Synthesis and Testing of Catalysts for Decrease of Toxic Emissions of Vehicles
Y. A. Aubakirov1, L. R. Sassykova1 , A. M. Nalibayeva2, K. Dossumov3,4, Zh. Kh. Tashmukhambetova1, A. S. Zhumakanova2, A. K. Zhussupova1 and N. K. Zhakirova1
, A. M. Nalibayeva2, K. Dossumov3,4, Zh. Kh. Tashmukhambetova1, A. S. Zhumakanova2, A. K. Zhussupova1 and N. K. Zhakirova1
1Al-Farabi Kazakh National University, 71, al-Farabi ave., 050040, Almaty, Kazakhstan.
2D.V.Sokolsky Institute of Fuel, Catalysis and Electrochemistry, 142, D.Kunaev str., 050010, Almaty, Kazakhstan.
3Scientific Research Institute of New Chemical Technologies and Materials, 95a, Karasaibatyr str., 050012, Almaty, Kazakhstan.
4The Combustion Problems Institute, 172, Bogenbaibatyr str., 050012, Almaty, Kazakhstan.
Corresponding Author E-mail: larissa.rav@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/330655
Catalysts on the metal block carriers for decrease exhaust gases of motor transport were synthesized and tested. Stability of the carrier and the active phase of the catalysts to poisons SO2 and oxygen in the processes of cleaning of exhaust gases was studied. In the presence of 3% oxygen, the catalyst activity was considerably increased, especially for Co+Mn and Ni+Mn, but increase in content of oxygen to 10% reduced effectiveness of catalysts. Nickel-manganese catalyst promoted with 0.1 wt.% Pt was the most stable against oxygen influence. The activity of catalysts based on Pt, Pt+Pd, Pd in presence of SO2 decreased at low test temperatures, but after calcination at 500°C with air blowing for 2 h activity of Pt catalyst was almost returned and reached 80%. After the tests of the catalysts at the stand on the basis of the diesel generator (5GF-LDE with power of 5 kVA) degree of conversion of CO was 99.6%, hydrocarbons-80.7 %, nitrogen oxides – 60-61.9%.
KEYWORDS:Ecology; Exhaust Gases; Catalysts; Block Carriers; Catalyst Poisons
Download this article as:| Copy the following to cite this article: Aubakirov Y. A, Sassykova L. R, Nalibayeva A. M, Dossumov K, Tashmukhambetova Z. K, Zhumakanova A. S, Zhussupova A. K, Zhakirova N. K. Synthesis and Testing of Catalysts for Decrease of Toxic Emissions of Vehicles. Orient J Chem 2017;33(6). |
| Copy the following to cite this URL: Aubakirov Y. A, Sassykova L. R, Nalibayeva A. M, Dossumov K, Tashmukhambetova Z. K, Zhumakanova A. S, Zhussupova A. K, Zhakirova N. K. Synthesis and Testing of Catalysts for Decrease of Toxic Emissions of Vehicles. Orient J Chem 2017;33(6). Available from: http://www.orientjchem.org/?p=40284 |
Introduction
In general, emissions of pollutants and greenhouse gases into the atmosphere from industrial activities and transport are formed from the emissions of stationary and mobile sources. The source of emission of harmful substances of a motor vehicle is the internal combustion engine installed on it. In the exhaust gases of the engine contain more than 200 toxic chemical compounds. Except direct negative impact on health of the person, emissions of the motor transport have greenhouse and ozone-depleting effect on the atmosphere of the earth1-4. It relates to the content in the fulfilled gases of the engine of the following substances: carbon dioxide CO2, the main component in the exhaust gases of the engine, creating a greenhouse effect in the atmosphere (greenhouse gas); methane CH4, ammonia NH3 and nitrous oxide N2O – greenhouse and ozone-depleting substances contained in the exhaust gases of the engine5-9. The qualitative and quantitative indicators of the release of harmful pollutants with exhaust gases of vehicles during their transport work are ambiguous and depend on many factors: on the type of the used fuel, from the design, conditions and operating conditions of the engine, on the amount of the done work, on the type and characteristics of the car’s movement10-13. Therefore real quantitative assessment of emissions of pollutants and greenhouse gases in the atmosphere from the motor transport is a difficult task. Growth of environmental pollution from the motor transport and of number of vehicle fleet were the reason of toughening of requirements to qualitative ecological characteristics of products of oil-processing industry14-16. Today reduction of harmful emissions of motor transport and industry to necessary standards is possible only with use of catalytic methods, which are the most effective means of cleaning. Monolithic ceramic and metallic blocks are one of the most suitable carriers of the catalysts from the list of materials applied for the solution of ecological issues. They are capable of withstanding loads produced during operation under actual operating conditions, as well as meet the requirements of the catalytic converters. Total removal of toxic gases and poisons in the presence of an effective catalyst is possible at the optimum ratio of oxidizing and reducing agents17-20.
