Published online May 26, 2024. doi: 10.12998/wjcc.v12.i15.2568
Revised: April 2, 2024
Accepted: April 8, 2024
Published online: May 26, 2024
The measurement of triceps skinfold (TSF) thickness serves as a noninvasive metric for evaluating subcutaneous fat distribution. Despite its clinical utility, the TSF thickness trajectories and their correlation with overall mortality have not been thoroughly investigated.
To explore TSF thickness trajectories of Chinese adults and to examine their associations with all-cause mortality.
This study encompassed a cohort of 14747 adults sourced from the China Health and Nutrition Survey. Latent class trajectory modeling was employed to identify distinct trajectories of TSF thickness. Subjects were classified into subgroups reflective of their respective TSF thickness trajectory. We utilized multivariate Cox regression analyses and mediation examinations to explore the link between TSF thickness trajectory and overall mortality, including contributory factors.
Upon adjustment for multiple confounding factors, we discerned that males in the ‘Class 2: Thin–stable’ and ‘Class 3: Thin–moderate’ TSF thickness trajectories exhibited a markedly reduced risk of mortality from all causes in comparison to the ‘Class 1: Extremely thin’ subgroup. In the mediation analyses, the Geriatric Nutritional Risk Index was found to be a partial intermediary in the relationship between TSF thickness trajectories and mortality. For females, a lower TSF thickness pattern was significantly predictive of elevated all-cause mortality risk exclusively within the non-elderly cohort.
In males and non-elderly females, lower TSF thickness trajectories are significantly predictive of heightened mortality risk, independent of single-point TSF thickness, body mass index, and waist circumference.
Core Tip: In this prospective cohort study, 14747 adults from the China Health and Nutrition Survey (1993-2015) were included and lower triceps skinfold (TSF) thickness trajectories in males and non-elderly females were significantly associated with an increased risk of all-cause mortality, independent of the one-point TSF thickness, body mass index, and waist circumference. When the TSF thickness in early adulthood was similar, even if the TSF thickness subsequently increased, there was no significant difference in the risk of all-cause mortality.
- Citation: Yang N, He LY, Li ZY, Yang YC, Ping F, Xu LL, Li W, Zhang HB, Li YX. Triceps skinfold thickness trajectories and the risk of all-cause mortality: A prospective cohort study. World J Clin Cases 2024; 12(15): 2568-2577
- URL: https://www.wjgnet.com/2307-8960/full/v12/i15/2568.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i15.2568
The body mass index (BMI) is commonly used in epidemiological studies as a primary anthropometric indicator for mortality risk assessment. Existing literature shows a J-shaped or U-shaped correlation between BMI and the incidence of mortality from all causes within the general population[1,2]. Yet, the utility of BMI as a mortality indicator remains under scrutiny due to its inability to accurately represent the regional distribution of body fat, which may lead to considerable variability among individuals a similar BMI[1-5]. As such, estimations of body composition have gained recognition as potentially significant prognosticators of mortality[6,7].
The triceps skinfold (TSF) thickness is an alternative anthropometric measure that is noninvasive, straightforward, readily accessible, and cost-effective, enabling the assessment of peripheral fat distribution[8]. This measure is increasingly considered a viable proxy for exploring the interplay between subcutaneous adiposity and mortality risk[4,9-14]. Unlike isolated or sporadic TSF measurements, longitudinal trajectory analysis captures the temporal evolution of TSF thickness, potentially elucidating a more comprehensive understanding of its association with mortality. Despite the apparent importance, there seems to be a gap in research endeavors examining the connection between TSF thickness trajectories and all-cause mortality.
Hence, the main objective of this investigation is to identify distinct TSF thickness trajectories within a substantial cohort of Chinese adults and to examine their potential associations with all-cause mortality.
The China Health and Nutrition Survey (CHNS) represents a comprehensive, ongoing prospective study, spanning cross China. Comprising 10 rigorous rounds of data collection, this survey encompasses a representative sample from nine provinces, accounting for nearly half of China’s population[15]. Ethical approval was secured from the respective Institutional Review Boards of the University of North Carolina at Chapel Hill and the National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention, with all participants providing informed consent. The methodology and survey design have been elaborated in prior publications[16].
Supplementary Figure 1 illustrates the study cohort's selection flowchart. Considering the absence of complete anthropometric data prior to 1993, our analysis included adult participants from eight CHNS waves between 1993 and 2015. Initially, 32963 individuals were deemed eligible; however, exclusions were made for pregnancy, incomplete or anomalous anthropometric data, baseline cardiovascular or oncological diseases, and lack of follow-up data.
Mid-arm anthropometric measurements: Standardized procedures were adhered to by skilled health workers to perform mid-arm measurements in triplicate, per individual, to ensure precision, with the mean value used for subsequent analyses. TSF thickness was measured at the posterior mid-point between the olecranon and acromion apex, with accuracy to 0.5 mm, utilizing a skinfold caliper. Mid-upper arm circumference (MUAC) was determined at the midpoint of the extended arm, precise to 0.1 cm. The mid-arm muscle circumference was computed by subtracting π times the TSF thickness from the MUAC[17].
