C0 – initial conc. of Cr(VI) and Ct – conc. of Cr(VI) after treatment with PAC
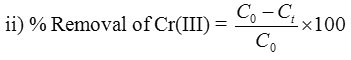
where, C0 – initial conc. of Cr(III) and Ct– conc. of Cr(III) after removal
Results and Discussion
Characteristics of PAC
The commercially available PAC used in the present study was purchased from Loba Chemie Pvt. Ltd. (India). The particle morphology and particle size of PAC was investigated by scanning electron microscopy (SEM). The SEM image (Fig.1) shows that the particles of the PAC possess irregular shapes with particle size in the range of 5 to 10 μm. The other characteristic of the PAC as quoted by the manufacturer are listed in Table 1.
 |
Figure 1: SEM image of PAC Click here to View figure |
Table 1: Characteristics PAC
|
Characteristic |
Value |
Characteristic |
Value |
| Water soluble substances | 1.5% | Loss on drying | 8.0% |
| Acid solubility | 2.0% | Iron | 300 ppm |
| Chloride | 0.01% | pH | 6.5 to 7.5 |
| Sulfate | 0.01% | M. B. value | 180 |
Characteristic of chrome electroplating effluent
The chemical analysis of the untreated electroplating effluent showed that it is acidic in nature (pH=1.62) and consist of Cr(VI) in dichromate form which is confirmed from electronic spectrum (Fig. 2) (Vogel, 1963). The observed concentration of Cr(VI) was 61.51±0.81 ppm. It is the commonly observed level of Cr(VI) in the electroplating effluent (Contreras-Ramos, 2004; Kumar et al., 2007; Kobya, 2004). The chromium and the acid content in the electroplating effluent arise due to the use of the chromic acid in the chrome electroplating solution (Mazumder et al., 2011). The electronic spectrum of the diluted effluent (Fig. 2) shows absorption bands at 348 and 445 nm and can be attributed to the charge transfer from (Figgis and Hitchman, 2000).
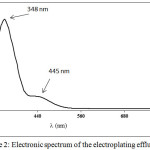 |
Figure 2: Electronic spectrum of the electroplating effluent Click here to View figure |
Preliminary Experiment
In the present study, preliminary experiment was performed to know the exact interaction taking place between the dichromate ions and the PAC. The interaction between dichromate ions and the PAC can result into the adsorption of the dichromate ions and or chemical reaction to form Cr(III). The analysis of PAC do not given the positive test for Cr(VI) with DPC reagent, indicating that dichromate ions are not present on PAC. At the same time, the analysis of PAC treated effluent showed the presence of Cr(III). This observation revealed that the interaction between PAC and the dichromate ions resulted into the chemical reaction. The presence of Cr(III) in the PAC treated effluent (faint green colour) was confirmed by the development of yellow colour to the effluent with H2O2 in alkaline condition. The development of yellow colour is due the oxidation of Cr(III) to CrO42- (Vogel, 1963). (1)
The reduction of Cr(VI) to Cr(III) is also supported by the electronic spectrum of the PAC treated effluent. In Fig. 3, electronic spectra of original electroplating effluent (dotted line) and the PAC treated effluent (solid line) are compared. The electronic spectra of the original effluent shows two absorption bands at 348 and 445 nm which are the characteristics bands of Cr(VI) in the dichromate form. The spectrum of the PAC treated effluent shows the weak and broad absorption bands at 411 and 576 nm which are the characteristics bands of hexaaquochromium(III) complex ion i.e. [Cr(H2O)6]3+ (Figgis and Hitchman, 2000).
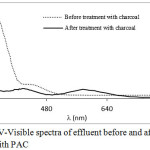 |
Figure 3: UV-Visible spectra of effluent before and after treatment with PAC Click here to View figure |
The observations of the preliminary experiment clearly showed that the chemical reaction takes place between Cr(VI) and the PAC to form Cr(III) which can be represented as given below. (2)
The first step in the formation of Cr(III) might be the adsorption of dichromate ions on the PAC which is immediately followed by the reduction reaction. The reduction of Cr(VI) to Cr(III) takes place at a cost of the oxidation of PAC. It was confirmed by the loss of dye adsorption ability of PAC. When the fully oxidized PAC was placed in the aqueous solution of a methyl orange (an azo dye), it did not adsorbed the dye while fresh PAC resulted into the complete adsorption of the dye.