The objective of this work was preparation of catalysts on the block metal carriers with the different active phase and different composition of washcoats and testing their activity and stability to different poisons in the processes of neutralization of toxic emissions of the motor transport.
Materials and Methods
In the work were used catalysts of purification of exhaust gases of motor transport and harmful emissions of the industry based on monolithic metal blocks. Catalysts have a cylindrical shape and are convenient in placing at the source of toxic emissions21. As primary inert carrier the tape from a steel foil (goffered and folded into the block) the H23Yu5, H15Yu5 brands containing about 5% of aluminum is applied. The foil is subjected to a heat treatment at a temperature 850-920oC in air or oxygen during 12-15 h. Formed on the surface the alumina is necessary for increase in adhesion of the intermediate covering to a surface of a steel foil. Intermediate (secondary) coating, a washcoat, is deposited by the suspension method on this treated primary carrier at room temperature. As the washcoats are used alumina with the addition of zeolite and alumina modified by additives of compounds on the base of Ce, Ti, Zr, La and Fe. Suspension for the catalysts preparation represents water alcohol solution (water-ethanol) with a ratio water-alcohol 1:1, in which it was dispersed 22.0-32.0 wt.% of Аl(ОН)3 and it was dissolved 2.0-4.0 wt.% of Al(NO3)3 and 2.0-5.0 wt.% of Се(NO3)2. Using this suspension allows for once (single immersion) applied to the support block from 7.0 to 14.0 wt.% of alumina as an intermediate coating which greatly reduces the duration of catalyst preparation. The addition of aluminum nitrate into the system is carried out for plasticizing the suspension to the required level, ensuring higher intermediate coating adhesion to the metal surface of the carrier. Directly into the suspension is also added Ce(NO3)2, forming during the heat treatment of it cerium oxide is a thermostabilizing additive of intermediate coating from alumina, particularly in the case of using the catalyst in a possible thermal shocks. To additionally increase the mass of washcoat, after drying the metal block was immersed into a slurry again and then the stages of drying and calcining are carried out. After preparation the prepared catalyst has the characteristics: Аl2O3content in the catalyst – 7.0-14.0 wt.%; Аl2O3 specific surface area – 120-130 m2/g; content of CeO2 in Аl2O3 – 8.0-15.0 wt.%. After supporting the intermediate coating the block is impregnated with aqueous solutions of the corresponding active metals. For preparation of solutions of the active components of the catalysts were applied oxides of Mn, Ni, Co, Fe, prepared from acetates and formates. Also the compounds on the base of Pt-group metals were used. The previously weighed blocks were immersed in a solution of salts (acetate or formate) of metal were shaken slightly from the excess solution between the blocks of channels, then were dried at 600°C for 2 h in a furnace, after that procedure they were calcined in an electric furnace at 600°C for 2 h. In this case metal salts are decomposed to form metal oxides on the surface of the carrier the block. In the work for increasing the activity of platinum catalysts in the reactions of oxidation of CO, hydrocarbons and nitrogen oxides decomposition as a rule platinum metals were transferred to a colloidal state by impregnation of catalysts by the previously prepared solutions of polymers with inclusion of solutions of the deposited metals with the subsequent thermal decomposition. In this work the platinum nano-size particles were prepared by reduction with hydrogen in an aqueous solution containing chloroplatinic acid and citric acid. As the stabilizer of colloid platinum particles is used isopropyl alcohol. Also in the synthesis of catalysts based on platinum and palladium as active components of catalysts acetates of Pt and Pd and their π-complexes are used. Solutions of acetate of palladium were prepared by dissolution