Synthesis, Identification and Biological Activity of Some New Chalcone derivatives from 8-Chlorotheophylline
Nabeel A. Abdul‐Reda1 and Salwa R. Abdul-Ameer2
and Salwa R. Abdul-Ameer2
1Department of Chemistry, College of Science, University of Al‐Qadisiyah, Iraq.
2Department of Chemistry, College of Education University of Al‐Qadisiyah, Iraq.
Corresponding Author E-mail: salwa22.razzaq@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340144
New derivatives of 8-Chloro theophylline (8-CTh) were synthesized by react 8-CTh and 4-amino acetophenone to prepared 8-(4-acetylphenylamino)-1,3-dimethyl-1H-purine-2,6(3H,7H)-dione then the result reacts with some substituted benzaldehyde derivatives to prepared different chalcone compound then the products were allowed to react with thiourea and hydrazine to give Pyrimidine and pyrazoline derivatives respectively. The reaction was monitored by thin‐layer chromatography (TLC) technique. All new compounds were characterized by melting points, elemental analysis, FT‐IR, 1H, 13C and 2D‐NMR spectroscopy. Antimicrobial activity of these derivatives was also determined.
KEYWORDS:8-Chlorotheophylline; Chalcone; Pyrazoline; Pyrimidine; Antimicrobial
Download this article as:| Copy the following to cite this article: Abdul‐Reda N. A, Abdul-Ameer S. R. Synthesis, Identification and Biological Activity of Some New Chalcone derivatives from 8-Chlorotheophylline. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Abdul‐Reda N. A, Abdul-Ameer S. R. Synthesis, Identification and Biological Activity of Some New Chalcone derivatives from 8-Chlorotheophylline. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=41845 |
Introduction
Xanthine a purine base produces in most human body tissues and fluids and in other organisms it is produced by purine metabolism. [1] The commonly used methyl xanthine theophylline, caffeine and theobromine are naturally occurring compounds in various plants. [2] These substances are components of most widely consumed beverages, coffee, tea and cocoa. Theophylline and its salts are used in the therapy of bronchial asthma and chronic obstructive pulmonary disease (COPD) 8-CTh (I) is a purine derivative which is obtained from theophylline (II) [3].
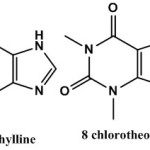 |
Scheme A Click here to View scheme |
In therapy, 8-CTh is used in combination with antihistamines in order to enhance their action against travel sickness and other hearing labyrinth diseases [4]. In this work prepared some derivatives from 8-CTh by chalcone reaction. Chalcone can be prepared by condensation reaction between different aldehyde compound and an acetophenone in the presence of opportunity base (aldol conditions) [5]. Recently, the heterocyclic compound of different ring with different heteroatoms are important for biologically active and industrial chemical products [6]. Pyrazoline (Py) and Pyrimidine (Pyr) derivatives are among the most prominent heterocyclic compounds, which have an important role in pharmaceutical and agrochemical industries as well as bioactive products because of their significant and wide spectrum of biological activities [7].
Experimental
All materials were of highest purity and supplied by Merck, Sigma Aldrich and Fluka- company. Melting points were measured on a Buchi melting point apparatus B-545 (Buchi Labortechnik AG, Switzerland). Microanalytical data were obtained with a Vario, Elementar apparatus (Shimadzu, Japan). The IR spectra were recorded on Schimadzu Fourier Transform Infra-red spectrophotometer (Model 270), using KBr discs. NMR spectra were recorded on 600 MHz (1H) and at 100 MHz and (13C) spectrometers (Bruker, Germany) with TMS as the internal standard and on δ scale in ppm. (TLC) was performed on silica gel for (TLC) and spots were visualized by Iodine vapors. The reagents used were of analytical grade while the solvents were purified before use.