Covariates: Demographics were obtained via structured interviews conducted by trained interviewers. Dietary assessments were ascertained based on a thorough three-day food consumption diary and household inventory[16]. Physical activity encompassed both occupational and domestic activity[18]. For participants of the 2009 survey wave, fasting venous blood samples were obtained[19]. The Geriatric Nutritional Risk Index (GNRI)[20] = 1.489 × serum albumin (Alb) (g/L) + 41.7 × present weight/ideal weight (kg). The ideal weight was derived from the Lorentz formula as follows: Ideal weight for females = 0.60 × height (cm) – 40, ideal weight for males = 0.75 × height (cm) – 62.5, and a present weight/ ideal weight ratio is set to 1 if it is no less than 1. Insulin resistance was assessed through the Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) index, based on fasting insulin and glucose levels[21].
Ascertainment of outcome: Mortality data, including the exact date of death, were ascertained from family reports during each survey wave. The baseline was set as the date of first entry with complete mid-arm measurements, with follow-up duration calculated up to death, loss to follow-up, or end of the study period, whichever occurred first.
Descriptive analysis: Baseline characteristics were categorized and analyzed, with continuous variables presented as means ± SD and categorical variables as counts (percentages). Comparative analyses across trajectory groups employed analysis of variance (ANOVA), Kruskal-Wallis, or Chi-square tests.
Trajectory modeling: Latent class trajectory models discerned TSF thickness trajectories for both genders, employing the lcmm package in R with a censored normal model[22]. Criteria for model selection included the lowest Bayesian information criterion, internal consistency as reflected by average posterior probability, and adequate trajectory class size (at least 5% of the sample population within each trajectory class) to evaluate mortality risk[15,23-26].
Mortality associations: Kaplan-Meier curves and log-rank tests examined TSF thickness trajectory-mortality associations. Cox models calculated hazard ratios (HRs) and 95% confidence intervals (95%CIs), adjusting multiple variables across four models. Model 1 was adjusted for age, marital status, educational attainment level, household income per capita level, and living in the city. Model 2 included the variables from Model 1 and also adjusted for: smoking status, alcohol consumption, physical activity level, total energy intake, fat intake, protein intake, carbohydrate intake, systolic blood pressure, and diastolic blood pressure. Model 3 included the variables in Model 2 and was adjusted for BMI, waist circumference (WC), TSF thickness, and MUAC. Model 4 was adjusted for the variables in Model 3, as well as the history of diabetes and hypertension.
Subgroup and mediation analyses: Subgroups were analyzed based on blood samples, using trajectory classifications from the total sample. Mediation analysis, conducted with the lavaan package in R, examined biomarkers as mediators between TSF thickness trajectories and mortality risk.
To ascertain the incremental value of trajectory analysis over single-time-point evaluations, we stratified baseline TSF thickness into tertiles for both genders, employing the lowest tertile as a referential baseline. Subsequently, the relationship between baseline TSF thickness and all-cause mortality was examined.
We undertook stratified analyses to investigate the influence of age (< 60 years, ≥ 60 years), BMI (< 24 kg/m2, ≥ 24 kg/m2)[27], and central adiposity WC ≥ 90 cm in males or ≥ 80 cm in females)[28]. The potential for effect modification was quantified by calculating interaction terms (P_interaction) within the multivariable framework, multiplying TSF thickness trajectory classifications by stratifying variables.
All statistical analyses were performed with SPSS for Windows (version 20.0; IBM Corporation, Chicago, IL, United States) and R version 4.0.2 (www.r-project.org/), and P < 0.05 (two-sided) was considered statistically significant.
From the CHNS dataset, a total of 32963 adults were initially identified. After applying the exclusion criteria, the study cohort was refined to 14745 adults (mean age 42.4 ± 15.1 years; 49.1% males), as shown in Supplementary Figure 1. There were 1019 documented mortalities during the study period.
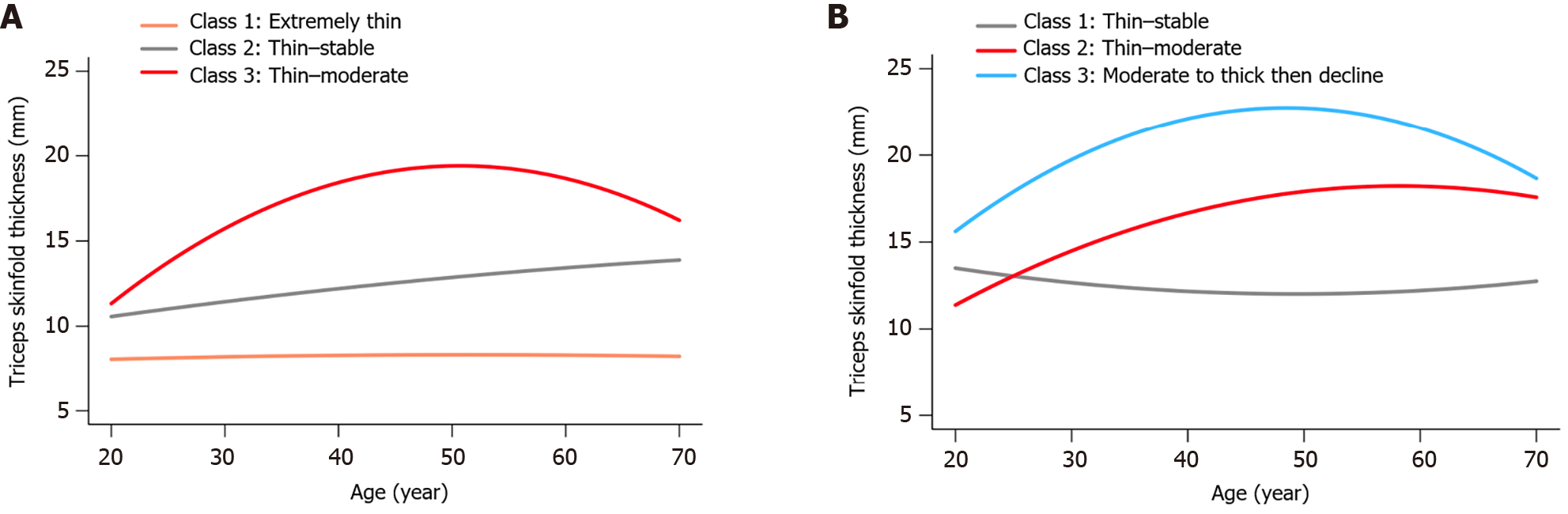
Figure 1 illustrates the TSF thickness trajectories among male and female participants, respectively. In males, 'Class 1: Extremely thin' denotes consistently extremely low TSF thickness through adulthood, 'Class 2: Thin–stable' represents consistently thin measurements, and 'Class 3: Thin–moderate' reflects an increase from thin to moderate values over time. There were 29.1% males in Class 1, 52.1% in Class 2, and 18.8% in Class 3. For females, 'Class 1: Thin–stable' indicates a steady thin TSF thickness measurement, 'Class 2: Thin–moderate' reflects an increase from thin to moderate, and 'Class 3: Moderate to thick then decline' describes an initial increase followed by a reduction in older age. There were 26.5% females in Class 1, 65.1% in Class 2, and 8.4% in Class 3.