of Pd in the acetic acid containing 3% of HNO3. Nitric acid was removed in the course of evaporation before complete cessation of release of nitrogen oxides. π-complexes of Pd and Pt were prepared by reacting of allyl alcohol with the salts of these metals with subsequent drying of the catalysts at 150°C and calcination at T= 500°C for 2 h. For increase of thermal stability catalysts were modified with additives of the second metal and oxides of refractory metals. In this work for the synthesis of active and sulfur-stable catalysts into the composition of carrier the modified natural clinoptillolit of Shankanay (Kazakhstan) field (5%) and high silica zeolite ZSM-5 (module 30) were added. As a part of the initial clinoptillolit-containing sample (% wt.) in particular such components were %: SiO2-65.0; Al2O3-6.0; Fe2O3-4.0; Na2O3-0.3; K2O3-0.5. At preparing of the modified clinoptillolit for decationation and dealumination the initial sample was treated with solutions of sulfuric acid 0.25-5.0 n for 3 hours at a temperature of 100°C and a ratio of the solid and liquid phases 1:10, then was washed with distilled water and was added 0.2 n ammonium chloride solution. The samples were then calcined at a temperature of 550°C for 4 h in an air atmosphere. Into catalyst composition as additives were added platinum, cobalt, nickel, manganese, iron, and their mixtures. The BET surface of the catalyst was 4.8 -15.0 m2/g.
Testing of catalysts was carried out by previously developed methodology, for laboratory tests the flowing catalytic installation with the tubular reactor of integrated type was applied21-23. The gas mixture was prepared by feeding of hydrocarbons from container and the compressed air from the line into the mixer. The content of hydrocarbon in the mixture was about 0.5%. The oxygen concentration was varied from 2.0 to 10.0 vol.%. The gas mixture was analyzed by GLC and gas analyzer “OPTOGAS-500.3” before and after the reaction. Crystal 2000M and Chrom 3700 chromatographs with the flame ionization detector are used. Total duration of analysis is 20-30 min. The activity of the catalysts was determined at temperatures of 150-500°C. Characteristic of activity of the catalyst was the degree of conversion (α) of initial substance (hydrocarbon, carbon monoxide, nitric oxide).
The samples of the catalysts were analyzed by EM on EM-125 K microscope by method of one-stage replicas. The X-ray phase analysis method of the synthesized catalysts was carried out on the X-ray DRON-4-0.7 diffractometer with the copper anode. Samples for research were prepared by mechanical destruction of the catalyst put on a block metal framework. The fallen part of the catalyst was crushed in an agate mortar up to 100 µ and was used for research by method XRD.
In this work the stand on the basis of the diesel generator of brand 5GF-LDE with power of 5 kVA for the test of full-size samples of converters23 was mounted. For these tests the block catalyst has diameter 30 mm, height 90 mm and volume 63.4 mm3. As a load device the rheostats are served. Samples before and after the catalyst were selected in all operating modes of the diesel engine (from idle up to 4 kVA) directly from exhaust pipe by a gas analyzer “OPTOGAS-500.3”.
Results and Discussion
The researches of stability of the synthesized catalysts to catalytic poisons were carried out. The poisoning action of SO2 in the process of cleaning of combustion gases was studied. XPS research of freshly prepared and waste (after the long-run tests of 50 h) catalysts showed that the reason of decrease of the activity of the Pt-containing catalysts in the course of purification of products of combustion of fuel is associated to accumulation of sulfur compounds24. As for activity and stability to SO2 (0.1% in air for 10 h at 350°C) of catalysts based on Pt, Pt+Pd, Pd it was revealed that the catalyst activity is reduced at low test temperatures, but after calcination at 500°C with air blowing for 2 h activity of Pt catalyst is almost recovered and activity of the Pd-catalyst was reached 80% (fig.1, 2).