Synthesis of 8-(4-acetylphenylamino) -1,3-dimethyl-1H-purine-2,6 (3H,7H) -dione (S1). [8]
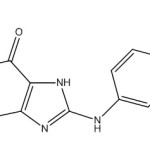 |
Scheme B |
8-CTh (0.21g/0.001 mol) it was dissolve with 4–amino acetophenone (0.135g /0.001mol) in 15 ml of THF and add 1ml of triethyl amine and then reflux reaction for 24 hours and monitored reaction by TLC using the solution (ethyl acetate: n-Hexane) (4:2) the reaction is neutralized by glacial acetic acid and the precipitate obtained was filtered, washed and recrystallized from ethanol.Yield (79%) as light yellow solid. m.p = 176-179oC. Rf =0.6 , IR (KBr, cm-1): N-H (3361), C-Harom.(3130), C-Hali.(2983, 2883), C=O (1716),C=O ketone (1700) C=Carom (1696), C=C (1635) C-N (1373),C=N (1519).1H-NMR (600 MHz, DMSO-d6) δ= 3.18 (s, 3H, N1-CH3), 3.53 (s, 3H, N2-CH3.), 9.72 (s ,1H, N10H), 14.54 (s,br, 1H, N9H), 2.33 (s, 3H, COMe), 7.57-7.60 (d, 2H, C2´+6´ J= 8.3 Hz), 7.89-7.91 (d, 2H, C3´+5´ J= 8.3 Hz) 13C-NMR (100 MHz, DMSO d6): δ= 27.3 (N1-CH3), 29.8 (N3-CH3), 24.6 (Me), 113.9 (C2´+6´), 115.0 (C5), 127.3 (C4´), 131 (C3´+5´), 143.8 (C1´), 149.4 (C4), 151.4 (C=O2), 156.5 (C=O 6) , 167.3 (C8), 196.5 (C=O).
General procedure for synthesis chalcone (S2-S4). [9,10]
(3.13 g / 0.01 mol) from S1 with different aromatic aldehydes (0.01 mol). The mixture dissolved in 10 ml of alcohol. Sodium hydroxide solution 3ml (10%) was added slowly and the mixture stirred for 2hr until the entire mixture becomes very cloud then the mixture was poured slowly into 20 ml of water with constant stirring and kept in refrigerator for 24 hours. The reaction was monitored by TLC (ethyl acetate: n-Hexane) (4:2). the reaction is neutralized by glacial acetic acid and, the precipitate obtained was filtered, washed and recrystallized from ethanol
8-(4-((E)-3-(4-hydroxy-3-methoxy phenyl) acryloyl) phenylamino)-1,3-dimethyl-1H-purine -2,6 (3H,7H)-dione (S2).
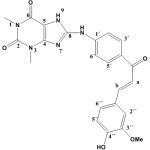 |
Scheme C |
(3.13 g / 0.01 mol) of S1 with Vanillin (1.52g / 0.01mol) Yield (74%) as Brown solid. m.p = 185-188oC. Rf = 0.51 ,FT‐IR (KBr, ν, cm‐1): 3336 (N-H), 3113, 2999, 2885 (C-H)ali, 1516 (C=C) cha, 1668 (C=O)cha, 3437 (O-H), 1593, 1471 (C=C)ar. 1267 (C-O-C), 3224 (N10-H) cha. 1H-NMR (600 MHz, DMSO-d6) δ =3.21 (s, 3H, N1-CH3), 3.60 (s, 3H, N3-CH3.), 3.80 (s, 3H, OMe), 7.64, 7.66 (d, 1H, Hb J=15.9 Hz), 8.0 ,8.03 (d, 1H, Ha J= 15.9 Hz), 9.84 (s, 1H, OH), 12.46 (s. br, 1H, N9H), 9.64 (s. br, 1H, N10H), 6.54-7.44 (m, 7Harom). 13C-NMR (100 MHz, DMSO d6) δ= 27.6 (N1-CH3), 29.7 (N3-CH3), 55.4 (OMe), 108.8 (C5), 112.6 (C2″), 114.9 (C2´+C6´+C5″), 121.5 (Ca +C6″), 123.3 (C1″), 124.8 (C4´), 130.4 (C3´+C5´), 144.2 (C1´+Cb), 147.1 (C4), 147.3 (C4″ C-OH), 150.8 (C3´(C-OMe) ,151.0 (C=O 2), 153.8 (C=O6), 167.1 (C8), 194.8 (C=O) cha. Anal. calc. for C23H21N5O5 (447.45): C 61.74, H 4.73, N 15.65. Found: 61.51, H 4.65, N 15.42.
8-(4-((E)-3-(4-chlorophenyl) acryloyl) phenylamino)-1,3-dimethyl -1H-purine-2,6 (3H,7H)–dione (S3).