The baseline characteristics of the participants, stratified by TSF thickness trajectories, are delineated in Supplementary Table 1. Using 'Class 1' as the reference group, Kaplan-Meier survival analysis revealed that individuals classified within 'Class 3' demonstrated superior cumulative survival rates (Supplementary Figure 2, P < 0.05) across both genders.
Table 1 elucidates the sex-specific association among TSF thickness trajectories and all-cause mortality, refined through multivariable-adjusted Cox regression models. For the male subset, when compared with 'Class 1,' the other trajectories were inversely correlated with mortality risk, persisting across all analytical models. Notably, 'Class 3' did not exhibit a statistically significant differential in mortality risk relative to 'Class 2' [HR 0.858 (95%CI: 0.582, 1.263)], post comprehensive adjustments. In the female subset, multivariate adjustments revealed no discernable correlation between TSF thickness trajectories and mortality risk.
| Trajectories | No. of participants/death | Model 1 | Model 2 | Model 3 | Model 4 |
| HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | ||
| Male | |||||
| Class 1 | 2132/335 | 1 | 1 | 1 | 1 |
| Class 2 | 3818/221 | 0.674 (0.564, 0.805) | 0.685 (0.572, 0.820) | 0.654 (0.532, 0.805) | 0.651 (0.529, 0.801) |
| Class 3 | 1379/40 | 0.670 (0.476, 0.942) | 0.675 (0.478, 0.954) | 0.628 (0.415, 0.949) | 0.635 (0.420, 0.961) |
| Female | |||||
| Class 1 | 1968/213 | 1 | 1 | 1 | 1 |
| Class 2 | 4828/206 | 0.827 (0.680, 1.007) | 0.844 (0.693, 1.029) | 0.982 (0.777, 1.241) | 0.984 (0.779, 1.244) |
| Class 3 | 620/4 | 0.335 (0.124, 0.910) | 0.341 (0.126, 0.927) | 0.441 (0.156, 1.246) | 0.447 (0.158, 1.264) |
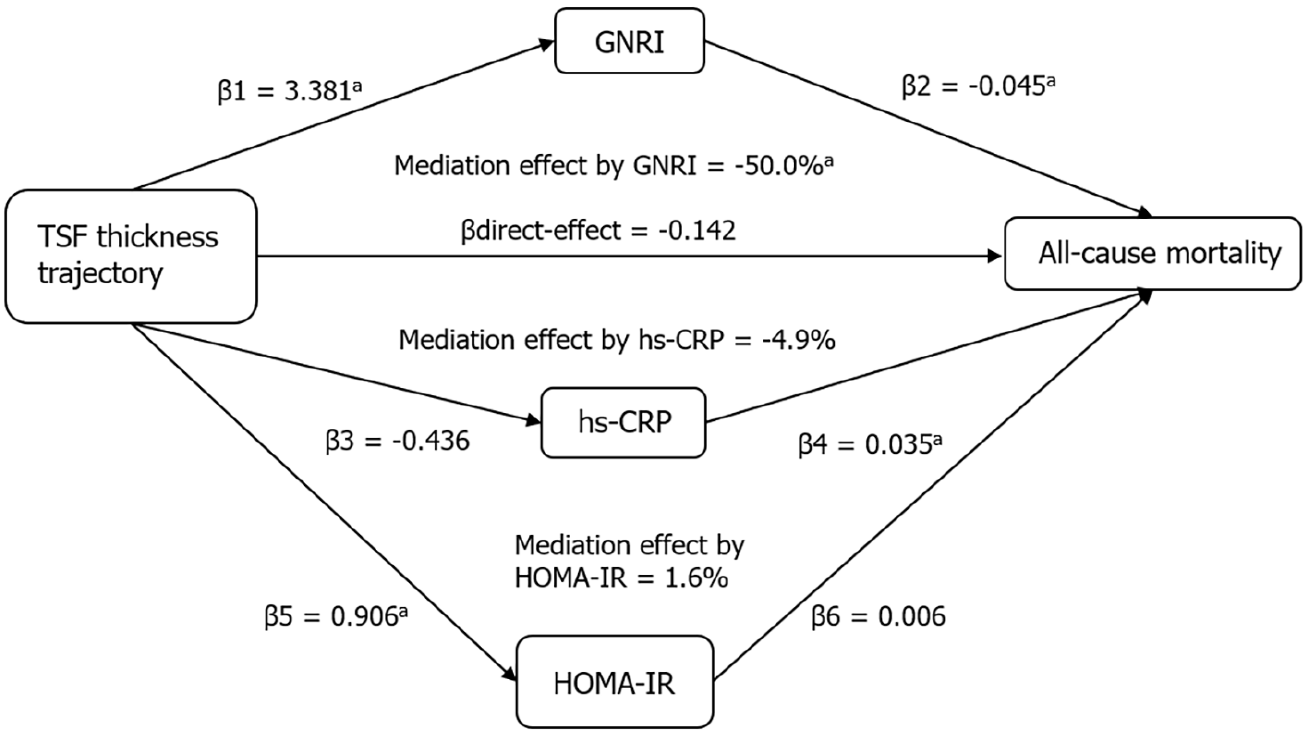
