Published online Jan 26, 2024. doi: 10.12998/wjcc.v12.i3.657
Peer-review started: November 18, 2023
First decision: December 5, 2023
Revised: December 17, 2023
Accepted: January 2, 2024
Article in press: January 2, 2024
Published online: January 26, 2024
Anaplastic lymphoma kinase (ALK)-positive large B-cell lymphoma (LBCL) is an aggressive and rare variant of diffuse LBCL. Herein, we report an uncommon case of stage IE extranodal ALK-positive LBCL initially originating in the bulbar con
A 63-year-old woman presented with a mass in the left bulbar conjunctiva that had persisted for six months, accompanied by swelling and pain that had per
Awareness of the condition presented in this case report is necessary for early and accurate diagnosis and appropriate treatment.
Core Tip: Anaplastic lymphoma kinase (ALK)-positive large B-cell lymphoma (LBCL) is an aggressive and rare variant of diffuse LBCL. Herein, we report an uncommon case of stage IE extranodal ALK-positive LBCL initially originating in the bulbar conjunctiva. This case represents the first example of primary extranodal ALK-positive LBCL presenting as a bulbar conjunctival mass, which is extremely rare and shares morphological and immunohistochemical features with a variety of other neoplasms that can result in misdiagnosis. Awareness of the condition presented in this case report is necessary for early and accurate diagnosis and appropriate treatment.
- Citation: Guo XH, Li CB, Cao HH, Yang GY. Primary anaplastic lymphoma kinase-positive large B-cell lymphoma of the left bulbar conjunctiva: A case report. World J Clin Cases 2024; 12(3): 657-664
- URL: https://www.wjgnet.com/2307-8960/full/v12/i3/657.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i3.657