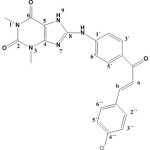 |
Scheme D Click here to View scheme |
(3.13 g / 0.01 mol) from S1 with p-chloro benzaldehyde (1.4g / 0.01mol) Yield (82%) as Yellow solid. m.p = 178-181oC. Rf = 0.55 FT‐IR (KBr, ν, cm‐1): 3460 (N-H), 2995, 2875 (C-H)ali, 1589 (C=C) cha, 1665 (C=O)cha, 1055 (C-O), 1016 (C-Cl)cha, 3219 (N10-H).1H NMR (600 MHz, DMSO-d6) δ= 3.17 (s, 3H, N1-CH3), 3.33 (s, 3H, N3-CH3.) , 7.33-7.68 (m,8H. arom),7.72, 7.76 (d,1H, Hb J=15.6 Hz), 7.99,8.03 (d,1H, Ha J=15.6 Hz), 8.69 (s, br, 1H, NH), 9.99 (s,1H, N9H) 13C-NMR (100 MHz, DMSO d6) δ = 27.3 (N1-CH3), 29.8 (N3-CH3), 115.0 (C5), 112.7 (C2´+C6´), 121.3 (Ca), 127.3 (C4´), 129.3 (C3″+C5″), 130.6 (C2″+C6″), 131.1 (C3´+C5´), 134.6 (C4″ CCl+C1″), 142.7 (C1´), 144.6 (Cb), 149.4 (C4), 151.4 (C=O 2), 156.5 (C=O 6), 166.9 (C8), 192.6 (C=O cha). Anal. calc. for C22H18ClN5O3 (435.87): C 60.62, H 4.16, N 16.07. Found: C 60.40, H 4.04, N 15.79.
8-(4-((E)-3-(4-(dimethylamino) phenyl) acryloyl) phenylamino)-1,3-dimethyl-1H-purine 2,6(3H,7H) dione (S4).
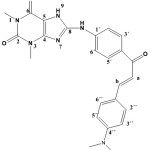 |
Scheme E Click here to View scheme |
(3.13 g / 0.01 mol) from S1 with p-dimethyl amino benzaldehyde (1.49g /0.01mol) Yield (78%) as Brown solid. m.p = 189-191oC. Rf = 0.42 FT‐IR (KBr, ν, cm‐1): 3336 (N-H), 3132, 2939, 2858 (C-H) ali, 3000 (C-H) 1540 (C=C) cha, 1658 (C=O) cha, 1604 (C=C) ar. 1H-NMR: (600 MHz, DMSO-d6) δ = 3.38 (s, 3H, N1-CH3), 3.21 (s, 3H, N3-CH3.), 3.06 (s,3H, NMe2), 7.50,7.51 (d,1H, Hb), 8.06 ,8.08 (d,1H, Ha), 6.54-6.56 (d,2H, C3″+C5″), 7.60-7.62 (d, 2H, C3´+C5´) ,7.64-7.66 (d, 2H, C2´+6´), 7.72-7.74 (C2″+C6″), 9.84 (s,1H, N9H), 12.46 (s, br, 1H, NH). 13C-NMR (100 MHz, DMSO-d6) δ = 27.3 (N1-CH3), 29.8 (N3-CH3), 43.5 (NMe2), 112.5 (C5), 111.9 (C2´+C6´), 122.9 (Ca) ,129.4 (C4´), 111.5 (C3″+C5″), 129.7 (C2″+C6″), 130.1 (C3´+C5´), 151.4 (C4″), 123.3 (C1″) 141.4 (C1´), 143.9 (Cb), 148.1 (C4), 150.1 (C=O 2), 156.2 (C=O 6), 167.2 (C8),191.5 (C=O) cha.
General procedure for synthesis Pyrimidine compounds derivatives (S5-S7).[11].
(0.001mol) of prepared chalcone was dissolved in ethanolic sodium hydroxide (10ml) and (0.001mol) was add slowly from thiourea, were stirred about 3-4 hours with a magnetic stirrer. This was then poured into 5 ml of cold water with continuous stirring for an hour and then kept in refrigerator for 24 hours, the reaction was monitored by TLC (ethyl acetate: n-Hexane) (4:2). The precipitate obtained was filtered, washed and recrystallized with ethanol.
8-(4-(6-(4-hydroxy-3-methoxy phenyl)-3,6-dihydro-2-mercapto pyrimidin-4-yl) phenyl amino)-1,3-dimethyl-1H-purine-2,6(3H,7H)-dione (S5).