Quantitative analysis of the PAC treated effluent showed the presence of 94.46±1.23% Cr(III) with respect to Cr(VI) content in the untreated effluent. Thus, preliminary experiment clearly demonstrated that the interaction of dichromate ions in the acidic medium with PAC resulted into the reduction of Cr(VI) to Cr(III).
The reduction of Cr(VI) (in dichromate form) to Cr(III) by the PAC is a contrast observation to the many other researchers where adsorption of dichromate ions is reported (Xiaoli and Xiali, 2013; Gottipati and Mishra, 2010; Tangujank et al., 2009; Gupta and Babu 2009; Alfa-Sika et al., 2010; Verga et al., 2013; Selvi et al., 2001; Shaochen et al., 2013). The reduction of Cr(VI) to Cr(III) by the activated charcoal was also reported by Selvi et al., (2001) and Kobya (2004) but they have not reported any detailed study on the same. Thus, further experiments were performed to optimize the experimental parameters so that reduction of Cr(VI) to Cr(III) and removal of Cr(III) can be performed, efficiently.
Effect of pH on reduction of Cr(VI)
Experimental results of effect of the pH on percent reduction revealed that strong acidic condition is required for reduction of Cr(VI) to Cr(III) by PAC. Results represented in figure-4 point out that pH 1.62 (original pH of the effluent) is the most favourable pH for the reduction of Cr(VI) to Cr(III). The reaction between dichromate ions and the PAC (equation-2) is catalysed by H+ ions therefore high H+ concentration (low pH) favours this reaction. Similar results were reported by Lackots et al., (2002) and Hu et al., (2009) on the reduction of Cr(VI) by different types of coal and carbon nano-tubes. Various reducing agents were utilized for the reduction of Cr(VI) to Cr(III) and all they requires acidic condition (Modhave and Shinde, 2014).
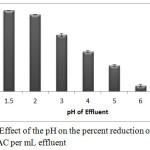 |
Figure 4: Effect of the pH on the percent reduction of Cr(VI) at 1mg PAC per mL effluent Click here to View figure |
Effect of the dose of PAC reduction of Cr(VI)
The results of this experiment (Fig.5) indicate that the percent reduction of Cr(VI) increases with increase in PAC dose. An increase in PAC dose results into increase in number of reaction sites, which is in turn increases Cr(VI) reduction. The percentage reduction increased from 26 to nearly 100% when PAC dose was increases from 25 to 100 mg per 100 ml of the effluent (i.e. 1 mg per mL). Thus, in further experiment to achieve complete reduction of Cr(VI) to Cr(III) 1 mg PAC per mL of the effluent was utilized.
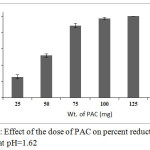 |
Figure 5: Effect of the dose of PAC on percent reduction of Cr(VI) at pH=1.62 Click here to View figure |
Kinetics of reduction
Figure 6 shows the effect of contact time on the percent reduction of Cr(VI) by PAC. Results of this experiment reveal that initially reduction reaction takes place at fast rate and rate of reaction goes on decreasing with increase in time. Initially, active reaction sites are largely and easily available on the surface of PAC which reflect in terms of high rate of reduction of Cr(VI). Once, all the reaction sites on the surface of PAC particles are exhausted, the reduction reaction continue with the utilization of less reactive groups or less accessible reaction sites of the PAC particle. This factor limits rate of reaction in later phase. In addition to this factor, decreased concentration of Cr(VI) ions in the reaction mixture is also responsible for slowing down the rate of reaction with time.