Anaplastic lymphoma kinase (ALK)-positive large B-cell lymphoma (LBCL) is an aggressive B-cell non-Hodgkin’s lym
Herein, we present the first uncommon case of primary extranodal ALK-positive LBCL manifesting as a bulbar con
A 63-year-old woman presented with a mass in the left bulbar conjunctiva that had persisted for six months, accom
The mass had not significantly increased in size since its discovery. It was not taken seriously at the time and was not treated. However, swelling and pain appeared 3 d ago.
The patient had undergone aortic valve replacement surgery 8 years ago.
The personal history had no abnormalities. There was no immunodeficiency, particularly human immunodeficiency virus (HIV) infection. The patient had no family history of lymphoid hematopoietic system tumors and hereditary diseases.
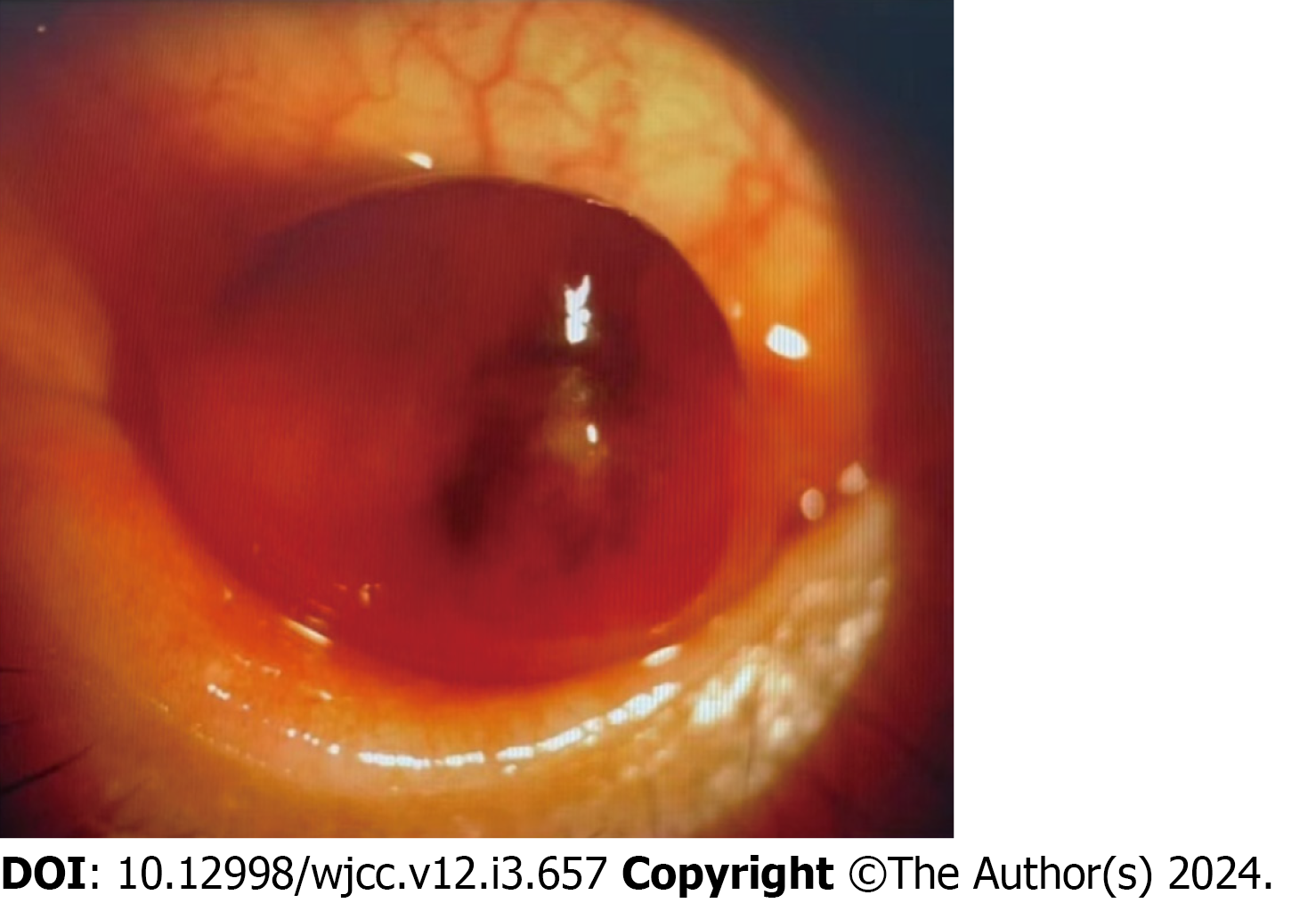
Eye examination revealed an 8 mm slightly elevated, sessile, pink-colored sub-epithelial mass described as a “salmon patch” in the conjunctival sac of the left eye (Figure 1, anterior segment image), which could move with eyeball rotation, accompanied by congestion and edema of the surrounding conjunctiva.
No abnormalities were found in routine blood, biochemical tests and lactose dehydrogenase levels.
For in-depth diagnosis and treatment of this patient, positron emission tomography/computed tomography examination was performed after conjunctival mass resection, and revealed no other positive signs in the body.
Macroscopically, the surface of the specimen was smooth and the cross section was solid, grayish-white, and focally darkish red, and it measured 1.3 cm × 0.9 cm × 0.6 cm.
