Synthesis of Oximes from the Corresponding of Organic Carbonyl Compounds with NH2OH.HCl and Oxalic Acid
Negin Piri Ghozlojeh and Davood Setamdideh*
Department of Chemistry, Mahabad Branch, Islamic Azad University, Mahabad, Iran. Correspondence Author Email: davood.setamdideh@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310365
Article Received on :
Article Accepted on :
Article Published : 21 Aug 2015
The oximation of a variety of aldehydes and ketones was carried out with NH2OH·HCl in the presence of oxalic acid as catalyst under reflux conditions. The reactions were performed in CH3CN with excellent yields(90-95%) of products in appropriate times (55-90 min).
KEYWORDS:Oximesaldoximes; acetophenoneoximes; H2NOH; HCl; Oxalic acid
Download this article as:| Copy the following to cite this article: Ghozlojeh N. P, Setamdideh D. Synthesis of Oximes from the Corresponding of Organic Carbonyl Compounds with NH2OH.HCl and Oxalic Acid. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Ghozlojeh N. P, Setamdideh D. Synthesis of Oximes from the Corresponding of Organic Carbonyl Compounds with NH2OH.HCl and Oxalic Acid. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10406 |
Introduction
Oximeshave been used for the protection and purification of carbonyl compounds in organic synthesis1.Also,these compounds have antimicrobial2-3, antioxidant4, antitumor5, anti-depressive6, antiviral agents and anticonvulsant properties7.Recently, the oximationmethods have been reviewed8by us. The lack of information for the oximation of carbonyl compounds in the presence of oxalic acid and our ongoing attentions to the development of modified methods in organic synthesis 8-15 encouraged us to investigate this transformation with NH2OH·HCl in the presence of oxalic acid.So,herein, we describe a convenient method for the oximation of a variety aldehydes and ketones to their corresponding alcohols with NH2OH·HCl/H2C2O4 system.
Results and Discussions
In order to determine optimization reaction conditions for the oximation of aldehydes and ketones,bezaldehyde and acetophenone have been used as model compounds. The results showed that using NH2OH.HCl (1mmol) and H2C2O4 (1 mmol) in CH3CN (3 ml) was the best conditions for the oximation of benzaldehyde. The reaction was completed in 60 minutes with the excellent yield (95%)of the product as shown in scheme 1.
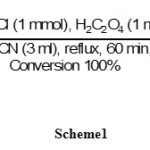 |
Scheme 1 Click here to View scheme |
In order to evaluate the generality of the process, a variety of aldehydes were ground with hydroxylamine hydrochloride under optimized reaction conditions. In this approach, the corresponding aldoximes were obtained in quantitative yield (90-95%). The results have been reported in table 1 (entries 1-6).
The oximation of ketones was also carried out well by NH2OH·HCl/H2C2O4 system, but due to the lower reactivity of ketones relative to aldehydes, the oximation requires higher molar amounts of NH2OH·HCl (2 mmol) and H2C2O4 (2mmol)vs. 1 mmol of the substrates (table 1, entries 7-12).The results showed that using NH2OH.HCl (2mmol) and H2C2O4 (2mmol) in CH3CN (3 ml) was the best conditions for the oximation of acetophenone. The reaction was completed in 90 minutes with the excellent yield (95%) as shown in scheme 2.
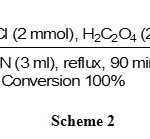 |
Scheme 2 Click here to View scheme |
Experimental
All substrates and reagents were purchased from commercially sources with the best quality. IR and 1H NMR spectra were recorded on Perkin Elmer FT-IR RXI and 300 MHz Bruker spectrometers, respectively. The products were characterized by their 1H NMR or IR spectra and comparison with authentic samples (melting points). All yields referred to isolated pure products. The purity of products was determined by TLC and 1H NMR. Also, reactions were monitored by TLCs utilizing plates cut from silica gel 60 F254 aluminum sheets.
A typical procedure for the oximation of aldehydes with NH2OH·HCl/H2C2O4system
In a round-bottomed flask (10 mL) equipped with a condenser, a mixture of benzaldehyde (0.106 g, 1mmol), NH2OH·HCl (0.07 g, 1 mmol)and oxalic acid (0.09 g, 1 mmol)in CH3CN (3 mL) was prepared. The mixture was stirred under reflux conditions for 60 min. The progress of the reaction was monitored by TLC. After completion of the reaction, H2O (10 mL) was added and the reaction mixture was continued to stirring for 5 min. The product has been extracted with CH2Cl2(3х15 mL).The mixture was dried over anhydrous Na2SO4. Evaporation of the solvent and a short column chromatography of the resulting crude material over silica gel (eluent; CCl4/Et2O: 5/2) afforded the purebenzaldoxime (0.115 g, 95 % yield, table 1, entry 1).