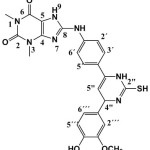 |
Scheme F |
(0.447g / 0.001 mol) from S2 with thiourea (0.076 g / 0.001 mol) Yield (72%) as light Brown solid. m.p = 195-199oC. Rf = 0.35, FT‐IR (KBr, ν, cm‐1), 2644 (S-H), 3394 (O-H), 1593 (C=C)ar 2993 (C-H) ar.1099 (C-O-C), 1523 (C=N)pyr. 1648 (C=C)pyr. 1H-NMR (600 MHz, DMSO-d6) δ =1.36 (s, 1H, SH) 3.34 (s, 3H, N1-CH3), 3.50 (s, 3H, N3-CH3.), 3.93 (s,3H. OMe), 4.78 (s.br,1H, C5″),5.20 (s.br,1H, C2″) 6.68 (s, br.,1H, C6‴), 6.88 (s,1H, br, C5‴), 6.96 (s, br,1H, C2‴),7.56 (s,1H, C3´+5´), 7.78 (s, br,1H, C2´+6´) 8.18 (s ,1H, NHpyr), 9.07 (s, br,1H, N9H), 9.66 (s,1H, OH) ,11.38 (s,1H, N10H). 13C-NMR (100 MHz, DMSO d6): δ= 27.6 (N1-CH3), 29.7 (N3-CH3), 55.4 (OMe), 62.7 (C2″), 103.4 (C5″), 108.1 (C5), 112.3 (C2‴), 114.9 (C5‴), 121.5 (C2´+C6´) ,123.3 (C6‴), 130.4 (C3´+C5´), 125.9 (C4´), 133.4 (C1‴), 136.5 (C1´), 142.3 (C6″), 148.8 (C3‴+C4‴), 149.7 (C4), 150.8 (C=O 2) 153.6 (C=O 6), 164.6 (C4″), 167.1 (C8). Anal. calc. for C24H25N7O4S (507.57): 56.79, H 4.96, N 19.32. Found: C 56.56, H 4.82, N 19.09.
8-(4-(6-(4-chlorophenyl)-3,6-dihydro-2-mercaptopyrimidin-4-yl) phenylamino)-1,3-dimethyl-1H-purine-2,6(3H,7H)-dione (S6).
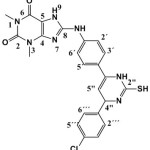 |
Scheme G |
(0.435 g / 0.001 mol) from S3 with thiourea (0.076g / 0.001mol) Yield (69%) as Brown solid. m.p = 190-192oC. Rf = 0.29 ,FT‐IR (KBr, ν, cm‐1): 2653 (S-H), 1604 (C=C) ar 1552 (C=N) 3084 (C-H) ar. 1523 (C=N)pyr. , 1652 (C=C)pyr. 1H-NMR (600 MHz, DMSO-d6) δ = 1.41 (s, 1H, SH), 3.20 (s, 3H, N1-CH3), 3.36 (s, 3H, N3-CH3.), 4.65 (s, 1H, C5″), 5.23 (s, 1H, C2″), 7.09-7.13 (d, 1H, C2‴+6‴ J =7.9 Hz), 7.21-7.24 (d, 1H, C3´+5´ J=8.2 Hz),7.41-7.44 (d, 1H, C3‴+5‴ J=7.9 Hz), 7.57-7.60 (d, 1H, C2´+6´ J=8.2 Hz), 8.71 (s ,1H, NH), 9.34 (s,br, 1H, N9H), 10.38 (s, 1H, N10H).13C-NMR (100 MHz, DMSO d6) δ=27.5 (N1-CH3), 29.7 (N3-CH3), 65.4 (C2″), 102.9 (C5″), 110.6 (C5), 120.8 (C2´+ C6´), 123.9 (C3‴+C5‴), 128.7 (C3´+ C5´), 129.4 (C2‴+C6‴), 130.1 (C4´), 131.1 (C1‴+C4‴), 135.6 (C1´), 142.9 (C6″), 146.6 (C4), 151.4 (C=O 2), 153.8 (C=O 6), 159.4 (C4″), 166.8 (C8).
8-(4-(6-(4-(dimethyl amino) phenyl)-3,6-dihydro-2-mercaptopyrimidin-4yl)phenylamino)-1,3-dimethyl-1H-purine-2,6(3H,7H)-dione (S7)
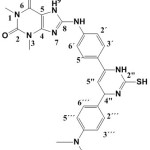 |
Scheme H |
(0.444g / 0.001 mol) from S4 with thiourea (0.076 g / 0.001 mol) Yield (78%) as Brown solid. m.p = 198-200oC. Rf = 0.25 ,FT‐IR (KBr, ν, cm‐1):2646 (S-H) 1604 (C=C) ar 3050 (C-H) ar. 1500 (C=N)pyr. , 1649 (C=C)pyr. 1H-NMR (600 MHz, DMSO-d6) δ = 1.37 (s, 1H, SH), 3.16 (s, 6H, NMe2) 3.20 (s, 3H, N1-CH3), 3.32 (s, 3H, N3-CH3.), 4.68 (s, 1H, C5″), 5.25 (s, 1H, C2″) 6.87-6.89 (d, 2H, C2‴+C6‴ J=7.8 Hz), 7.09-7.13 (d, 2H, C3´+5´ J=8.2 Hz), 7.22-7.24 (d, 2H, C3‴+5‴ J=7.8 Hz), 7.34-7.36 (d, 2H, C2´+6´ J=8.2 Hz), 9.57 (s ,1H, NH), 9.76 (s, 1H, N9H), 12.41 (s,1H, NH). 13C-NMR (100 MHz, DMSO d6): δ= 27.7 (N1-CH3), 29.8 (N3-CH3), 43.6 (NMe2) , 65.8 (C2″), 103.6 (C5″), 111.1 (C3‴+C5‴), 112.6 (C5), 120.6 (C2´+ C6´), 124.1 (C1‴), 128.7 (C3´+ C5´), 129.4 (C2‴+C6‴), 130.8 (C4´), 135.6 (C1´), 142.9 (C6″), 144.4 (C4), 146.4 (C4‴), 152.6 (C=O 2), 154.8 (C=O 6), 159.6 (C4″), 167.5 (C8).