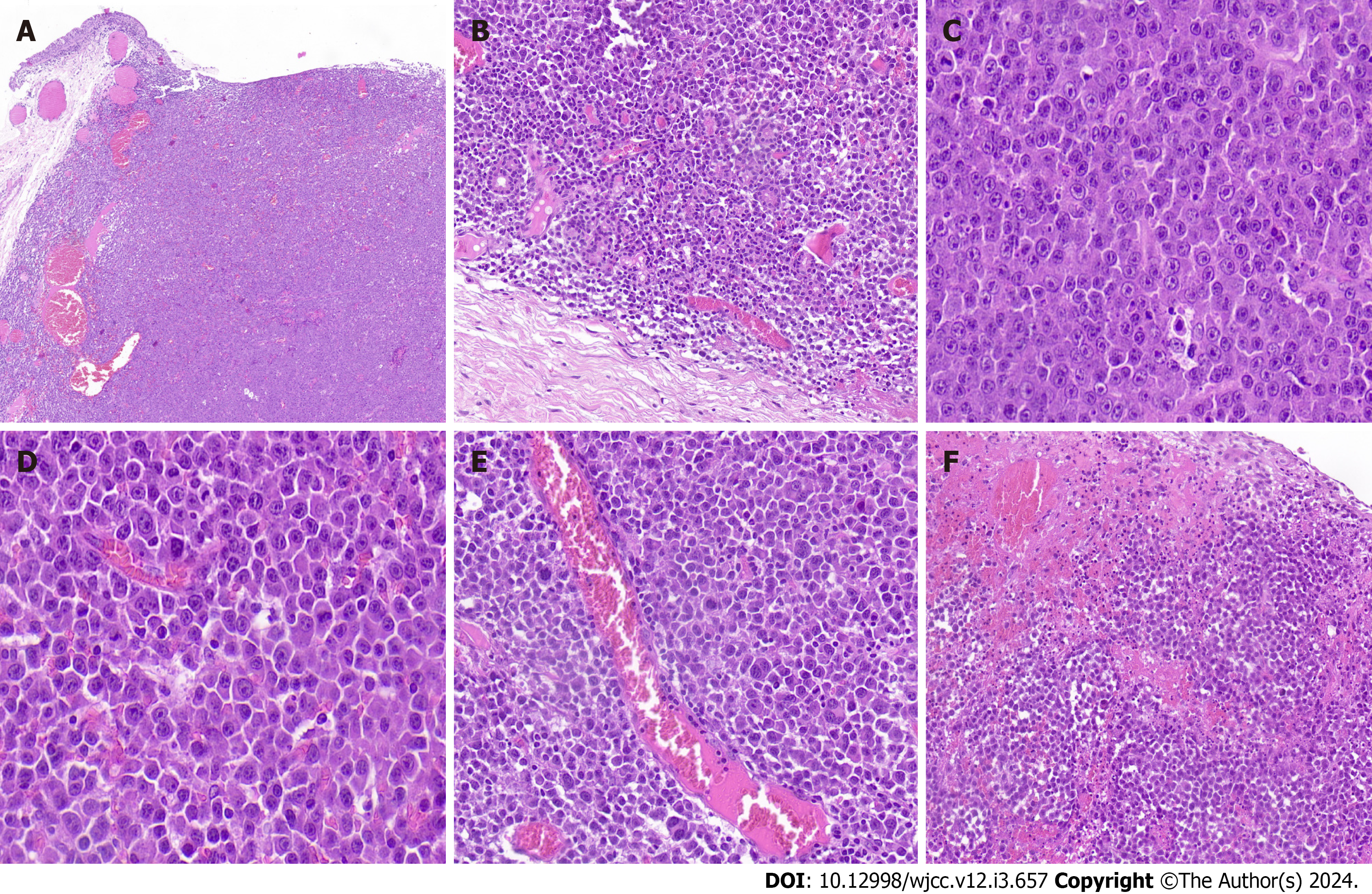
Microscopically, the tumor was well-demarcated in low-power fields and showed diffuse infiltration of monomorphic lymphoid cells in the stroma of the conjunctival subepithelium (Figure 2A) and focal involvement of the accessory la
A polymerase chain reaction-based clonality study was performed for immunoglobulins (Ig) and TCR according to BIOMED-2 protocols. The results showed monoclonal IGH/IGK/IGL and TCRD rearrangements; however, monoclonal TCRB and TCRG rearrangements were not detected. Fluorescence in situ hybridization (FISH) studies did not reveal any BCL2, BCL6, and MYC rearrangements. Furthermore, in situ hybridization did not detect an Epstein-Barr virus (EBV) infection in the lesions.
Given that the first IHC only showed CD79a patchily weak positive, an extensive IHC panel was performed using a streptavidin-peroxidase assay in batches to further confirm the nature of the neoplasm with appropriate reactive controls.