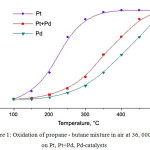 |
Figure 1: Oxidation of propane – butane mixture in air at 36, 000 h-1 on Pt, Pt+Pd, Pd-catalysts Click here to View figure |
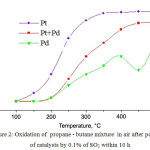 |
Figure 2: Oxidation of propane – butane mixture in air after poisoning of catalysts by 0.1% of SO2 within 10 h |
The activity of the freshly prepared catalyst and spent (exhausted) catalyst sample in the presence of SO2 (after long-term tests for 50 h) in the reaction of selective catalytic reduction (SCR) of nitrogen oxides was studied. Studies of catalysts by XPS showed in the spectra of spent (exhausted) catalyst intense bands characteristic of the sulfur-p-2 electrons, the concentration of which is comparable with the concentration of the active metal. SO2 reaction braking effect is manifested both in the presence of oxygen and in the absence thereof. The maximum activity in the reaction demonstrated previously treated with acid samples of catalysts. Such catalysts are more resistant to the effects of sulfur dioxide. Activity of catalysts samples which were pretreated with acids in the presence of SO2 in the reaction mixture was even slightly higher than in its absence24. Processing of a catalyst sample with more dilute acid (0.25-0.5 N) increases the concentration of strong acid sites, thereby increasing the activity of these samples during the oxidation.
Emission spectral analysis results of samples treated with sulfuric acid of various concentrations, showed a different degree of dealumination, i.e. reduction of the total concentration of acid sites is associated with significant decrease in the content of Al2O3 in clinoptillolite treated with sulfuric acid. The maximal activity was shown by H-forms of a natural clinoptillolit at which processing the solutions of sulfuric acid with 0.25-0.5 N were applied (fig.3).
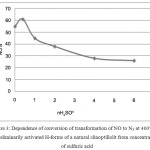 |
Figure 3: Dependence of conversion of transformation of NO to N2 at 400°C on preliminarily activated H-forms of a natural clinoptillolit from concentration of sulfuric acid Click here to View figure |
Activity of the H-form of samples of a natural clinoptillolit at which modifying were used solutions of sulfuric acid of various concentration in the direct ratio increased a catalytic activity with a decrease of concentration of sulfuric acid. At modifying of the washcoat on the base of clinoptillolit of H-form with Fe- and Co-containing components the permanence of activity of these catalysts in the reaction mixtures with SO2 at NO reduction is observed.
Table 1: Influence of conditions of modifying on activity of the H-form of a natural clinoptillolit in selective catalytic reduction (SCR-process)
| Concentration of sulfuric acid | The degree of dealumination, % | The degree of NO conversion, %(dealuminated samples) | The degree of NO conversion, % (activated samples) |
|
6.0 |
38.5 |
12.5 |
25.0 |
|
2.0 |
31.1 |
30.0 |
43.0 |
|
1.0 |
23.2 |
38.0 |
42.0 |
|
0.5 |
13.7 |
46.0 |
70.0 |
|
0.25 |
5.9 |
– |
62.0 |
In this work influence of oxygen on activity and stability of metal block catalysts was studied. It was found that in the presence of 3% oxygen the catalyst activity with increasing from 200o to 300oC was considerably increased, especially it was noticeable for Co+Mn and Ni+Mn. Increase in content of oxygen to 10% reduces effectiveness of catalysts. Influence of oxygen was considerably shown in the range of temperatures 250-300oC (tab.2). The most resistant against oxygen influence – nickel-manganese catalyst promoted with 0.1 wt.% Pt.