General procedure for synthesis pyrazoline compounds derivatives (S8-S10) [12].
A mixture a prepared chalcone (0.001 mol), and (0.001 mol) and hydrazine hydrate (99%) (0.001 mol) in (25 ml) ethanol was refluxed with stirring about 5 hrs, the reaction was monitored by TLC (ethyl acetate: n-Hexane) (4:2). The precipitate obtained was filtered, washed and recrystallized with ethanol
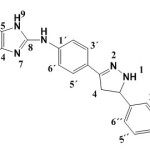
8-(4-(4,5-dihydro-5-(4-hydroxy-3-methoxyphenyl)-1H-pyrazoline-3-yl) phenylamino)-1,3-dimethyl-1H-purine-2,6(3H,7H)-dione. (S8)
 |
Scheme I Click here to View scheme |
(0.447 g / 0.001 mol) from S2 with hydrazine (0.032g /0.001mol) Yield (80%) as brown solid. m.p = 191-193oC. Rf = 0.38 FT‐IR (KBr, ν, cm‐1): 2960, 2926, 2879 (C-H) ali, 3363, 3219 (N-H) Py, 3039 ,1595 (C=C)ar., 3069 (C-H)ar.Py , 3462 (N-H), 3564 (O-H). 1H NMR (600 MHz, DMSO-d6) δ= 3.22 (s, 3H, N1-CH3), 3.37 (s, 3H, N3-CH3.) 3.63 (s, 3H, OMe), 3.78 (s, 1H ,C5 py) , 3.82 (s,1H, C4 Py), 6.77-7.83 (d, 8H. arom), 9.23 (s ,1H, NH py.), 10.29 (s,1H, N9H), 13.38 (s1H, NH), 9.50 (s, 1H, OH),.13C-NMR (100 MHz, DMSO d6): δ= 27.6 (N1-CH3), 29.7 (N3-CH3), 42.4 (C 4Py), 58.8 (OMe), 49.7 (C5py), 108.8 (C5), 112.6 (C2″) ,114.9 (C2´+C6´+ C5″), 121.5 (C6″) ,123.3 C4´) ,130.4 (C3´+C5´), 137.5 (C1″), 142.6 (C1´), 147.5 (C4″ C-OH ) ,147.7 (C4), 149.7 (C3″), 150.8 (C3py) , 150.0 (C=O 2 +C3´(C-OMe), 151.0 (C=O 6) ,168.2(C8)
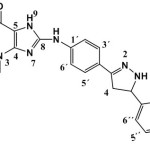
8-(4-(5-(4-chlorophenyl)-4,5-dihydro-1H-pyrazoline-3-yl) phenylamino)-1,3-dimethyl-1H-purine-2,6(3H,7H)-dione (S9)
 |
Scheme J Click here to View scheme |
(0.435 g / 0.001 mol) from S3 with hydrazine (0.032g / 0.001mol) Yield (77%) as Yellow solid. m.p = 189-193oC. Rf = 0.42 FT‐IR (KBr, ν, cm‐1): 3130,2981, 2926, 2879 (C-H) ali, 3429 (N-H) ,3346,3227 (N-H) py ,1597 (C=C) ar., 3462 (N-H), 3059 (C-H) ar. 1H-NMR (600 MHz, DMSO-d6) δ = 3.21 (s, 3H, N1-CH3), 3.37 (s, 3H, N3-CH3.), 3.87 (d, 1H, C5), 7.43-7.85 (m, 8H. arom), 9.15 (s ,1H, NH py.), 10.09 (s,1H, N9H), 3.71 (s,1H, C4 Py) .13C-NMR (100 MHz, DMSO d6): ): δ= 27.6 (N1-CH3), 29.7 (N3-CH3), 108.5 (C5), 113.1 (C2´+C6´ ), 123.4 (C4´) ,127.5 (C3´+C5´ +C2″ +C6″), 129.4 (C3″+ C5″ ), 141.4 (C1″), 134.0 (C4″), 136.9 (C1´), 147.2 (C4), 150.2 (C3), 48.6 (C5Py), 151.7 (C3py) , 42.3 (C4 Py), 150.8 (C=O 2), 153.7 (C=O 6), 167.1 (C8)
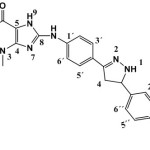
8-(4-(5-(4-(dimethylamino) phenyl)-4,5-dihydro-1H-pyrazoline-3-yl) phenylamino)-1,3-dimethyl-1H-purine -2,6(3H,7H) dione. (S10)
 |
Scheme K Click here to View scheme |