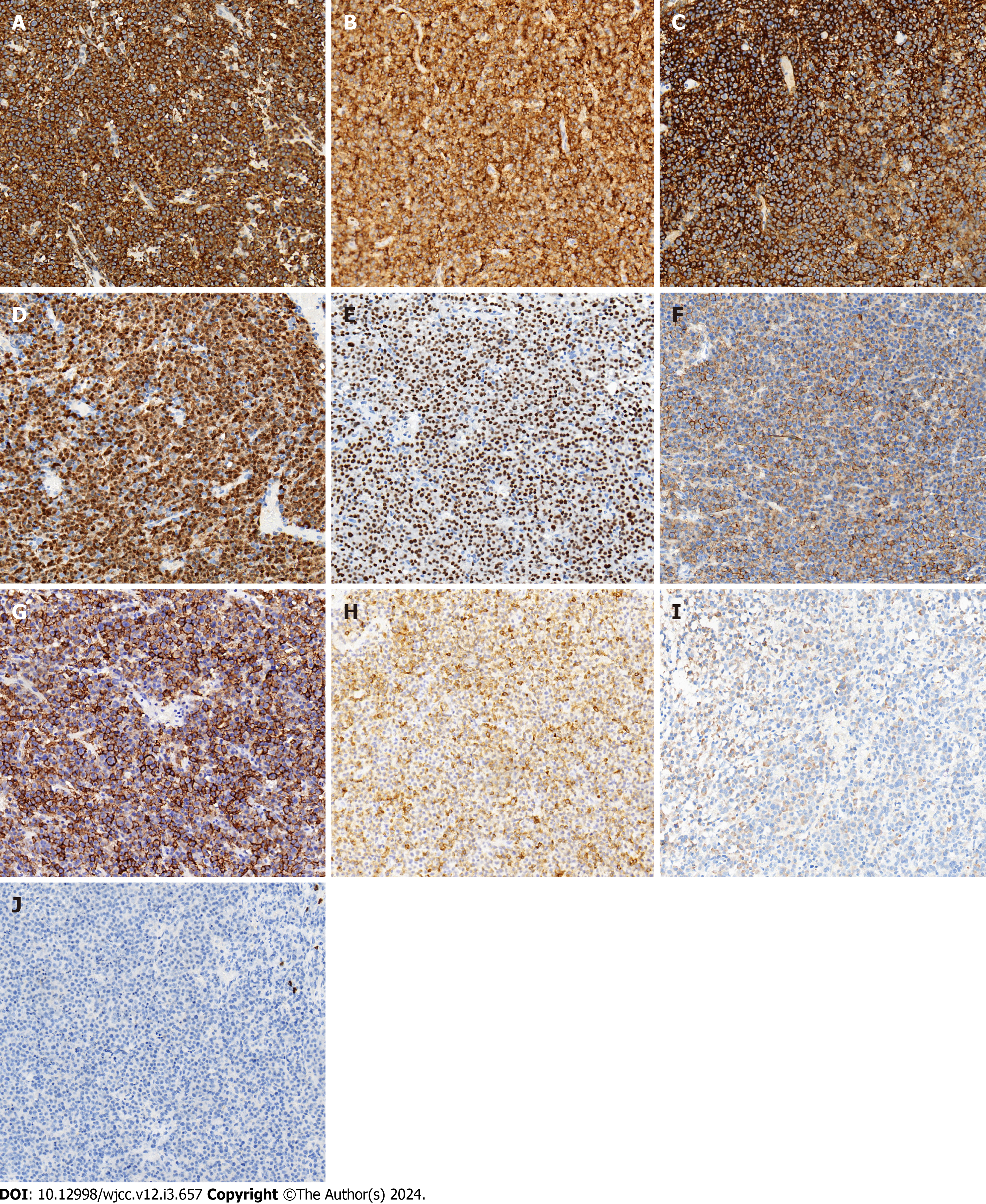
Immunophenotypically, the neoplastic cells were diffusely positive for ALK (cytoplasmic with perinuclear intensification) (Figure 3A), CD10 (Figure 3B), CD138 (Figure 3C), Kappa, MUM1, BOB.1 (Figure 3D), OCT-2 (Figure 3E), CD4 (Figure 3F), CD45, EMA (Figure 3G), and Vimentin. Furthermore, they were focally weak positive for CD79a (Figure 3H), CD38, IgA (Figure 3I), CK (AE1/AE3), and CD99 (plasmalemma staining) and negative for CD20 (Figure 3J), PAX5, Lambda, IgG, IgM, BCL6, CD30, CD21, CyclinD1, c-MYC, BCL2, CD3, CD5, CD43, CD8, TIA-1, CD56, CD117, MPO, TDT, S-100, HMB45, Desmin, INI-1, and BRG1. The proliferation index (Ki67) was approximately 70%. ALK showed a cyto
Based on the above observations, a final diagnosis of primary ALK-positive LBCL of the left bulbar conjunctiva was made, and the neoplasm was subsequently classified as stage IE using the Ann Arbor staging system, and the International Prognostic Index score was 0.