The reduction of Cr(VI) to Cr(III) by the PAC is also supported by UV-Visible spectroscopic study (Fig. 7). The decrease in dichromate ion concentration in solution with time is clearly reflected in terms of the drop off absorbance at 350 nm (Fig.7). As Cr(VI) concentration decreases in the solution, Cr(III) concentration goes on increasing which is indicated by the increase in absorbance at 576 nm (Fig.7 magnified view).
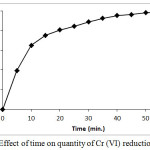 |
Figure 6: Effect of time on quantity of Cr(VI) reduction Click here to View figure |
From experimental data, first order rate constant of reduction reaction was calculated (Fig. 8) and it was found to be 0.0783 min-1.
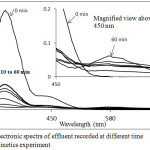 |
Figure 7: Electronic spectra of effluent recorded at different time interval in kinetics experiment Click here to View figure |
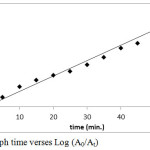 |
Figure 8: Graph time verses Log (A0/At) Click here to View figure |
Per gram reduction capacity of PAC
This experiment was performed to evaluate the reduction capacity of the PAC towards Cr(VI). In the experiment, 1 g PAC was used repeatedly in a batch of the 300 mL effluent and it was observed that up to 7 batches complete reduction of Cr(VI) was taken place but in 8th batch it remained incomplete. Thus, 1 g PAC can be used for the complete reduction of Cr(VI) from 2100 mL i.e. 137.65±3.17 mg Cr(VI). The observed reduction capacity of the PAC is comparable with reducing agent like FeSO4.7H2O, Na2S, Fe metal, etc. (Modhave and Shinde, 2014).
Removal of Cr(III)
The effluent treated with PAC consists of Cr(III) ions. Thus, further treatment was given to this effluent to remove Cr(III). The removal of Cr(III) was achieved by the adsorption of Cr(III) as hydroxide on the PAC. It has been observed that the adsorption of Cr(III) on the PAC is highly dependent on the pH of the effluent (Fig. 9). The highest Cr(III) removal efficiency was observed at pH 6.5 to 7. Similar pH range was reported by Shen-Fong et al., (2012) and Fonseca-Correa et al., (2013) for the removal of Cr(III) by adsorption. Hydrolysis of Cr(III) ions take place above the pH 5 and gives rise to the formation of water insoluble species like Cr(OH)2+, Cr(OH)2+ and Cr(OH)3 (Lakatos, et al., 2002; Fonseca-Correa, et al., 2013). An independent experiment was performed to observe the formation of water insoluble species from Cr(III) (Fig. 10) where turbidity of Cr(III) solution was determined as a function of pH. It was observed that the turbidity of solution containing Cr(III) starts increasing from pH 5 and the highest turbidity was observed at pH 7. It is due to the formation of water insoluble species by hydrolysis of Cr(III). At pH=7, precipitation of Cr(III) might be taking place completely as Cr(OH)3 hence increase in turbidity was not observed above the pH 7. Cr(OH)3 is a neutral species, which get adsorbed on PAC by the van der Waal’s forces of attraction. This might be the reason for the highest adsorption of Cr(III) at pH 6.5 to 7. At these pH values, the observed Cr(III) removal efficiency was found to be 97.60±0.57%.
The recovery of Cr(III) was achieved by washing of PAC with 1 N H2SO4. The recovered PAC was then dried and reused in the subsequent adsorption cycle. We do not observed appreciable decrease in the adsorption capacity of the recovered PAC at least for three adsorption cycles.