(0.444g / 0.001mol,) from S4 with hydrazine (0.032g / 0.001m) Yield (76%) as brown solid .m.p =199-102oC. Rf = 0.61 FT‐IR (KBr, ν, cm‐1), ,3334,3223 (N-H) py ,1597 (C=C) ar,1560 (C=N) 3039 (C-H) ar.Py ,3334 (N-H)Py, 2953, 2922, 2856 (C-H)ali.1H-NMR (600 MHz, DMSO-d6) δ= 2.98 (s, 3H, N(Me)2), 3.20 (s, 3H, N1-CH3), 3.36 (s, 3H, N3-CH3.) ,3.47 (s,1H ,C4 Py), 3.82 (s,1H ,C5 Py), 6.57-6.59 (d, 2H, C3″+5″J=8.6 Hz), 6.74-6.77 (d,2H, C2´+6´ J=8.5 Hz), 7.32-7.34 (d, 2H, C2″+6″J=8.6 Hz), 7.61-7.64 (d,2H, C3´+5´ J=8.5 Hz), , 8.36 (s ,1H, NHpy.), 10.60 (s,1H, N9H), 13.06 (s,1H,NH) 5.98 (s, 1H, C4 Py). 13C-NMR (100 MHz, DMSO d6): δ= 27.5 (N1-CH3), 29.7 (N3-CH3), 45.5 (NMe)2, 43.0 (C4) Py, 49.9 (C5)py, 108.2 (C5), 113.0 (C3″+C5″), 118.1 (C2´+C6´), 125.7 (C4´) ,127.5 (C3´+C5´) 127.8 (C2″+C6″), 133.5 (C1″), 138.7 (C1´), 147.3 (C4), 149.0 (C4″), 150.2 (C3)py 151.4 (C=O 2), 153.4 (C=O 6), 166.8 (C8). Anal. calc. for C24H24N8O2 (456.20): 63.15, H 5.30, N 24.55. Found: C 62.92, H 5.21, N 24.31
Antimicrobial Evolution
The newly synthesized compounds were selected for their antimicrobial activities against different bacteria and fungi. The microorganisms used were Staphylococcus aureus (Gram positive), Escherichia coli, (Gram negative) and Candida albicans by using the agar diffusion method [13] to select the most potent compounds. 5 mg of each compound was dissolved in dimethyl sulfoxide (DMSO, 1 mL) then complete up to 10 mL with distal water to give a concentration of 500 μg/mL. The bacteria were maintained on Muller hentone agar media, the dishes incubated at 37°C for 24 hr for bacteria while 72 hr for fungal. [14,15] The efficiency of the tested compounds was compared to that of water and DMSO, zone of inhibition measured by ruler.
Results and Dicussion
8-CTh was a starting material for the synthesis of new chalcone compound over a reaction with (P-amino acetophenone) scheme [1]
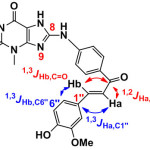
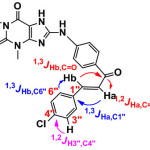
The appearance of stretching band of secondary aromatic amine (NH) at (3361) cm-1and disappearance of C-Cl of 8-CTh at (760) cm-1, and showed- 1H-NMR spectrum for N-H 14.54 (s, br,1H, NH), are attributed to the formation amine compound. 13C-NMR spectrum for 24.6 (Me), 113.9 (C2´+ 6´), 127.3 (C4´), 131 (C3´+5´), 143.8 (C1´) [16]. The reaction of S1 compond with different aromatic aldehydes in the presence NaOH to formation chalcone in scheme 2 showed appearance of the band 1516, 1589 and 1525 to (C=C) cha. for S2, S3 and S4 respectively, in other hand 1668, 1665 and 1658 to (C=O)cha. for S2, S3 and S4 respectively. 1H-NMR showed the deplete of CH=CH proton at about 7.64, 7.66 for Hb, 8.0 ,8.03 for Ha to S2 and 7.72, 7.76 Hb, 7.99, 8.03 Ha to S3 and 7.50,7.51 for Hb, 8.06 ,8.08 for Ha to S4.13C-NMR spectrum for (C=O)cha. 194.8, 192.6 and 191.5 for S2, S3 and S4 respectively [17]. Some of prepared compounds studding the 2D-NMR (HMBC) and we found for compound S2 three coupling for type 1,3 JH, C the first; between C6″ to be the distance d=120 ppm and proton Hb d=7.5 ppm, and the second between C1″ for the distance δ =124 ppm and proton Ha δ =8 ppm. Third of coupling 1,3 JH, C between C=O for the distance δ =195 ppm and proton Hb for the distance δ = 7.5 ppm. or the distance δ = 195 ppm and proton Ha for the distance δ = 8 ppm.