Differences in biomarkers across TSF thickness trajectory classes are shown in Supplementary Table 2. Figure 2 shows the mediation effects of GNRI, high-sensitivity C-reactive protein (hs-CRP), and HOMA-IR on the association between trajectories and the risk of all-cause mortality in males. The direct effect of the TSF thickness trajectory was estimated to be 0.142. β1 to β6 were used to calculate the overall indirect effect of these factors (βind = −0.152 for GNRI, P < 0.05; −0.015 for hs-CRP, P = 0.06; and 0.005 for HOMA-IR, P = 0.176). The percentages of the total effects mediated by GNRI were estimated to be 50.0%.
The Kaplan-Meier estimate of the all-cause mortality of males by one-point TSF thickness showed no significant differences among tertiles (P = 0.297, Supplementary Figure 3A). Supplementary Table 3 shows that TSF thickness measured at a single time point was not significantly associated with all-cause mortality for males or females.
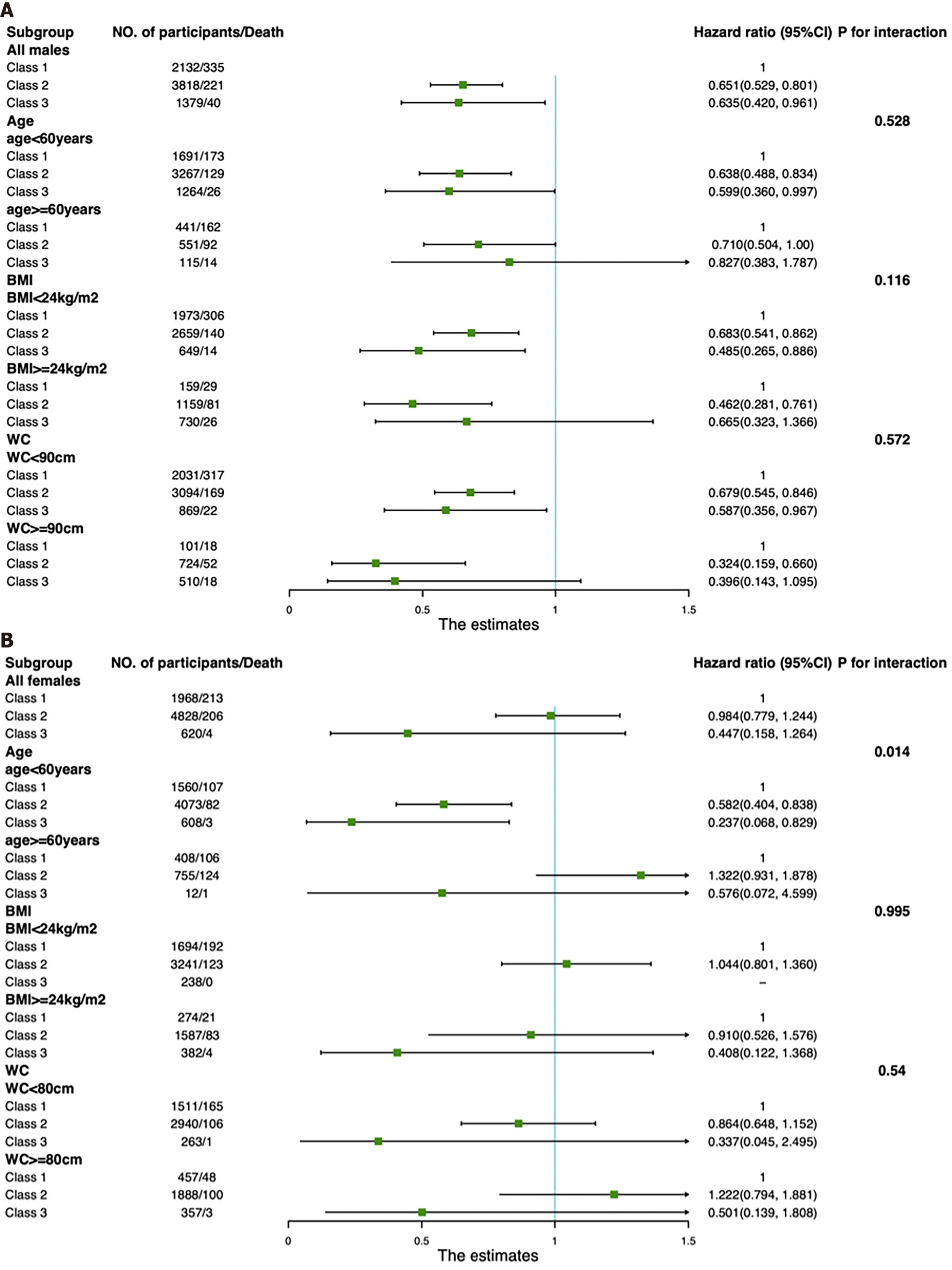
Subgroup analyses were conducted to evaluate the association between TSF thickness trajectories and all-cause mortality (Figure 3). Although a significant difference was only observed in the age < 60 years group, there was no significant interactive effect of TSF thickness trajectories and age group in males (Pinteraction = 0.528). Notably, for females, only those in the age < 60 years group had a significantly decreased risk of all-cause mortality in Class 2 and Class 3 compared with Class 1 [HR 0.582 (95%CI: 0.404, 0.838) for Class 2; HR 0.237 (95%CI: 0.068, 0.829) for Class 3], indicating that the effect of TSF thickness trajectories for females on all-cause mortality was greater in those with younger age, which was also supported by our finding that there was a significant interactive effect of TSF thickness trajectories and age group on all-cause mortality (Pinteraction = 0.014). There was no significant effect of BMI or WC modification on the association between TSF thickness trajectories and all-cause mortality for males or females (Pinteraction > 0.05).