Table 2: The effect of oxygen concentration on the nitrogen oxide conversion by propane-butane mixture
| Catalyst | The degree of conversion of nitrogen oxide, % at various oxygen content,% | |||||||
|
3.0 |
5.0 |
7.0 |
10.0 |
|||||
| 250oC | 300oC | 250oC | 300oC | 250oC | 300oC | 250oC | 300oC | |
| Ni+Mn+Pt |
65.0 |
75.0 |
62.0 |
70.0 |
40.0 |
58.0 |
12.0 |
25.0 |
| Co+Mn+Pt |
69.0 |
75.0 |
23.0 |
65.0 |
0 |
35.0 |
0 |
20.0 |
| Fe+Mn+Pt |
71.0 |
80.0 |
0 |
38.0 |
0 |
16.0 |
0 |
0 |
| Co+Mn |
23.0 |
72.0 |
0 |
32.0 |
0 |
0 |
0 |
0 |
| Ni+Mn |
35.0 |
88.0 |
0 |
38.0 |
0 |
10.0 |
0 |
0 |
| Fe+Mn |
52.0 |
60.0 |
0 |
25.0 |
0 |
0 |
0 |
0 |
Research of Pt and Pd catalysts on thermo stability was carried out by maintaining of the catalyst with an interval of 5 h at Т=500°C in a reactionary gas mixture with the contents of 0.5 % of propane-butane with the subsequent analysis of products of reaction. The total duration of researches was 100 h. It was found that the most stable appeared the catalysts obtained from acetates Pt, less stable – on basis Pd.
High thermal stability of the oxide washcoat is provided with introduction to it of the modifying additives. For example, into the alumina washcoat are introduced cations of cerium, zirconium, lanthanum, which do not only stabilize γ-Al2O3 phase, but also provide resistance to poisons or sintering of Pt, Pd, Rh-active components of the catalysts25-27. In this work metal block Pd-Mo-catalysts on Al2O3 carrier modified by additives of Ce, Ti, Zr, La and Feare prepared and researched in reaction of NOx+C3H6+O2. Activity of Pd-Mo of catalysts increases when modifying the washcoat with cations of Ce4+, Zr4+. Activity of Pt-Cu catalysts on the zeolite-containing washcoats NaY, ZSM-5 and their hydrogen forms was studied.
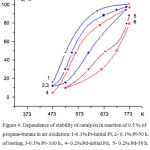 |
Figure 4: Dependence of stability of catalysts in reaction of 0.5 % of propane-butane in air oxidation: 1-0.1% Pt-initial Pt, 2- 0.1% Pt-50 h. of testing, 3-0.1% Pt- 100 h., 4- 0.2% Pd-initial Pd, 5- 0.2% Pd-50 h. of testing, 6- 0.2% Pd-100 h. of testing Click here to View figure |
It was found that adding to the composition of washcoats of titanium dioxide considerably increased extent of reduction of nitrogen oxides by means of propylene on both compositions of oxidic catalysts in all interval of the studied temperatures (150-500°C). Promotion with platinum improved the reduction ability of cobalt-manganese catalyst on alumina only at temperatures higher than 400°C. On the titanium-containing sample in the presence of platinum degree of NO conversion decreased.
The X-ray phase analysis of Pd and Pt showed dispersion of a spectrum that testified the high dispersion of the metals23. The metal-organic Pd and Pt complexes at magnification of 33,000 times represented translucent areas of the clots of polymer filled with dispersed particles of ~3 nm in size. At higher magnification (by 62,000 times) the small rare congestions of more dense particles of approx. 5 nm in size were observed as well. In Fig.5, 6 the nano-dimensional particles of Pt and Pd obtained by introduction of the corresponding salts to the PEG water solution and applied on a carrier surface, are revealed. The images were made at 300,000-fold magnification. The sizes of Pt and Pd particles were approx. 7-8 nm and 11 nm respectively.
 |
Figure 5: Fragment of Pd cluster |
 |
Figure 6: Small congestions of denser particles of Pd Click here to View figure |
At a diffractogram of catalysts on the basis of a cobalt (fig.7) there are diffraction peaks which are correspond to oxide of a cobalt of structure of a spinel Co3O4 (СоСо2О4): 2θ = 31.35; 36.90; 44.90; 59.40; 55.90.
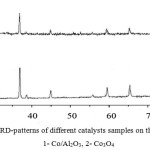 |
Figure 7: XRD-patterns of different catalysts samples on the base of Co: 1- Co/Al2O3, 2- Co3O4 |
For the catalysts based on Pd supported on different washcoats the (111) peak of Pd (40 2θ) and peaks of Pd 46.5 (plane 200), 68.2 (plane 220) were found. Results of XRD showed that there were clear particles of Pd21.