Table 1: Oximation of Carbonyl Compounds (1 mmol) by NH2OH.HCl/H2C2O4 under Reflux Conditions in CH3CN (3 mL).
| Table 1. Oximation of Carbonyl Compounds (1 mmol) by NH2OH.HCl/H2C2O4 under Reflux Conditions in CH3CN (3 mL). | |||||
|
Entry |
Aldehydes | Products |
Time/min. |
Yieldc/% |
|
|
1a |
benzaldehyde | benzaldehydeoxime |
60 |
95 |
|
|
2a |
4-bromobenzaldehyde | 4-bromo benzaldehydeoxime |
55 |
95 |
|
|
3a |
4-nitrobenzaldehyde | 4-nitrobenzaldehydeoxime |
60 |
90 |
|
|
4a |
4-methylbenzaldehyde | 4-methyl benzaldehydeoxime |
60 |
94 |
|
|
5a |
2-methoxylbenzaldehyde | 2-methoxy benzaldehydeoxime |
60 |
91 |
|
|
6a |
4-methoxybenzaldehyde | 4-methoxy benzaldehydeoxime |
60 |
94 |
|
|
7b |
acetophenone | acetophenoneoxime |
90 |
95 |
|
|
8b |
4-methylacetophenone | 4-methylacetophenone oxime |
90 |
93 |
|
|
9b |
benzalacetone | benzalacetoneoxime |
90 |
94 |
|
|
10b |
Benzophenone | Benzophenoneoxime |
80 |
90 |
|
|
11b |
9H-fluoren-9-one | 9H-fluoren-9-one oxime |
80 |
91 |
|
|
12b |
cyclohexanone | cyclohexanoneoxime |
60 |
95 |
|
| a The reaction has been carried out by NH2OH.HCl (1 mmol)/H2C2O4 (1mmol). bThe reaction has been carried out byNH2OH.HCl (2mmol)/H2C2O4 (2mmol). c Yields refer to isolated pure products (±3%).
|
|||||
A typical procedure for the oximation of ketones with NH2OH·HCl/H2C2O4system
In a round-bottomed flask (10 mL) equipped with a condenser, a solution of acetophenone (0.120 g, l mmol) NH2OH·HCl (0.14 g, 2mmol) and oxalic acid (0.18 g, 2mmol) in CH3CN (3 mL) was prepared. The mixture was stirred under reflux conditions for 90 min. The progress of the reaction was monitored by TLC. After completion of the reaction, H2O (10 mL) was added and the reaction mixture was continued to stirring for 5 min. The product has been extracted withCH2Cl2(3х15 mL).The mixture was dried over anhydrous Na2SO4. Evaporation of the solvent and a short column chromatography of the resulting crude material over silica gel (eluent; CCl4/Et2O: 5/2) afforded the pureacetophenoneoxime (0.128 g, 95% yield, table 1, entry 7).
Conclusion
In this context, the oximation of a variety of aldehydes and ketones was carried out efficiently with NH2OH·HCl/H2C2O4 system. The reactions were performed in CH3CN under reflux conditions.This new protocol for the oximation of carbonyl compounds could be a useful addition to the present methodologies.
Acknowledgements
The authors gratefully appreciated the financial support of this work by Islamic Azad University branch of Mahabad.
References
- Xia, J. J.; Wang, G. W.Molecules 2007, 12, 231-236.
- Li, H. Q.; Xiao, Z. P.; Yin, L.; Yan, T.; Lv, P.C.; Zhu , H. L. . Eur. J. Med. Chem. 2009, 44, 2246-2251.
- Karakurt, A.; Sevim, D.; Özalp, M.; Özbey, S.; Kendi, E.; Stables, J. P. Eur. J. Med. Chem. 2001, 36, 421-433.
- Puntel, G. O.; de Carvalho, N. R.; Gubert, P.; Palma, A. S.; Corte, C. L. D.; Ávila, D. S.; Pereira, M. E.; Carratu, V. S.; Bresolin, L.; J. Da Rocha, B. T.; Soares, F. A. A. Chem. Biol. Interact. 2009, 177, 153-160.
- Wang, T. C.; Chen, I. L.; Lu, C. M.; Kuo, D. H.; Liao, C. H Chem. Biodivers. 2005, 2, 253-263.
- De Sousa, D. P.; Schefer, R. R.; Brocksom U.; Brocksom, T. J. Molecules 2006, 11, 148-155.
- Ouyang, G.; Chen, Z.; Cai, X. J.; Song, B. A.; Bhadury, P. S.; Yang, S.; Jin, L. H.; Xue, W.; Hu, D.Y.; Zeng, S. Bioorg. Med. Chem. 2008, 16, 9699-9707.
- Setamdideh, D.; Khezri, B.; Esmaeilzadeh, S. J. Chin. Chem. Soc.2012, 59, 1119-1124.
- Setamdideh, D.; Khezri, B.; Alipouramjad, A. J. Chin. Chem. Soc. 2013, 60, 590-596.
- Sofighaderi, S.; Setamdideh, D. Orient. J. Chem. 2013, 29, 1135-1137.
- Setamdideh, D.; Sepehraddin, F. J. Mex. Chem. Soc. 2014, 58, 22-26.
- AziziAsl, P.; Setamdideh, D. J. Chin. Chem. Soc. 2014, 59, 940-944. f) Setamdideh, D. J. Mex. Chem. Soc. 2014, 58, 230-234.
- Azimzadeh, M.; Setamdideh, D. Orient. J. Chem., 2015, 31, 1085-1089.
- Rezaeekhoredehforosh, R.; Khezri, B.; Setamdideh, D. Orient. J. Chem., 2015, 31, 1205-1209.
- Mahmoudi, M.; Setamdideh, D. Orient. J. Chem., 2015, 31, 1215-1218.

This work is licensed under a Creative Commons Attribution 4.0 International License.