Surgical resection was performed with no complications. No further treatments were administered for any indication.
During the return visit and four weeks postoperative follow-up, a scar was present in the conjunctiva; however, the patient had no clinical symptoms and blood counts, biochemical tests, and lactose dehydrogenase levels were within normal ranges. At 6, 15, and 21 mo postoperative follow-up visits, the patient was in good condition without obvious discomfort and remained in regular follow-up.
Based on the best of our knowledge and extensive literature search, we believe that ALK-positive LBCL is a rare variant of DLBCL that has not been previously reported in the conjunctiva. Its identification in routine pathological examinations remains challenging; however, recognition of this condition is important, as it possesses an aggressive clinical course and the conventional therapy used for typical DLBCL has limited efficacy. Therefore, pathologists should be aware of this lymphoma to make an accurate diagnosis.
Clinically, ALK-positive LBCL can occur in all age groups (range: 9–90 years old). It affects men more than women (male/female: 5:1), and usually presents as a mediastinal mass or diffuse lymphadenopathy. Approximately half of the patients present with B symptoms[1-3,6], whereas a male patient had spontaneous tumor lysis syndrome[7]. Most cases have no association with immunosuppression, whereas one case was comorbid with an HIV infection[8]. Notably, one case was treated with azathioprine for ulcerative colitis[9]. At present, there are no reports of human herpesvirus-8 infection; however, two cases of EBV infection have been reported[10,11].
Histopathologically, ALK-positive LBCL features a monomorphic immunoblastic and/or plasmablastic appearance, diffuse or sinusoidal infiltrative patterns in the lymph nodes, and diffuse or sheet-like growth patterns in extranodal sites. Small lymphocytes and mature plasma cells are present in the background, whereas in a rare case, this condition was accompanied by prominent neutrophilic infiltration[12].
The immunophenotype of ALK-positive LBCL is consistent with plasmablastic differentiation; therefore, the neoplastic cells are diffusely positive for plasma cell markers and CD45 and B-cell-specific transcriptional factors (including BOB.1 and OCT-2), whereas the mature pan-B cell markers are downregulated and present as mostly negative or focally weakly positive occasionally. Neoplastic cells are usually negative for T-cell and cytotoxic markers; however, the T helper marker CD4 is abnormally positive in half of the cases. The EMA is generally positive, whereas cytokeratin AE1/AE3 is patchy and weakly positive (perinuclear dots or cytoplasm), which may raise the hypothesis of a poorly differentiated carci
Furthermore, germinal center-associated antigens, comprising CD10 and BCL6, are either expressed by themselves or together in some cases, similar to the reported case here. ALK-positive LBCL is postulated to originate from post-germinal center B cells with plasmablastic differentiation. This raises the following possibilities that some subpopulations may: (1) Be derived from germinal center B cells; (2) be influenced by the germinal center microenvironment; or (3) acquire CD10 expression through somatic mutations. Coexpression of CD10 and BCL6 has also been reported, supporting the hypothesis that at least a subset of ALK-positive LBCL may be derived from germinal center B cells (germinal center plasmablasts)[5]. However, in this case, the patient was CD10-positive, BCL6-negative, and MUM-1-positive. Considering that BCL-6 expression is predominantly restricted to germinal center B cells, the absence of BCL-6 in the present case may indicate that these cells did not originate from germinal center B cells.