In Table 3 the characteristics of catalysts on base of Pt and Pd received with use of method TPDNH3 are presented. The data show that a total (summarized) concentration of acid centers Pt-and Pd-catalysts with the additives of nickel and manganese oxides, above, than in initial Al-platinum and Pd-catalysts.
Table 3: The characteristics of the Pt- и Pd-catalysts
| Catalyst | Characteristic of the porous structure | Total concentration of the acid centres by results of NH3 adsorption, µmol/g | |
| Specific surface, m2/g | The pores volume, cm3/g | ||
| Pt/Al2O3 |
200 |
0.348 |
240 |
| Pd/ Al2O3 |
205 |
0.356 |
110 |
| Pd/Ni-Mn/Al2O3 |
350 |
0.274 |
620 |
| Pt/ Ni-Mn/Al2O3 |
370 |
0.290 |
660 |
In fig.8 the comparative diagrams for catalysts with various concentrations of the acid centers of different force were submitted. The catalysts on basis Pt/Ni-Mn/Al2O3 were characterized by the greatest concentration of the strong acid centers (280 µmol/g), atPd/Ni-Mn/Al2O3 catalyst the greatest concentration of the weak acid centers – 250 µmol/g.
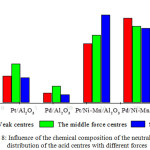 |
Figure 8: Influence of the chemical composition of the neutralizers on distribution of the acid centres with different forces Click here to View figure |
Also it was found, that the oxide catalysts represented spinel with cubic latticeNiMnO4 with peaks 2Å, 52Å, 148Å, 203Å. There were small intensive peaks of CeO2 (308Å) and alumina (160Å, 256Å)21.
The activity of full-size samples of catalysts was investigated at the stand on the basis of diesel generator. Test data of the full-size block catalyst in the bifunctional mode at various loadings of operation of the engine are presented in Table 4. For the catalyst with 0.1% Pt effectiveness was already at 267°C, the degree of conversion of CO was equal to 99.6%, hydrocarbons – 80.7%, nitrogen oxide was 44.4%. With an increase in engine power of up to 3-4 kVA is observed high activity on NOx (61.1-61.9%).
Table 4: Results of the analysis of toxic emissions on the diesel generator on the platinum-containing block catalyst at various loads
| Power consumption, kVA | Temperature of exhaust gases, °C | The content of toxic components in the exhaust gas, % | ||
|
CO |
CxHy |
NOx |
||
| Idling (0) |
20 |
90.6 |
21.5 |
3.0 |
|
2 |
267 |
99.6 |
80.7 |
44.4 |
|
3 |
308 |
100 |
95.6 |
61.1 |
|
4 |
427 |
100 |
99.0 |
61.9 |
Thermal stability of full-size samples of catalysts was determined by definition of activity of the initial catalyst on the diesel generator working under loading 3 kVA with the following fractional calcination of converters at 600°C with an interval of 5 h in a muffle furnace. During 100-h test high heat stability of catalysts was revealed.
Conclusion
Catalysts for neutralization of toxic gases of the industry and motor transport on metal carriers with the honey comb structure of channels were prepared. As the washcoat was used alumina with the additives of zeolite or compounds of Ce, Ti, Zr, La and Fe. For preparation of solutions of the active components of catalysts are also applied oxides of Mn, Ni, Co, Fe (obtained from acetates and formats) and the samples of catalysts on the base of the platinum group metals. Stability of the carrier and the active phase of catalysts to poisons SO2 and oxygen in processes of cleaning of exhaust gases was studied. The braking effect of reduction reaction of nitrogen oxide is shown by adding SO2 both in the presence of oxygen and in the absence thereof. Adding into a composition of the washcoat of a clinoptilolit of the Shankanaysky field increased resistance of catalysts to poisoning with catalytic poisons. The catalysts stability stability against poisoning with compounds of sulfur are developed. Influence of oxygen was considerably shown in the range of temperatures 250-300oC. The Ni-Mn catalyst with additive of 0.1% (wt.) Pt was the steadiest against oxygen influence. At tests of full-size samples at the stand on the basis of the diesel generator degree of conversion of CO was 99.6%, hydrocarbons-80.7 %, nitrogen oxides – 60-61.9%.