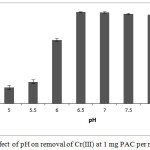 |
Figure 9: Effect of pH on removal of Cr(III) at 1 mg PAC per mL effluent Click here to View figure |
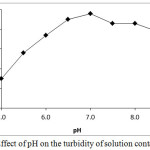 |
Figure 10: Effect of pH on the turbidity of solution containing Cr(III) Click here to View figure |
Pilot Scale Removal of Chromium
After the bench scale experiments, the reduction and the removal of chromium was achieved from 100 litre synthetic effluent. The experiment was performed in a tank of 120 L capacity. In first step the reduction of Cr(VI) was achieved to Cr(III) by the reaction with PAC (1 g/L). It required about 60 minutes. In the next step the removal of Cr(III) was accomplished at pH 6.5 on the PAC as an adsorbent. In this process more than 97% removal of the chromium was observed. These results clearly demonstrated that PAC based two step process can be effectively used for large scales treatment of chrome electroplating effluent. The recovery of Cr(III) from PAC was done by washing it with 1 N H2SO4. The washing of the PAC resulted into the formation of about 0.8 L solution containing Cr(III). Thus, process allows the recovery of PAC as well as Cr(III) and sludge is not produced. The recovered Cr(III) can be recycled as it does not consists of the impurities.
Conclusions
By proposed two stage PAC based process more than 97% removal of chromium is possible from electroplating effluent. Use of PAC as a reducing agent is adventitious over other chemical reducing agents as it does not results addition of any new chemical species into the effluent. Recovery of Cr(III) and used PAC in a adsorption step is possible which makes process economic. The process allows removal and recovery of chromium efficiently. Therefore, proposed process could be used as viable alternative to present methods of removal of Cr(VI) from the chrome electroplating effluent to diminish chromium discharge, recovery of Cr(III) and adsorbent, and to trim down pollution due to chromium.
References
- Agency For Toxic Substances And Disease Registry (Atsdr) (2000) Toxicological Profile for Chromium.
- Alfa-Sika M. S., Liu F and Chen H (2010) Optimization of key parameters for chromium (VI) removal from aqueous solutions using activated charcoal. J. of Soil Sci. and Environ. Manag. 1(3) 55-62.
- Beukes Jp, Pienaar Jj, Lachmann G and Giesekke Ew (1999) The reduction of hexavalent chromium by sulphite in wastewater. Water SA. 25(3) 363-370.
- Chulsung K, Qunhui Z, Baolin D, and Edward CT HUIFANG XU (2001) Chromium(VI) reduction by hydrogen sulfide in aqueous media: Stoichiometry and kinetics. Environ. Sci. and Technol. 35 2219-2225.
CrossRef - Contreras-Ramos Sm, Alvarez-Bernal D, and Trujillo-Tapia N, (2004) Dendooven L, Composting of tannery effluent with cow manure and wheat straw. Bioresour. Technol. 94 223-228.
CrossRef - Figgis Bn and Hitchman Ma (2000) Ligand Field Theory and Its Applications, Wiley VCH; 2nd Ed. 205-207.
- Fonseca-Correa R, Giraldo L and Moereno-Pirajan JC (2013) Trivalent chromium removal from aqueous solution with physically modified corncob waste. Journal of Analytical and Appl. Pyroly. 101 132-141.
CrossRef - Gang Q, Michael J, Mcguire, Nicole Kb, Seidel C, and Fong L (2005) Hexavalent chromium removal by reduction with ferrous sulfate, coagulation, and filtration: A pilot-scale study. Environ. Sci. Technol. 39 6321-6327.
CrossRef - Gottipati R and Mishra S (2010) Process optimization of adsorption of Cr(VI) on activated carbon prepared from plant precursors by a two level full factorial design. Chem. Eng. J. 160 99-107.
CrossRef - Gupta S and Babu Bv (2009) Modelling, simulation and experimental validation for continuous Cr(VI) removal from aqueous solution using sawdust as an adsorbent. Bioresour. Technol. 100 5633-5640.
CrossRef - Hu J, Wang Sw, Shao Dd, Dong Yh, Li Xl, And Wnak Xk (2009) Adsorption and reduction of cr(VI) from aqueous solution by multiwalled carbon nanotubes, the open Env. Pollut. and toxicol. J. 1 66-73.