 |
Scheme L Click here to View scheme |
For S3 also studding the HMBC-NMR and we found three coupling for type 1,3JH, C the first; between C6″ to be the distance d=115 ppm and proton Hb δ=7.5 ppm, and the second between C1″ for the distance δ=135 ppm and proton Ha δ=8 ppm. Third of coupling 1,3JH, C between C=O for the distance δ= 194 ppm and proton Hb for the distance δ=7.5 ppm. The second type of coupling is 1,2JH,C there are two coupling between C=O for the distance δ=194 ppm and proton Ha for the distance δ= 8 ppm and between C4″ for the distance δ= 135 ppm and proton H3″ for the distance δ= 7.3 ppm. [18,19] .The second type of coupling is 1,2 JH, C there are one coupling between C=O for the distance δ = 195 ppm and proton Ha for the distance δ = 8 ppm. of Chalcone (S2-S4) that reacted with thiourea in the presence ethanolic Sodium hydroxide to give corresponding. Pyrimidine as well as reacted with hydrazine in the presence absolute ethanol to give pyrazoline derivatives. Pyrimidine and pyrazoline can be prepared by Scheme 3. 1H-NMR and 13C-NMR of Pyrimidine and pyrazoline compounds can be show in Table [1] and [2] respectively.
 |
Scheme M Click here to View scheme |
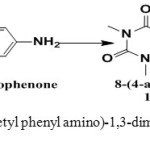 |
Scheme 1: Synthesis of 8-(4-acetyl phenyl amino)-1,3-dimethyl-1H-purine-2,6 (3H,7H) –dione. |
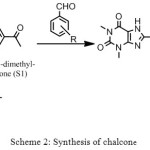 |
Scheme 2: Synthesis of chalcone Click here to View scheme |
Table 1: Characteristic FTIR absorption, 1H-NMR and 13C-NMR bands of compounds (S5-S7)
| No. of comp. | IR | 1H-NMR | 13C-NMR | ||||
| N-H py | N-H py | C4py | C5py | C3py | C4py | C5py | |
| S8 | 3219,3363 | 9.15 | 6.21 | 3.97 | 151.3 | 42.7 | 50.1 |
| S9 | 3346,3227 | 9.15 | 3.71 | 3.78 | 151.7 | 42.3 | 50 |
| S10 | 3223 ,3334 | 9.63 | 3.75 | 3.9 | 150.5 | 42.9 | 42.3 |
Table 2: Characteristic FTIR absorption, 1H-NMR and 13C-NMR bands of compounds (S8-S10)
| IR | 1H-NMR | 13C-NMR | |||||||
| No.of comp. | S-H | C=C pyr. | C=N pyr. | N-H | S-H | C2″ | C4″ | C5″ | C6″ |
| S5 | 2644 | 1648 | 1523 | 8.18 | 1.36 | 62.7 | 103.4 | 164.6 | 142.3 |
| S6 | 2653 | 1649 | 1523 | 8.71 | 1.41 | 65.4 | 102.9 | 159.4 | 142.9 |
| S7 | 2646 | 1652 | 1500 | 9.68 | 1.37 | 65.8 | 103.6 | 159.6 | 142.9 |
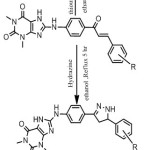 |
Scheme 3: Synthesis of Pyrimidine and Pyrazoline derivatives Click here to View scheme |
Biological part
Control of microbial population is necessary to prevent show of disease, infection, decomposition, spoilage and contamination and caused by them. The newly synthesized compounds were screened for their antimicrobial activity invitro against bacteria (Staphylococcus aureues, Escherichia coli) and fungal (Candida albicans) as show in Figure 1. The antimicrobial activity results discovered that most of the tested compounds have moderate to strong activity. The most effective compounds are S3, S5 and S8 When these compounds were compared with the reference compounds (DMSO, distill water) we found that they have an antimicrobial activity higher or almost equal to them. While candida albicans not responsive for all compounds.