In this longitudinal analysis of the Chinese population over two decades, our findings indicate a significant association between lower TSF thickness trajectories for males and an elevated risk of all-cause mortality. Notably, initial TSF thickness measurements in early adulthood, even when similar among individuals, did not yield significant predictive differences in mortality risk over time. Additionally, GNRI was identified as a partial mediator in the relationship between TSF thickness trajectories and mortality outcomes. For females, the association of lower TSF thickness with increased mortality was confined to the non-elderly demographic.
Our analysis revealed a heightened risk of all-cause mortality associated with persistently low TSF thickness trajectories compared to others. This association persisted even controlling for baseline TSF thickness. Moreover, in our cohort, a one-time measurement of TSF thickness did not correlate with mortality in either gender, underscoring that TSF thickness trajectories may be a more indicative measure of mortality risk than a single measurement. Previous literature reports inconsistent findings on the relationship between TSF thickness and mortality[9,12,13,29-32], with many studies being cross-sectional or focused on particular cohorts such as older adults, hemodialysis patients, or those with liver cirrhosis. Our study extends the exploration to a comprehensive adult population, contributing novel insights to the discourse. Notably, the extremely low TSF thickness trajectory was prevalent in 18.8% of the male participants, meriting further investigation. Additionally, we observed that similar TSF thickness levels in early adulthood did not result in mortality risk divergence in later years, regardless of subsequent increases. These findings challenge prior studies that highlighted the significance of TSF thickness predominantly in older populations[4,10], and instead, accentuates the detrimental effects of lower TSF thickness in early adulthood and the necessity of monitoring TSF thickness progression throughout life.
This study unveils an intriguing gender-specific observation: The novel association between TSF thickness and mortality was exclusively noted for males and non-elderly females. This gender disparity may stem from the fact that females typically exhibit higher body fat percentages than males at equivalent BMI levels[33]. Additionally, estrogen is known to modulate fat distribution, preferentially augmenting subcutaneous rather than visceral fat accumulation in females[7,34,35]. This finding diverges from some prior studies which suggested a more pronounced protective role of TSF thickness for females[29]. For instance, a prospective study highlighted that females with colorectal cancer presenting with low TSF thickness faced a 40% increased risk of adverse outcomes compared to those with normal TSF thickness[30], whereas TSF thickness did not emerge as a prognostic determinant for males. Our data also revealed a predominance of extremely low TSF thickness trajectories for males, while for females the trajectory was more frequently characterized by thicker TSF thickness, corroborating earlier assertions of gender-based differences in subcutaneous fat distribution. Moreover, our subgroup analyses suggest a significant association between TSF thickness trajectories and mortality in younger females, hinting that TSF thickness may exert a more substantial impact earlier in life, thereby underscoring the need for vigilant monitoring of TSF thickness from early adulthood. Nonetheless, the physiological underpinnings of regional fat accumulation are intricate and not yet fully elucidated[36], necessitating further investigation in this domain.
Previous studies on TSF thickness and mortality risk have largely focused on elderly populations, indicating an increased risk of all-cause mortality with thinner skinfolds[12], However, our study suggests that the trajectory of TSF thickness may have a greater impact on younger individuals, highlighting the importance of early and continuous monitoring of skinfold thickness changes to potentially influence mortality risk. Our study data lacks specific causes of death, but a review of previous literature indicates that previous studies have explored the role of TSF thickness in predicting mortality risk associated with cancer, cardiovascular and cerebrovascular diseases, and dialysis. Data from NHANES suggest that lower TSF thickness is associated with a higher risk of cardiovascular disease mortality but not with cerebrovascular disease mortality[4]. A multicenter study demonstrated a significant correlation between low skinfold thickness and increased risk of cancer-related mortality[36]. Another study on colorectal cancer mortality risk indicated that lower TSF thickness was associated with increased mortality risk for females but not for males[30]. In dialysis patients, lower TSF thickness was linked to higher mortality risk[37]. However, there is currently a lack of research on the relationship between TSF thickness trajectories and specific disease mortality risks, necessitating further studies.