- Khezami L and Capart R (2005) Removal of chromium (VI) from aqueous solution by activated carbon: Kinetic and equilibrium studies. J. Hazard. Mater. 123(1-3) 223-231.
CrossRef - Kobya M (2004) Removal of Cr(VI) from aqueous solution by adsorption on hazelnut shell activated carbon: kinetics and equilibrium studies. Bioresour. Technol. 91 317-321.
CrossRef - Kumar R, Bishnoi NR. and Bishnoi GK (2007) Biosorption of Cr(VI) from aqueous solution and electroplating waste water using fungal biomass. Chem. Eng. J. 135 202-208.
CrossRef - Lakatos J, Brown SD and Snape CE (2002) Coal as sorbent for the removal and reduction of hexavalent chromium from aqueous waste stream. Fuel, 81 691-698.
CrossRef - Levankumar L, Muthukumaranv and Gobinath Mb (2009) Batch adsorption anf kinetics of of chromium (VI) removal from aqueous solution by Ocimum americanum Seed Pods. J. Hazard. Mater. 161 709-713.
CrossRef - Malkoc E, Nuhoglu Y and Dundar M (2006) Adsorption of Cr(VI) on pomac-An olive oil industry waste: Batch and column studies. J. Hazard. Mater. B. 138 142-151.
CrossRef - Mazumder D, Ghosh D, and Bandyopandhyay P (2011) Treatment of electroplating wastewater by adsorption technique. Int. J. of Civil and Environment. Eng. 3(2) 101-109.
- Modheve Ss And Shinde DR (2014) Evaluation of plant biomass as reducing agent to Cr(VI) from electroplating effluent and removal of Cr(III) by adsorption on hematite ore. Ascian J. of Chem., 26 (23), in press.
- Niekerk Wv, Pienaar Jj, Lachmann G, Van Eldik R and Hamza M (2007) A kinetic and mechanistic study of the chromium (VI) reduction by hydrogen peroxide in acidic aqueous solution Water SA 33(5) 619-626.
- Selvi K, Pattabhi S and Kadirvelu K (2001) Removal of Cr(VI) from aqueous solution by adsorption onto activated carbon. Bioresour. Technol. 80 87-89.
CrossRef - Selvraj K, Manonmani S, and Pattabhi S (2003) Removal of hexavalent chromium using distillery sludge. Bioresour. Technol. 89 207-211.
CrossRef - Shaochen W, Dongtian L, Zhe H, Yaqin H, and Feng W (2013) High capacity adsorption of Cr(VI) from aqueous solution using a hierarchical porous carbon obtained from pig bone. Bioresour. Technol. 134 407-411.
CrossRef - Sheng-Fong L, Song-Yung W, Ming-Jer T, and Lang-Dong L (2012) Adsorption capacity and removal and removal efficiency of heavy metal ions by Moso and Ma bomboo activated carbon. Chem. Eng. Res. and Design. 90 1397-1406.
CrossRef - Tangujank S, Insuk N, Udeye V, and Tontrakoon J (2009) Cr(III) sorption from aqueous solution using activated carbon prepared from cashew nut shell, Int. J. Physic. Sci. 4(8) 412-417.
- Verga M, Takacs M, Zaray G, and Varga I (2013) Comparative study of sorption kinetics and equilibrium of Cr(VI) on charcoal prepared from different low cost materials. Microchem. J. 107 25-30.
CrossRef - Vogel AI, (1963) In Quantitative Inorganic Analysis Including Elementary Instrumental Analysis, 3rd Ed., ELBS.
- Xiaaoshu L, Jiang X, Guangming J, Jieang T And Xinhua X (2012) Highly active nanoscale zerovalent iron-Fe2O3 nanoparticles for removal of Cr(VI) from aqueous solution. J. Colloid and Interface Sci. 369 460-469.
CrossRef - Xiaoli X and Xiali G (2013) Preparation of bio-charcoal from sewage sludge and its performance on removal of Cr(VI) from aqueous solution. J. Mol. Liq. 183 26-30.
CrossRef

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