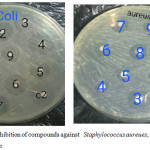 |
Figure 1: Zone of inhibition of compounds against Staphylococcus aureues, Escherichia coli and Candida albican
|
Table 3
| Gram-positive | Gram-negative | Fungal | |
| No. of Comp. | S. Aureus | E-coli | Candida ablicans |
| S1 | 12 | 18 | – |
| S2 | 14 | 22 | – |
| S3 | 17 | 23 | – |
| S4 | 14 | 25 | – |
| S5 | 13 | 20 | – |
| S6 | 19 | 23 | – |
| S7 | 15 | 17 | – |
| S8 | 16 | 24 | – |
| S9 | 13 | 16 | – |
| S10 | 15 | 18 | – |
| Control/DMSO | 0 | 0 | 0 |
| Distill water | 0 | 0 | 0 |
Zone of inhibition measured in mm: no activity (0.0), very weak activity (< 7 mm), weak activity (7–10), moderate activity (11–15 mm), strong activity (> 15 mm).
Conclusion
In this study we are reported synthesis of many chalcone derivatives from 8-CTh. The work included preparation of chalcone compounds and then prepared Pyrimidine when react with thiourea, in other hand react with hydrazine to prepared pyrazoline. These derivatives were found active angst bacterial and not response to fungal. These derivatives confirmed from spectral data analysis; FT-IR, H1-NMR, C13-NMR and 2D-NMR.
Acknowledgment
The authors are thankful prof. Dr. Najim A.AL-Masoudi, Chemistry Department, Konstanz University, Germany, for making and analyzer NMR spectra. Also we are really appreciating the help of Miss Zahra khthair, College of education -Biology, AL-Qadisiya University. The antimicrobial activity results discovered that most of the tested compounds have moderate to strong activity. The most effective compounds are 5, 8 and 10 When these compounds were compared with the reference compounds (DMSO, distill water) we found that they have an antimicrobial activity higher or almost equal to them.While candida albicans not responsive for all compounds.
References
- Szentmiklosi, A. J; Cseppento, A; Gesztelyi, R; Zsuga, J; Körtvely, A; Harmati G; Nanasi P.P. Current Medicinal Chemistry. 2011 .18, 3695-3706.
- Barnes, P. Theophylline. Pharmaceuticals, 2010, 3, 725-747
CrossRef - Riksen, N.P.; Smits, P.; Rongen, G.A. The cardiovascular effects of methyl xanthines. Handb. Exp. Pharmacol., 2011, 200, 413-437.
CrossRef - Mosselhi, A .M; Abdallah, M. A; Metwally, N.H, El-Desoky I. A; Break, L. M. ARKAT USA, Inc .2009, 1551-7012, 53 -63.
- Mamedov, I, G; Khrustalev, V.N; Bayramov, M.R; Maharrmov, A.M .INDIAN J.CHEM .2017.56B,192-196.
- Solankee, A; Tailor, R. Chemistry International 2016. 2(4) 189-200.
- Tawfiq, M. T; Zaier A. J. Journal of College of Basic Education,2015.21.18
- Younis, F.M. College of Basic Education Researchers Journal. 2011.11, 711-721.
- Khazaal, M. S. Tomma ,J. H. IBN AL- HAITHAM J. FOR PURE & APPL. SCI .2011.24.2.
- Hsieh, C.T. Bioorganic & Medicinal Chemistry Letters .2012. 22, 3912-3915.
CrossRef - Elarfi.M. J. Sci. Revs. Chem. Commun. 2012 .22, 103-107.
- Voskiene, A; Mickevicius, V; and Mikulskiene, G. ARKIVOC .2007. xv ,303-314.
- Abdalla, M.; Gomha, S; Abd el-aziz, M; Serag, N. Turk J Chem.2016. 40 .441-453.
- Dabholkar, V.V; Ansari, Y.F. Indian Journal of chemistry. 2008. 47. 1758-1761.
- Joshi, V. D; Kshirsagar, M. D.; Singhal, S. International Journal of ChemTech Research.2012. 4,971-975.
- Abdul‐Reda N.A; Abass, A. K and Gebur, N. A. European Journal of Chemistry .2016. 2 ,206‐212.
CrossRef - Savant, S. S, Patel, S.M, Gamit, E. A, HaJiyani, H. U, Patel, S. A, Patel, P. S and Patel, K.C. Int J Chem Sci. 2017 ,15. 2 .139.
- Abdul-Rida, N. A; Mohammed, T. I; Al-Masoudi, N.A;Frotscher, M. Medical chemistry research.2017.22.
- Nakanishi, K; Mill Valley, California: university Science Books.1990.136.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