References
- O’Neill, B.C. J.Science. 2002, 5575, 1971
CrossRef - Sendilvelan, S.; Bhaskar, K.; Nallusamy S. Rasayan J. Chem. 2017, 10(2), 454 – 460
- Alexandrov, Yu.A.; Ivanovskaya, K.E.; Vorozheykin, I.A. J. of Appl.Chem.2003, 8, 1298
- Rauch, S.; Harold, H.F.; Barbante, C.; Masanori, O.; Morrison, G.M.; Peucker-Ehrenbrink, B.; Wass, U. J.Environ.Sci.Technol. 2005, 1, 8156
CrossRef - Takami, A.; Ichikawa, T. J. Zeolites. 1995, 15(3), 283
CrossRef - Sendilvelan, S.; Bhaskar K. Rasayan J. Chem. 2017, 10(3), 1043-1049
- Val’dberg, A.Yu; Kosogorova, T.O.; Tsedilin, A.N.; Pokrovskii, D.D.; Yakimychev, A.A.; J.Chemical and Petroleum Engineering. 2007, 5-6, 287-291
- Awofeso, N. American Journal of Respiratory and Critical Care Medicine. 2011, 10, 1437
CrossRef - Prabhahar, M.; Murali Manohar, R.; Sendilvelan, S.; European Journal of Scientific Research, 2012, 73(4), 504-511
- McGrath, M. Four major cities move to ban diesel vehicles by 2025. http://www.bbc.com/news/science-environment-38170794
- Yang, S.; He, L-Y. J.Energy & Environment. 2016.
- http://dx.doi.org/10.1177/0958305×15627545.
CrossRef - Sendilvelan, S.; Bhaskar, K. Orient J Chem. 2017, 33(4), 2111
- Karol, I.L.; Kisselev, A.A. Priroda. 2003, 6, 18
- Inozemtsev, V.L. Priroda, 2001, 1, 20
- Murali Manohar, R.; Prabhahar, M.; Sendilvelan, S. European Journal of Scientific Research. 2012, 76(3), 327-334
- Sendilvelan, S.; Rajan, K. Rasayan Journal of Chemistry, 2017, 10 (1), 190
- Lee, B.Y.; Inoue, Y.; Yasimori, I. J Bull Chem Soc Jpn. 1981, 54, 3711
CrossRef - In, B.D.; Kim, H.; Son, J.; Lee, K. Intern. J. Heat and Mass Transf. 2015, 86, 667 – 680
CrossRef - Sendilvelan, S.; Bhaskar, K. Rasayan J.Chem. 2016, 9(4), 692-696
- Sassykova, L.; Gil’mundinov, Sh.; Nalibayeva, A.; Bogdanova, I. Revue Roumaine de Chimie, 2017, 2, 107-114
- Sassykova, L.R.; Nalibayeva, A.; Gil’mundinov, Sh.A. Bulgarian Chemical Comm. 2017, 49(3)
- Sassykova, L.; Nalibayeva, A; Aubakirov, Y.; Tashmukhambetova, Zh.; Otzhan, U; Zhakirova, N.; Faizullaeva, M., Orient J Chem. 2017, 33(4), 1941-1948
- Gil’mundinov, Sh.A.; Sassykova, L.R.; Nalibayeva, A.M.; Bunin, V.N. News of the NAS of the RK, ser.chemistry and technology. 2006, 3, 26-28
- Shuy, J.S.; Weber, W.H.; Gandhi, H.S. J.Phys.Chem. 1988, 17, 4964
CrossRef - Burdeinaya, T.N.; Matyshak, V.A.; Tret’yakov, V.F.; Glebov, L.S.; Zakirova, A.G.; Carvajal, G., Villanueva, A.M.E. J.Appl.Catalysis B:Environmental. 2007, 1-4, 128.
CrossRef - Kramer, M.; Schmidt, T.; Stowe, K.; Maier, W.F. Applied Catalysis A: General, 2006, 302, 257–263.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