Our findings reveal that the GNRI serves as a partial mediator in the correlation between TSF thickness trajectories and all-cause mortality, suggesting that the impact of TSF thickness on mortality may predominantly be channeled through nutritional status rather than metabolic factors such as insulin resistance. While Alb is a recognized clinical marker of nutritional status, its circulating level can be influenced by a multitude of factors[38]. Hence, we delved deeper into the relationship between GNRI and various TSF thickness trajectories. Incorporating Alb, weight, and height, GNRI is advocated as an economical yet effective instrument for evaluating nutritional status[39,40]. This study discovered an inverse relationship between TSF thickness trajectories and GNRI, with GNRI exhibiting a significant mediating role in the association between TSF thickness trajectory and mortality. This observation suggests that TSF thickness may act as an indirect indicator of overall nutritional and health status. Aligning with previous studies, our findings suggest that a greater TSF thickness may confer protection against malnutrition and cachexia, potentially mitigating mortality risks associated with chronic and malignant conditions[29,41].
This investigation stands as the first study examining the trajectory of TSF thickness in relation to all-cause mortality, distinguished by its extensive cohort and prolonged observation period. Our research indicates that TSF thickness trajectories offer additional predictive insights on mortality risk, transcending the data provided by single TSF thickness measurements, BMI, and WC. Moreover, our inclusive approach, encompassing adults across a wide age spectrum, has demonstrated that the implications of TSF thickness trajectories extend well beyond geriatric demographics. We also delved into potential underlying mechanisms for our findings, contributing to a deeper understanding of this area. Nevertheless, this study had several limitations. Primarily, it is imperative to acknowledge that our cohort consisted exclusively of individuals from China, which may limit the applicability of our findings across different ethnic groups. We recognize this as a limitation and suggest that future research should aim to include a more ethnically diverse population. As an observational study, it precludes the establishment of causality. The lack of cause-specific mortality data precludes a comprehensive analysis, as deaths due to extraneous causes could introduce an element of bias. This gap in data restricts our ability to dissect and discuss the multifactorial nature of mortality in relation to TSF thickness trajectory. We acknowledge this as a critical area for future studies and aim to explore these aspects further in subsequent studies, provided that relevant data become accessible.
In conclusion, trajectories with lower TSF thickness for males and non-elderly females were significantly associated with an increased risk of all-cause mortality, independent of the one-point TSF thickness, BMI, and WC. This study additionally emphasizes the harmful effects of lower TSF thickness in early healthy adulthood and the importance of monitoring TSF thickness over the adult life course. Further studies in different ethnic populations are required to verify this conclusion.
This research uses data from China Health and Nutrition Survey (CHNS). We thank the National Institute of Nutrition and Food Safety, China Center for Disease Control and Prevention, Carolina Population Center, the University of North Carolina at Chapel Hill, the NIH (R01-HD30880, DK056350, and R01-HD38700) and the Fogarty International Center, NIH for financial support for the CHNS data collection and analysis. We also thank the China-Japan Friendship Hospital and Ministry of Health for their support with the CHNS 2009 survey.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Soriano-Ursúa MA, Mexico S-Editor: Lin C L-Editor: A P-Editor: Yu HG
| 1. | Lee DH, Keum N, Hu FB, Orav EJ, Rimm EB, Willett WC, Giovannucci EL. Predicted lean body mass, fat mass, and all cause and cause specific mortality in men: prospective US cohort study. BMJ. 2018;362:k2575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 227] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 2. | Global BMI Mortality Collaboration, Di Angelantonio E, Bhupathiraju ShN, Wormser D, Gao P, Kaptoge S, Berrington de Gonzalez A, Cairns BJ, Huxley R, Jackson ChL, Joshy G, Lewington S, Manson JE, Murphy N, Patel AV, Samet JM, Woodward M, Zheng W, Zhou M, Bansal N, Barricarte A, Carter B, Cerhan JR, Smith GD, Fang X, Franco OH, Green J, Halsey J, Hildebrand JS, Jung KJ, Korda RJ, McLerran DF, Moore SC, O'Keeffe LM, Paige E, Ramond A, Reeves GK, Rolland B, Sacerdote C, Sattar N, Sofianopoulou E, Stevens J, Thun M, Ueshima H, Yang L, Yun YD, Willeit P, Banks E, Beral V, Chen Zh, Gapstur SM, Gunter MJ, Hartge P, Jee SH, Lam TH, Peto R, Potter JD, Willett WC, Thompson SG, Danesh J, Hu FB. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776-786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1610] [Cited by in F6Publishing: 1496] [Article Influence: 187.0] [Reference Citation Analysis (0)] |