Cytogenetically, ALK-positive LBCL shows characteristic ALK gene rearrangements, all of which lead to overexpression and oncogenic activation of the ALK protein. Clonal gene rearrangement tests for Ig/TCR clonality assays in B-NHL showed that the sensitivity of IgH and IgK gene rearrangement detection was 91.18%, and the rate of TCR gene rearrangement detection was 3.68%[17]. ALK-positive LBCL usually shows Ig rearrangements and the absence of TCR gene rearrangements. However, monoclonal Ig rearrangements are absent in some cases[2,18], which may be the result of a lack of consensus on target sequences or target site alterations as a result of somatic hypermutation. Moreover, TCR rearrangements are detected in some reported cases[19], as well as in our case showing Ig/TCR rearrangements, which may be formed from the earliest stages of B- and T-cell development. Otherwise, only a few reactive T cells within a high load of B-cell lymphoma are not sufficient to produce a polyclonal background[20]. Traditionally, TCRG and TCRB are the gold standard targets, whereas TCRD (generally combined with TCRG) should only be used as a target for well-defined clinical requests, and the paucity of TCRD templates may easily give rise to preferential amplification and pseudoclonality. Moreover, authentic clonal TCRD rearrangements may not be associated with malignant lymphoid proliferation[20]. This case can be explained from this viewpoint.
As the condition presented in this case originated in the conjunctiva, the main differential diagnoses may have included poorly differentiated carcinoma, small round cell tumors such as rhabdomyosarcoma and amelanotic mela
Herein, we report an extremely rare case of ALK-positive LBCL that presented as a bulbar conjunctival mass and shared morphological and IHC features with a variety of other neoplasms, which could result in misdiagnosis. Our experience suggests that awareness of this condition is necessary for an early and accurate diagnosis and appropriate treatment. For any subtype of conjunctival lymphoma, long-term follow-up is necessary because systemic disease may develop months or years after the initial diagnosis.
We wish to thank the timely help given by Hao Tang in figure editing.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pathology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chuang SS, Taiwan S-Editor: Zhang H L-Editor: A P-Editor: Xu ZH
| 1. | Campo E, Gascoyne RD. ALK-positive large B-cell lymphoma. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon: International Agency for Research on Cancer, 2017: 319–320. [Cited in This Article: ] |
| 2. | Pan Z, Hu S, Li M, Zhou Y, Kim YS, Reddy V, Sanmann JN, Smith LM, Chen M, Gao Z, Wang HY, Yuan J. ALK-positive Large B-cell Lymphoma: A Clinicopathologic Study of 26 Cases With Review of Additional 108 Cases in the Literature. Am J Surg Pathol. 2017;41:25-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 3. | Jiang XN, Yu BH, Wang WG, Zhou XY, Li XQ. Anaplastic lymphoma kinase-positive large B-cell lymphoma: Clinico-pathological study of 17 cases with review of literature. PLoS One. 2017;12:e0178416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Kushwaha P, Singh M, Vindal A, Verma N, Jain S. Primary ALK-Positive Large B Cell Lymphoma of Pancreas. J Gastrointest Cancer. 2022;53:830-833. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 5. | Yan M, Khattab R, Meyerson H. ALK-positive Large B-Cell Lymphoma Presenting as a Circumscribed Breast Mass with Germinal Center Immunophenotype. Int J Surg Pathol. 2023;31:233-238. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 6. | Xiong H, Liu SY, Yang YX, Tan XX, Luo QP, Peng J, Xiong ZT, Chen H, Chen J, Li Z, Jiang QP. An unusual case of anaplastic lymphoma kinase-positive large B-cell lymphoma in an elderly patient: A case report and discussion. Exp Ther Med. 2016;11:1799-1802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Chapman-Fredricks J, Blieden C, Sandoval JD, Ernani V, Ikpatt OF. Acute spontaneous tumor lysis syndrome as the initial presentation of ALK-positive diffuse large B-cell lymphoma. Appl Immunohistochem Mol Morphol. 