| 3. | Jayedi A, Soltani S, Zargar MS, Khan TA, Shab-Bidar S. Central fatness and risk of all cause mortality: systematic review and dose-response meta-analysis of 72 prospective cohort studies. BMJ. 2020;370:m3324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 149] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 4. | Li W, Yin H, Chen Y, Liu Q, Wang Y, Qiu D, Ma H, Geng Q. Associations Between Adult Triceps Skinfold Thickness and All-Cause, Cardiovascular and Cerebrovascular Mortality in NHANES 1999-2010: A Retrospective National Study. Front Cardiovasc Med. 2022;9:858994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 5. | Neeland IJ, Poirier P, Després JP. Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation. 2018;137:1391-1406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 479] [Cited by in F6Publishing: 444] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 6. | Liu XC, Liu L, Yu YL, Huang JY, Chen CL, Lo K, Huang YQ, Feng YQ. The Association of Subscapular Skinfold with All-Cause, Cardiovascular and Cerebrovascular Mortality. Risk Manag Healthc Policy. 2020;13:955-963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020;8:616-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 292] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 8. | Nickerson BS, Fedewa MV, Cicone Z, Esco MR. The relative accuracy of skinfolds compared to four-compartment estimates of body composition. Clin Nutr. 2020;39:1112-1116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Loh WJ, Johnston DG, Oliver N, Godsland IF. Skinfold thickness measurements and mortality in white males during 27.7 years of follow-up. Int J Obes (Lond). 2018;42:1939-1945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | He L, Yang N, Wang J, Huang J, Li W, Xu L, Ping F, Li Y, Zhang H. Mid-Arm Muscle and Subcutaneous Fat Associated with All-Cause Mortality Independent of BMI: A Prospective Cohort Study. Obesity (Silver Spring). 2021;29:1203-1214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Kim J, Meade T, Haines A. Skinfold thickness, body mass index, and fatal coronary heart disease: 30 year follow up of the Northwick Park heart study. J Epidemiol Community Health. 2006;60:275-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Enoki H, Kuzuya M, Masuda Y, Hirakawa Y, Iwata M, Hasegawa J, Izawa S, Iguchi A. Anthropometric measurements of mid-upper arm as a mortality predictor for community-dwelling Japanese elderly: the Nagoya Longitudinal Study of Frail Elderly (NLS-FE). Clin Nutr. 2007;26:597-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Huang CX, Tighiouart H, Beddhu S, Cheung AK, Dwyer JT, Eknoyan G, Beck GJ, Levey AS, Sarnak MJ. Both low muscle mass and low fat are associated with higher all-cause mortality in hemodialysis patients. Kidney Int. 2010;77:624-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | de Almeida Roediger M, de Fátima Nunes Marucci M, Quintiliano Scarpelli Dourado DA, de Oliveira C, Licio Ferreira Santos J, de Oliveira Duarte YA. Body Composition Changes and 10-Year Mortality Risk in Older Brazilian Adults: Analysis of Prospective Data from the SABE Study. J Nutr Health Aging. 2019;23:51-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Han T, Zhang S, Duan W, Ren X, Wei C, Sun C, Li Y. Eighteen-year alcohol consumption trajectories and their association with risk of type 2 diabetes and its related factors: the China Health and Nutrition Survey. Diabetologia. 2019;62:970-980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | He J, Fang A, Yu S, Shen X, Li K. Dietary Nonheme, Heme, and Total Iron Intake and the Risk of Diabetes in Adults: Results From the China Health and Nutrition Survey. Diabetes Care. 2020;43:776-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Barazzoni R, Jensen GL, Correia MITD, Gonzalez MC, Higashiguchi T, Shi HP, Bischoff SC, Boirie Y, Carrasco F, Cruz-Jentoft A, Fuchs-Tarlovsky V, Fukushima R, Heymsfield S, Mourtzakis M, Muscaritoli M, Norman K, Nyulasi I, Pisprasert V, Prado C, de van der Schuren M, Yoshida S, Yu Y, Cederholm T, Compher C. Guidance for assessment of the muscle mass phenotypic criterion for the Global Leadership Initiative on Malnutrition (GLIM) diagnosis of malnutrition. Clin Nutr. 2022;41:1425-1433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 94] [Article Influence: 47.0] [Reference Citation Analysis (1)] |
| 18. | Xue Y, Shen Q, Li C, Dai Z, He T. The Visceral Adipose Index in Relation to Incidence of Hypertension in Chinese Adults: China Health and Nutrition Survey (CHNS). Nutrients. 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Zhu B, Li Y, Shi Y, Song N, Fang Y, Ding X. Long-term drinking behavior change patterns and its association with hyperuricemia in chinese adults: evidence from China Health and Nutrition Survey. BMC Public Health. 2022;22:1230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Riveros C, Jazayeri SB, Chalfant V, Ahmed F, Bandyk M, Balaji KC. The Geriatric Nutritional Risk Index Predicts Postoperative Outcomes in Bladder Cancer: A Propensity Score-Matched Analysis. J Urol. 2022;207:797-804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Wang T, Li M, Zeng T, Hu R, Xu Y, Xu M, Zhao Z, Chen Y, Wang S, Lin H, Yu X, Chen G, Su Q, Mu Y, Chen L, Tang X, Yan L, Qin G, Wan Q, Gao Z, Wang G, Shen F, Luo Z, Qin Y, Huo Y, Li Q, Ye Z, Zhang Y, Liu C, Wang Y, Wu S, Yang T, Deng H, Zhao J, Shi L, Ning G, Bi Y, Wang W, Lu J. Association Between Insulin Resistance and Cardiovascular Disease Risk Varies According to Glucose Tolerance Status: A Nationwide Prospective Cohort Study. Diabetes Care. 2022;45:1863-1872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 22. | Lennon H, Kelly S, Sperrin M, Buchan I, Cross AJ, Leitzmann M, Cook MB, Renehan AG. Framework to construct and interpret latent class trajectory modelling. BMJ Open. 2018;8:e020683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 140] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 23. | Wang Y, Hou W, Siddiqi SM, Sun C, Han T, Yang J. Association of sleep trajectory in adulthood with risk of hypertension and its related risk factors: the China Health and Nutrition Survey. J Clin Sleep Med. 2020;16:515-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Sanchez-Sanchez JL, Izquierdo M, Carnicero-Carreño JA, García-García FJ, Rodríguez-Mañas L. Physical activity trajectories, mortality, hospitalization, and disability in the Toledo Study of Healthy Aging. J Cachexia Sarcopenia Muscle. 