2014;22:317-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Shi M, Miron PM, Hutchinson L, Woda BA, Nath R, Cerny J, Yu H. Anaplastic lymphoma kinase-positive large B-cell lymphoma with complex karyotype and novel ALK gene rearrangements. Hum Pathol. 2011;42:1562-1567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Quesada AE, Huh YO, Wang W, Medeiros LJ, Thakral B. Anaplastic lymphoma kinase (ALK)-positive large B-cell lymphoma in a patient treated with azathioprine for ulcerative colitis. Pathology. 2016;48:513-515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Liu H, Hu S. EBV+ ALK+ large B-cell lymphoma. Blood. 2021;138:2741. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 11. | Wu WN, Xiang CX, Ma DS, Liu GZ, Liu H. [ALK-positive large B-cell lymphoma with EBV infection or cyclin D1 expression: a clinicopathological analysis of 3 cases]. Zhonghua Bing Li Xue Za Zhi. 2022;51:506-511. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 12. | Lin SY, Chuang SS, Jhuang JY, Sakamoto K, Takeuchi K, Bahrami A, Tsai CC. ALK positive large B-cell lymphoma with a massive neutrophilic infiltrate: report of a case mimicking epithelioid inflammatory myofibroblastic tumour. J Clin Pathol. 2015;68:496-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Castillo JJ, Beltran BE, Malpica L, Marques-Piubelli ML, Miranda RN. Anaplastic lymphoma kinase-positive large B-cell lymphoma (ALK + LBCL): a systematic review of clinicopathological features and management. Leuk Lymphoma. 2021;62:2845-2853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Valera A, Colomo L, Martínez A, de Jong D, Balagué O, Matheu G, Martínez M, Taddesse-Heath L, Jaffe ES, Bacchi CE, Campo E. ALK-positive large B-cell lymphomas express a terminal B-cell differentiation program and activated STAT3 but lack MYC rearrangements. Mod Pathol. 2013;26:1329-1337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Khanlari M, Medeiros LJ. ALK+ large B-cell lymphoma with aberrant expression of CD3. Blood. 2020;136:3086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Corean J, Li KD. A Rare Case of ALK-Positive Large B-Cell Lymphoma with CD33 Expression. Case Rep Hematol. 2018;2018:5320590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Liu X, He H, Li Y, Huang Y, Li G, Yu Q, Li W, Li D. The application of antigen receptor gene rearrangement of BIOMED-2 in the pathologic diagnosis of 348 cases with non-Hodgkin lymphoma in a single institution in Southwest of China. Pathol Res Pract. 2019;215:152615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Van Roosbroeck K, Cools J, Dierickx D, Thomas J, Vandenberghe P, Stul M, Delabie J, De Wolf-Peeters C, Marynen P, Wlodarska I. ALK-positive large B-cell lymphomas with cryptic SEC31A-ALK and NPM1-ALK fusions. Haematologica. 2010;95:509-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | De Paepe P, Baens M, van Krieken H, Verhasselt B, Stul M, Simons A, Poppe B, Laureys G, Brons P, Vandenberghe P, Speleman F, Praet M, De Wolf-Peeters C, Marynen P, Wlodarska I. ALK activation by the CLTC-ALK fusion is a recurrent event in large B-cell lymphoma. Blood. 2003;102:2638-2641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Langerak AW, Groenen PJ, Brüggemann M, Beldjord K, Bellan C, Bonello L, Boone E, Carter GI, Catherwood M, Davi F, Delfau-Larue MH, Diss T, Evans PA, Gameiro P, Garcia Sanz R, Gonzalez D, Grand D, Håkansson A, Hummel M, Liu H, Lombardia L, Macintyre EA, Milner BJ, Montes-Moreno S, Schuuring E, Spaargaren M, Hodges E, van Dongen JJ. EuroClonality/BIOMED-2 guidelines for interpretation and reporting of Ig/TCR clonality testing in suspected lymphoproliferations. Leukemia. 2012;26:2159-2171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 336] [Article Influence: 28.0] [Reference Citation Analysis (0)] |