2020;11:1007-1017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Charles A, Detilleux J, Buckinx F, Reginster JY, Gruslin B, Bruyère O. Physical performance trajectories and mortality among nursing home residents: results of the SENIOR cohort. Age Ageing. 2020;49:800-806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Chen Y, Cai C, Tan J, Lei X, Chen Q, Zhang J, Zhang Y. High-risk Growth Trajectory Related to Childhood Overweight/Obesity and Its Predictive Model at Birth. J Clin Endocrinol Metab. 2022;107:e4015-e4026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Gao M, Lv J, Yu C, Guo Y, Bian Z, Yang R, Du H, Yang L, Chen Y, Li Z, Zhang X, Chen J, Qi L, Chen Z, Huang T, Li L; China Kadoorie Biobank (CKB) Collaborative Group. Metabolically healthy obesity, transition to unhealthy metabolic status, and vascular disease in Chinese adults: A cohort study. PLoS Med. 2020;17:e1003351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 28. | Huang G, Xu J, Zhang Z, Cai L, Liu H, Yu X. Total cholesterol and high density lipoprotein cholesterol ratio is associated with metabolic syndrome in a very elderly Chinese population. Sci Rep. 2022;12:15212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Wu SE, Chen WL. Not the enemy: potential protective benefits of superficial subcutaneous adipose tissue. Pol Arch Intern Med. 2022;132. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 30. | Xie H, Ruan G, Zhang H, Ge Y, Zhang Q, Lin S, Song M, Zhang X, Liu X, Li X, Zhang K, Yang M, Tang M, Deng L, Gan J, Shi H. Sex differences in the association of triceps skinfold thickness and colorectal cancer mortality. Nutrition. 2022;103-104:111811. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 31. | de Santana FM, Domiciano DS, Gonçalves MA, Machado LG, Figueiredo CP, Lopes JB, Caparbo VF, Takayama L, Menezes PR, Pereira RM. Association of Appendicular Lean Mass, and Subcutaneous and Visceral Adipose Tissue With Mortality in Older Brazilians: The São Paulo Ageing & Health Study. J Bone Miner Res. 2019;34:1264-1274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 32. | Oppert JM, Charles MA, Thibult N, Guy-Grand B, Eschwège E, Ducimetière P. Anthropometric estimates of muscle and fat mass in relation to cardiac and cancer mortality in men: the Paris Prospective Study. Am J Clin Nutr. 2002;75:1107-1113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Liu Y, Sun G, Li Y. A prospective cohort study on the association of lean body mass estimated by mid-upper arm muscle circumference with hypertension risk in Chinese residents. J Clin Hypertens (Greenwich). 2022;24:329-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Tang HK, Bowe SJ, Nguyen THHD, Dibley MJ. Triceps and subscapular skinfold thickness percentiles of a school-based sample of adolescents in Ho Chi Minh City, Vietnam. Eur J Clin Nutr. 2020;74:1483-1487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Ebadi M, Tandon P, Moctezuma-Velazquez C, Ghosh S, Baracos VE, Mazurak VC, Montano-Loza AJ. Low subcutaneous adiposity associates with higher mortality in female patients with cirrhosis. J Hepatol. 2018;69:608-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 36. | Yin L, Zhang L, Li N, Guo J, Liu L, Lin X, Fan Y, Liu J, Zhang M, Chong F, Chen X, Wang C, Wang X, Liang T, Liu X, Deng L, Li W, Yang M, Yu J, Yang S, Zuo Z, Yuan K, Yu M, Song C, Cui J, Li S, Guo Z, Shi H, Xu H. Several anthropometric measurements and cancer mortality: predictor screening, threshold determination, and joint analysis in a multicenter cohort of 12138 adults. Eur J Clin Nutr. 2022;76:756-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Noori N, Kopple JD, Kovesdy CP, Feroze U, Sim JJ, Murali SB, Luna A, Gomez M, Luna C, Bross R, Nissenson AR, Kalantar-Zadeh K. Mid-arm muscle circumference and quality of life and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:2258-2268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 226] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 38. | Hayama T, Hashiguchi Y, Ozawa T, Watanabe M, Fukushima Y, Shimada R, Nozawa K, Matsuda K, Fujii S, Fukagawa T. The preoperative geriatric nutritional risk index (GNRI) is an independent prognostic factor in elderly patients underwent curative resection for colorectal cancer. Sci Rep. 2022;12:3682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 39. | Xiang Q, Li Y, Xia X, Deng C, Wu X, Hou L, Yue J, Dong B. Associations of geriatric nutrition risk index and other nutritional risk-related indexes with sarcopenia presence and their value in sarcopenia diagnosis. BMC Geriatr. 2022;22:327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Güç ZG, Alacacıoğlu A, Kalender ME, Oflazoğlu U, Ünal S, Yıldız Y, Salman T, Küçükzeybek Y, Tarhan MO. HALP score and GNRI: Simple and easily accessible indexes for predicting prognosis in advanced stage NSCLC patients. The İzmir oncology group (IZOG) study. Front Nutr. 2022;9:905292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Pinto AC, Sousa AS, Amaral TF, Guerra RS. Association Between Anthropometric Indicators of Nutrition Status and Length of Hospital Stay in Hospitalized Patients. JPEN J Parenter Enteral Nutr. 2021;45:381-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |