Published online May 16, 2024. doi: 10.12998/wjcc.v12.i14.2350
Revised: March 9, 2024
Accepted: April 7, 2024
Published online: May 16, 2024
This study presents an evaluation of the computed tomography lymphangio
To improve the diagnosis of lymphatic PB or primary chylothorax, a retrospective analysis of the clinical features and CTL characteristics of 71 patients diagnosed with lymphatic PB or primary chylothorax was performed.
The clinical and CTL data of 71 patients (20 with lymphatic PB, 41 with primary chylothorax, and 10 with lymphatic PB with primary chylothorax) were collected retrospectively. CTL was performed in all patients. The clinical manifestations, CTL findings, and conventional chest CT findings of the three groups of patients were compared. The chi-square test or Fisher's exact test was used to compare the differences among the three groups. A difference was considered to be statistically significant when P < 0.05.
(1) The percentages of abnormal contrast medium deposits on CTL in the three groups were as follows: Thoracic duct outlet in 14 (70.0%), 33 (80.5%) and 8 (80.0%) patients; peritracheal region in 18 (90.0%), 15 (36.6%) and 8 (80.0%) patients; pleura in 6 (30.0%), 33 (80.5%) and 9 (90.0%) patients; pericardium in 6 (30.0%), 6 (14.6%) and 4 (40.0%) patients; and hilum in 16 (80.0%), 11 (26.8%) and 7 (70.0%) patients; and (2) the abnormalities on conventional chest CT in the three groups were as follows: Ground-glass opacity in 19 (95.0%), 18 (43.9%) and 8 (80.0%) patients; atelectasis in 4 (20.0%), 26 (63.4%) and 7 (70.0%) patients; interlobular septal thickening in 12 (60.0%), 11 (26.8%) and 3 (30.0%) patients; bronchovascular bundle thickening in 14 (70.0%), 6 (14.6%) and 4 (40.0%) patients; localized mediastinal changes in 14 (70.0%), 14 (34.1%), and 7 (70.0%) patients; diffuse mediastinal changes in 6 (30.0%), 5 (12.2%), and 3 (30.0%) patients; cystic lesions in the axilla in 2 (10.0%), 6 (14.6%), and 2 (20.0%) patients; and cystic lesions in the chest wall in 0 (0%), 2 (4.9%), and 2 (4.9%) patients.
CTL is well suited to clarify the characteristics of lymphatic PB and primary chylothorax. This method is an excellent tool for diagnosing these two diseases.
Core Tip: Lymphatic plastic bronchitis and primary chylothorax are rare lymphatic drainage disorders. The pathological and physiological mechanisms, clinical manifestations, and imaging findings of the two are similar, making differential diagnosis difficult. Computed tomography lymphangiography (CTL) is an appropriate method to detect abnormal lymphatic vessels and pulmonary abnormalities. CTL is an effective diagnostic method for distinguishing between the two diseases.
- Citation: Li XP, Zhang Y, Sun XL, Hao K, Liu MK, Hao Q, Wang RG. Lymphatic plastic bronchitis and primary chylothorax: A study based on computed tomography lymphangiography. World J Clin Cases 2024; 12(14): 2350-2358
- URL: https://www.wjgnet.com/2307-8960/full/v12/i14/2350.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i14.2350
Lymphatic plastic bronchitis (PB) is a rare lung disease. Abnormal lymph flow caused by various causes leads to the abnormal accumulation of lymph in the bronchus and the formation of bronchial casts. This disease is often accompanied by local or widespread bronchial obstruction or varying degrees of respiratory dysfunction, leading to severe and life-threatening respiratory distress[1,2]. Chylothorax refers to the accumulation of chylous fluid in the pleural cavity. It is mainly formed when lymph leaks from the thoracic duct or other lymphatic vessels into the pleural cavity due to various causes. According to the aetiology, chylothorax can be divided into primary and secondary chylothorax[3]. The former is related to congenital lymphatic abnormalities, such as generalized lymphatic anomalies, lymphatic malformations in Gorham-Stout disease, and channel-type lymphatic malformations[4-7]. Both lymphatic PB and primary chylothorax are lymphatic reflux disorders caused by primary lymphatic vessel dysplasia. The morbidity is low, and most cases have been reported in small samples[8-10].
Direct lymphangiography (DLG) can be used to intuitively and dynamically observe abnormal changes such as tortuous lymphatic vessels, lymph leakage, or reflux. DLG is the gold standard for visualizing lymphatic vessels, cisterna chyli, and the thoracic duct and detecting lymphatic fistulas; it can help diagnose lymphatic diseases and anatomical abnormalities[11-13]. Computed tomography lymphangiography (CTL) was performed on the chest and abdomen after DLG. CTL can compensate for the limitations of DLG in affecting image overlap and is valuable for revealing changes in the lung, abnormal deposits and reflux of lipiodol, pleural effusion, and extrathoracic lymphatic malformations and their degree[14-16]. Although scholars have applied CTL to the study of lymphatic PB and primary chylothorax[17], we had the largest sample size.
The purpose of our study was to retrospectively analyse the clinical characteristics and CTL features of 71 patients diagnosed with lymphatic PB or primary chylothorax.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Beijing Shijitan Hospital Affiliated to Capital Medical University Hospital, and informed consent was waived because the study was a retrospective review. Between January 2009 and December 2021, 295 consecutive patients with suspected lymphatic PB and chylothorax admitted to Shijitan Hospital were retrospectively analysed.
The inclusion and exclusion criteria are shown in Figure 1. Patients without CTL data (n = 3) were excluded. The diagnostic criteria for lymphatic PB are the presence of bronchial casts and the abnormal development of lymphatic vessels. Chylothorax patients met at least one of the following criteria: (1) Had a positive chylous test in the effusion; (2) had a triglyceride level > 1.25 mmol/L in the effusion; (3) had a DLG showing contrast medium in the pleural cavity; and (4) had nuclear imaging showing contrast medium entry into the pleural space[18,19]. Patients who met the following criteria were selected for the study: Patients who were clinically diagnosed with lymphatic PB and primary chylothorax. The exclusion criteria were as follows: (1) Had chylothorax or PB caused by secondary factors (tumour, infection, trauma, surgery); (2) had chylous ascites; and (3) did not undergo DLG or CTL. The clinical and CTL data of 71 patients (20 with lymphatic PB, 41 with primary chylothorax, and 10 with lymphatic PB with primary chylothorax) were collected retrospectively.
The main demographic and clinical variables of the patients in the three groups are shown in Table 1.
| Characteristic or symptoms | Group A (n = 20) | Group B (n = 41) | Group C (n = 10) | P value |
| Ages (yr) | 33.1 ± 17.3 | 34.0 ± 19.0 | 48.3 ± 11.7 | 0.060 |
| Gender | 0.472 | |||
| Male | 11 | 16 | 5 | |
| Female | 9 | 25 | 5 | |
| Course (m) | 36.0 (6.5-129.0)a | 6 (2.5-20.0)a | 33 (5-108) | 0.005 |
| Cough | 20 (100)a | 9 (22.0)a,b | 9 (90.0)b | < 0.001 |
| Sputum | 20 (100)a | 0 (0)a,b | 10 (100)b | < 0.001 |
| Chest tightness | 12 (60.0) | 29 (70.7) | 8 (80.0) | 0.501 |
| Fever | 5 (25.0) | 4 (9.8) | 0 (0) | 0.125 |
| Hemoptysis | 4 (20.0)a | 0 (0)a | 0 (0) | 0.008 |
In our study, 61 of 71 patients underwent thoracic duct outlet surgery. Twenty patients in group A, 33 patients in group B, and 8 patients in group C underwent this operation.
Before the lower extremity DLG, consent was obtained from the patients or their guardians. DLG was performed using a GE Innova 2000-IQ DSA machine (AXIOM, Siemens, Erlangen, Germany). The patient was placed in the supine position. The surgeon punctured the skin between the first and second toes of the healthy or less oedematous side of the foot. Methylene blue dye (2.5% Patent Blue V dye; 1 mL, Guerbet Laboratories, Aulnay-Sous-Bois, France) and 1 mL of 2% lidocaine (1:1) were injected into the skin. Subsequently, the superficial lymphatic vessels were examined under a microscope and injected via fine needle puncture with 8-20 mL of superfluid contrast agent (Lipiodol; Guerbet Laboratories, Villepinte, France). The reflex of the contrast agent (lipiodol) along the lymphatic vessels can be observed dynamically. Depending on the patient’s age and the development of lymphatic vessels, the injection time was approximately 2 h. In normal people, lipiodol cannot enter tissues or lesions through the lymphatic vessel wall and can only be removed through the thoracic duct. The DLG lasted approximately 5-6 h.
All patients underwent CTL within 30 min-2 h after DLG. CT (Somatom Sensation Cardiac 16, Siemens Healthcare; Brilliance iCT, Philips Medical Health Care, Best, the Netherlands) scans were performed bilaterally from the inferior border of the thyroid cartilage in the neck to the lower border of the lungs. Patients were asked to hold their breath after deep inhalation during the scan, and a CT scan was performed at the end of inhalation. After the scan, the patients breathed freely. The scanning parameters were as follows: Tube voltage of 120 kVp, collimation of 1 mm, pitch of 1, and tube current of 110 mAs. After scanning, the raw data were transferred to the workstation for three-dimensional reconstruction with a layer thickness of 2 mm and a layer spacing of 2 mm.
The patient was placed in the supine position and under general anaesthesia. The patient's shoulders are padded with pillows so that the head is tilted slightly to the right. After disinfection of the neck area with iodine, a sterile towel, a medium sheet, and a small opening sheet were laid out. A transverse skin incision is made from the left sternoclavicular joint to the left external jugular vein between the body projection lines, approximately one finger above the clavicle. Under the microscope (12.5 /8-10 magnification), the subcutaneous layer, the broad cervical muscle, and the left sternocleidomastoid muscle were cut with an electric knife. The cut edge was treated with electrocoagulation to stop the bleeding. The left jugular vein angle (located under the clavicle) was revealed by posterior dissection, and the thoracic duct was explored and released from the obstruction. The wound surface was checked for obvious blood and fluid spillage, and sodium hyaluronate was injected locally to prevent adhesions. The gauze and instruments were counted, and silk sutures were interrupted to close the sternocleidomastoid dissection, the cervical brevis layer, and the subcutaneous layer. The skin incision was glued with tissue glue, and the wound was covered with a sterile dressing. At the end of the operation, the tracheal tube and gastric tube were removed upon awakening from anaesthesia.
All 71 patients underwent DLG successfully, and the quality of the CTL images met the criteria for disease assessment. All CTL images were double-blind reviewed by two radiologists (diagnostic radiologists with over 15 years of experience).
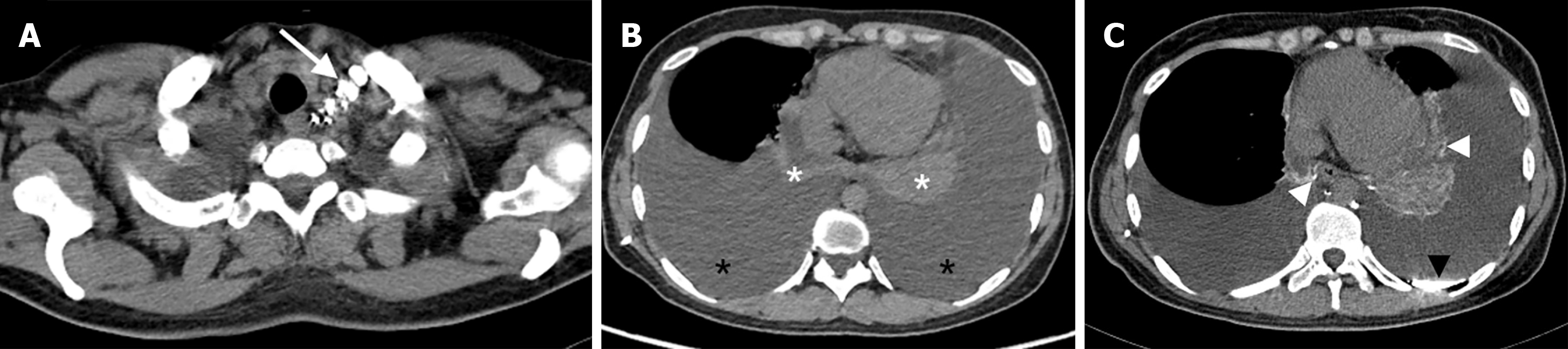
After the contrast agent (lipiodol) has been injected into the superficial lymphatic vessels of the unilateral foot, the lipiodol follows the lymphatic fluid up to the lumbar trunk and converges into the cisterna chyli, then into the thoracic duct, and finally into the blood at the angle of the left jugular vein. During this process, the distribution of lipiodol deposits in any other part of the body, such as the diaphragm, peritracheal region, pericardium, pleura, and hilum, is considered abnormal. Such deposits represent dilated lymphatic vessel hyperplasia, reflux, or fistula.
CTL and conventional chest CT were performed to assess the following features: (1) Abnormal distribution of lipiodol in the peritracheal region, pleura, pericardium, hilum, and thoracic duct outlet; and (2) ground-glass opacity, atelectasis, interlobular septal thickening, bronchovascular bundle thickening, pleural effusion, and mediastinal and extrapleural soft tissue changes.
Statistical analysis was performed by using software (SPSS, version 26.0; IBM, Armonk, NY, United States). Normally distributed quantitative data are expressed as the mean ± SD, and nonnormally distributed continuous variables are expressed as the median (25th-75th percentiles). Normally distributed and nonnormally distributed variables were evaluated by using one-way analysis of variance and the Kruskal-Wallis H test, respectively. The chi-square test or Fisher's exact test was used to compare the differences in abnormal CTL lipiodol deposition and abnormal changes in the lungs among the three groups. The Bonferroni correction was used for pairwise comparisons when the overall difference was statistically significant. Statistical significance was established at P values less than 0.05 for overall and pairwise adjusted tests of significance.
The data of 71 patients (mean age: 35 ± 18 years; age range: 1-73 years; 32 men) with a diagnosis of lymphatic PB (n = 20), primary chylothorax (n = 41), or lymphatic PB with primary chylothorax (n = 10) were reviewed. For further characteristics of the patients, see Table 1.
All DLG examinations (100%) were technically successful at visualizing anatomic variations or lymph reflux.
In 1 patient in group A, the lipiodol did not continue upward at the first lumbar vertebra. In the remaining 19 patients, reflux was observed in 3 patients in the left bronchomediastinal lymphatic trunk, 5 patients in the bilateral bronchomediastinal lymphatic trunk, 3 patients in the left subclavian lymphatic trunk, 1 patient in the right subclavian lymphatic trunk, 3 patients in the bilateral subclavian lymphatic trunk, 2 patients in the left jugular lymphatic trunk and 4 patients in the bilateral jugular lymphatic trunk.
In group B, 4 patients did not experience continued upward movement of lipiodol at the level of the 10th thoracic vertebra to the first lumbar vertebra. Among the remaining 37 patients, 7 had reflux in the left bronchomediastinal lymphatic trunk, 15 had reflux in the left subclavian lymphatic trunk, 1 had reflux in the bilateral subclavian lymphatic trunk, 14 had reflux in the left jugular lymphatic trunk, and 2 had reflux in the bilateral jugular lymphatic trunk. During lymphangiography, lipiodol leakage into the pleural cavity was observed in 2 patients.
In group C, 1 patient experienced lipiodol obstruction at the level of the second lumbar vertebra, and 1 patient experienced lipiodol obstruction at the level of the sixth thoracic vertebra. In the remaining 8 patients, reflux was observed in 2 patients with a left bronchomediastinal lymphatic trunk, 5 patients with a left subclavian lymphatic trunk, 4 patients with a left jugular lymphatic trunk, and 1 patient with a bilateral jugular lymphatic trunk.
The abnormal deposition of lipiodol in patients in groups A, B and C was as follows: Terminal thoracic duct in 14, 33 and 8 patients (P = 0.674); peritracheal region in 18, 15 and 8 patients (P < 0.001); pleura in 6, 33 and 9 patients (P < 0.001); pericardium in 6, 6 and 4 patients (P = 0.134); and hilum in 16, 11 and 7 patients (P < 0.001) (Figures 2-4).
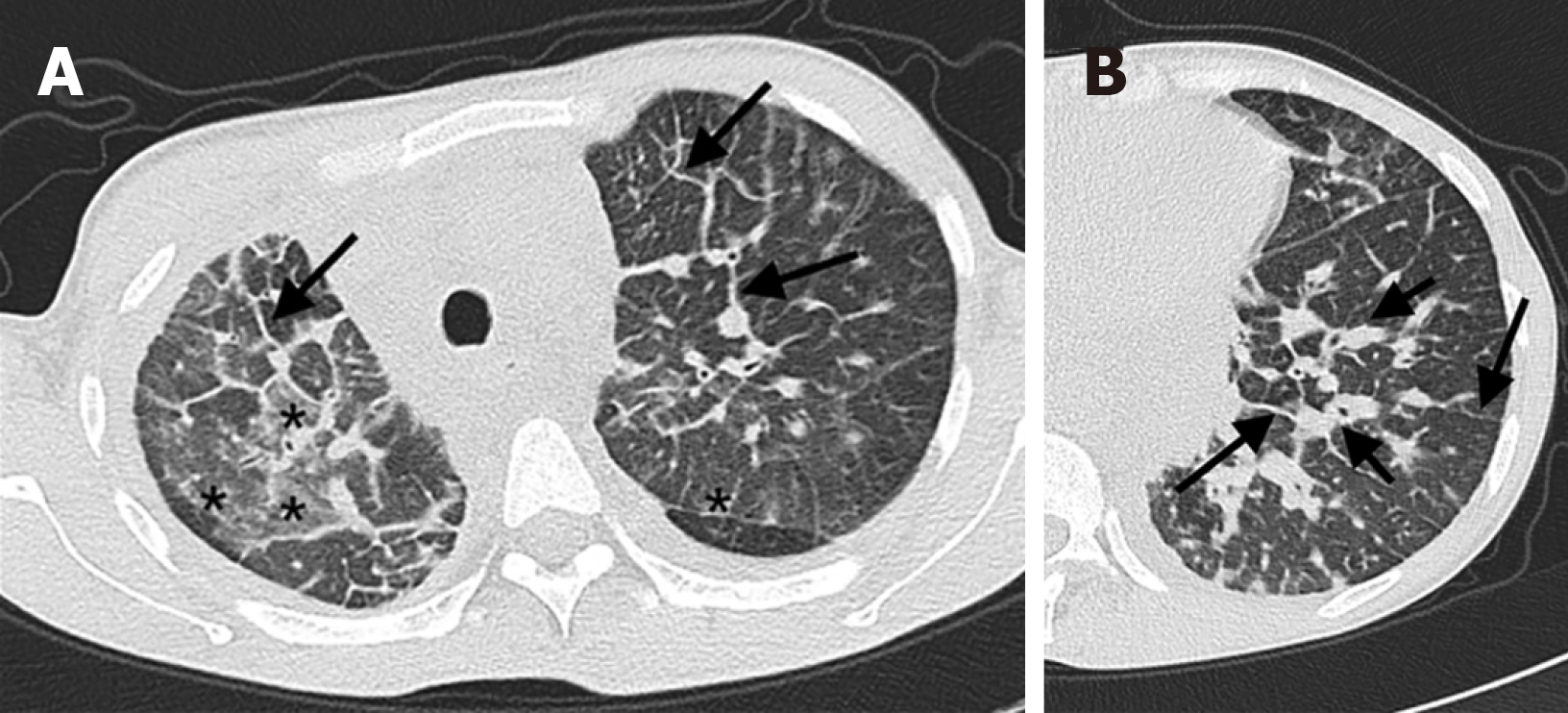
The abnormalities on conventional chest CT in the three groups were as follows: Ground-glass opacity in 19, 18 and 8 patients (P < 0.001); atelectasis in 4, 26 and 7 patients (P = 0.003); interlobular septal thickening in 12, 11 and 3 patients (P = 0.037); bronchovascular bundle thickening in 14, 6 and 4 patients (P < 0.001); mediastinal confined speckle or tubular shadow in 14, 14, and 7 patients (P = 0.012); diffuse mediastinal changes in 6, 5, and 3 patients (P = 0.146); cystic hypodense foci in the axilla in 2, 6, and 2 patients (P = 0.796); and cystic hypodense foci in the chest wall in 0, 2, and 2 patients (P > 0.999) (Figure 5).
The proportions of imaging features in the three groups of patients are detailed in Table 2.
| Imaging features | Group A (n = 20) | Group B (n = 41) | Group C (n = 10) | P value |
| Thoracic duct outlet obstruction | 14 (70.0) | 33 (80.5) | 8 (80.0) | 0.674 |
| Location of lymphatic reflux | ||||
| Peritracheal region | 18 (90.0)a | 15 (36.6)a | 8 (80.0) | < 0.001 |
| Pleura | 6 (30.0)a | 33 (80.5)a | 9 (90.0) | < 0.001 |
| Pericardium | 6 (30.0) | 6 (14.6) | 4 (40.0) | 0.134 |
| Hilum | 16 (80.0)a | 11 (26.8)a | 7 (70.0) | < 0.001 |
| Pulmonary abnormalities | ||||
| Ground-glass opacity | 19 (95.0)a | 18 (43.9)a | 8 (80.0) | < 0.001 |
| Atelectasis | 4 (20.0)a,b | 26 (63.4)a | 7 (70.0)b | 0.003 |
| Interlobular septal thickening | 12 (60.0)a | 11 (26.8)a | 3 (30.0) | 0.037 |
| Bronchovascular bundles thickening | 14 (70.0)a | 6 (14.6)a | 4 (40.0) | < 0.001 |
| Localized changes in mediastinum | 14 (70.0)a | 14 (34.1)a | 7 (70.0) | 0.012 |
| Diffuse changes in mediastinum | 6 (30.0) | 5 (12.2) | 3 (30.0) | 0.146 |
| Cystic lesions in the axilla | 2 (10.0) | 6 (14.6) | 2 (20.0) | 0.796 |
| Cystic lesions in the chest wall | 0 (0.0) | 2 (4.9) | 2 (4.9) | < 0.999 |
There were significant differences between groups A and B in the following presentations: Lipiodol deposition in the peritracheal region (P < 0.001), lipiodol deposition in the pleura (P < 0.001), lipiodol deposition in the hilum (P < 0.001), ground-glass opacity (P < 0.001), atelectasis (P < 0.001), interlobular septal thickening (P = 0.036), and bronchovascular bundle thickening (P < 0.001). These significant differences were also detected between groups A and C in terms of the following manifestations: Lipiodol deposition on the pleura (P = 0.006) and atelectasis (P = 0.045).
The pairwise comparison of patients between the three groups is shown in Table 2.
In our study, 61 of 71 patients underwent thoracic duct outlet surgery. Twenty patients in group A, 33 patients in group B, and 8 patients in group C underwent this operation. Among them, 12 patients in group A underwent chest CT reexamination 3-19 d after the operation, and the signs were alleviated in 10 patients, similar in 1 patient, and aggravated in 1 patient. In group B, 8 patients were reexamined with chest CT 4-10 d after the operation, and the chest CT signs were alleviated in 3 patients, similar in 1 patient, and aggravated in 4 patients. Twenty-seven patients in group C were reexamined via chest CT 5-9 d after the operation; 13 patients were relieved, 9 patients were similar, and 5 patients were worse than before.
In our study, we retrospectively analysed the clinical features, CTL findings, and conventional chest CT findings of patients with thoracic lymphatic reflux disorder. Lymphatic PB, primary chylothorax, and lymphatic PB with primary chylothorax showed no differences according to sex or age. There were some differences in clinical symptoms among the three groups. In addition, there were tortuous and dilated lymphatic vessels in the corresponding lesion sites in the three groups, and fistulas formed in the adjacent airway cavity or pleural cavity. This finding supports a common cause of the formation of lymphatic PB and chylothorax.
It has been reported in the literature[4,20-22] that both lymphatic PB and primary chylothorax are more prevalent in children and adolescents. Clinical symptoms, such as cough, sputum, chest tightness, and fever, are nonspecific. The incidence of lymphatic PB is slightly greater in males than in females, and the specific clinical manifestation is the formation of bronchial casts in the bronchi. There are no sex differences or specific clinical manifestations in patients with primary chylothorax. In our study, the mean ages of the patients in groups A, B, and C were all over 30 years, with a wide age distribution that was not limited to only children and adolescents. The ratios of male to female patients were 11:9, 16:25, and 5:5, respectively. There were no statistically significant differences in age or sex among the three groups. The above characteristics were different from those reported in the literature. In our study, there were statistically significant differences in cough (P < 0.001), sputum (P < 0.001), and haemoptysis (P = 0.027) between group A and group B. The differences in cough (P < 0.001) and sputum (P < 0.001) were statistically significant between group B and group C. There were no significant differences in chest tightness or fever among the three groups. The differences between the clinical symptoms of these diseases have not been reported in the literature.
In our study, lipiodol deposition at the thoracic duct outlet was not significantly different among the three groups: 14 patients (70.0%) in group A, 33 patients (80.5%) in group B, and 8 patients (80%) in group C (P = 0.674). The differences in lipiodol deposition around the bronchus and pleura were statistically significant among the three groups. There were statistically significant differences in abnormal lipiodol deposition around the bronchi and pleura among the three groups. Pairwise comparison revealed that there was more abnormal lipiodol deposition around the bronchi in group A (n = 18, 90.0%) than in group B (n = 15, 36.6%), and there was significantly more abnormal lipiodol deposition in the pleura in group B (n = 33, 80.5%) than in group A (n = 6, 30.0%). More importantly, 61 patients underwent thoracic duct outlet surgery in this study. We found that the dense fibrous tissue surrounding the thoracic duct outlet compressed the surrounding structures and obstructed the distal thoracic duct. When the obstruction was removed, the flow of lymph increased significantly. Combining the findings of the patients’ CTL and thoracic duct outlet surgery, we speculate that one of the aetiologies of lymphatic PB and primary chylothorax may be as follows: Thoracic duct obstruction leads to poor lymph return into the bloodstream, which results in slow lymph return or even reflux downstream. The lymph refluxes into the peritraceal lymphatics or pleural lymphatics via the bronchomediastinal lymphatic trunk and subclavian lymphatic trunk. Subsequently, the lymph infiltrates or leaks into the corresponding lumen, forming bronchial plastic substances or pleural effusion. This finding is similar to the findings of abnormal pulmonary lymphatic perfusion proposed by O’Leary et al[23]; they suggested that obstruction at the thoracic duct outlet or large branches results in the backflow of lymph from the thoracic duct into the mediastinum, lungs, pleura, and submucosa of the bronchi, which is the common cause of the formation of lymphatic PB and chylothorax.
In addition, in our study, the differences in pulmonary signs (ground-glass opacity, atelectasis, interlobular septal thickening, and bronchovascular bundle thickening) were statistically significant between group A and group B. The difference in atelectasis between group A and group C was also statistically significant. More ground-glass opacity, interlobular septal thickening, and bronchovascular bundle thickening were observed in group A than ingroup B. Atelectasis was more common in group B than in group A. These differences have not been reported in the literature. We speculate that 3 reasons may account for its occurrence: (1) Congenital tortuosity, dilatation, and hyperplasia of the pulmonary lymphatic vessels are more common in group A than in group B patients. This developmental abnormality can lead to thickening of interstitial lymphatic tissues, such as thickening of interlobular septa and bronchovascular bundles; (2) leakage of lymph into the bronchi, small branches, or even pulmonary acinus manifests as limited or diffuse ground-glass opacity in the lungs; and (3) patients in group A may cough up part of the tubular substances on their own, and the volume of the sputum may be reduced on admission. Thus, relatively few patients have obstructive pulmonary atelectasis. In contrast, compressive atelectasis caused by moderate to massive pleural effusion compression was more common in group B than in group B.
There are several limitations of our study. First, this was a retrospective study with a small sample size and possible selection bias. Further studies with larger sample sizes are needed in the future. Second, this study only explored primary chylothorax and did not include secondary patients. Future studies will explore this disease further. Finally, the study was a single-center study.
In conclusion, lymphatic PB and primary chylothorax are rare lymphatic drainage disorders. The physiological mechanisms of the two diseases have both similarities and differences. Similarities: Both are caused by obstruction of the thoracic duct, resulting in slow lymphatic reflux and even downstream reflux. However, the location of the lymph reflux differed between the two groups. CTL is an appropriate method for detecting abnormal lymphatic vessel positioning and pulmonary abnormalities. It can provide a crucial imaging basis for diagnosing lymphatic PB and primary chylothorax and provide information for treatment.
lymphatic PB and primary chylothorax are rare lymphatic drainage disorders. The physiological mechanisms of the two diseases have both similarities and differences. Similarities: Both are caused by obstruction of the thoracic duct, resulting in slow lymphatic reflux and even downstream reflux. Difference: The location of lymph reflux between the two is different. CTL is an appropriate method to detect abnormal lymphatic vessels position and pulmonary abnormalities. It can provide a crucial imaging basis for diagnosing lymphatic PB and primary chylothorax and provide information for treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade C
P-Reviewer: Tovichien P, Thailand S-Editor: Liu H L-Editor: A P-Editor: Chen YX
| 1. | Rubin BK. Plastic Bronchitis. Clin Chest Med. 2016;37:405-408. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Ntiamoah P, Mukhopadhyay S, Ghosh S, Mehta AC. Recycling plastic: diagnosis and management of plastic bronchitis among adults. Eur Respir Rev. 2021;30. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Agarwal S, Anderson BK, Mahajan P, Fernandes CJ, Margolin JF, Iacobas I. Sirolimus efficacy in the treatment of critically ill infants with congenital primary chylous effusions. Pediatr Blood Cancer. 2022;69:e29510. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Cholet C, Delalandre C, Monnier-Cholley L, Le Pimpec-Barthes F, El Mouhadi S, Arrivé L. Nontraumatic Chylothorax: Nonenhanced MR Lymphography. Radiographics. 2020;40:1554-1573. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Guo Y, Chen J, Xu B, Zheng Y, Shen K. Causes and manifestations of chylothorax in children in China: Experience from a children's medical center, 2007-2017. Pediatr Investig. 2018;2:8-14. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Riley LE, Ataya A. Clinical approach and review of causes of a chylothorax. Respir Med. 2019;157:7-13. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Tutor JD. Chylothorax in infants and children. Pediatrics. 2014;133:722-733. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Mehta I, Patel K. Lymphatic Plastic Bronchitis. N Engl J Med. 2022;386:e19. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Albitar HAH, Vassallo R. Lymphatic Plastic Bronchitis Secondary to Thoracic Duct Stenosis. Mayo Clin Proc. 2019;94:1141-1142. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Grönroos M, Palomäki A. Young adult with Gorham's disease presenting in an emergency department: a case report. J Med Case Rep. 2021;15:427. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Majdalany BS, Murrey DA Jr, Kapoor BS, Cain TR, Ganguli S, Kent MS, Maldonado F, McBride JJ, Minocha J, Reis SP, Lorenz JM, Kalva SP; Expert Panel on Vascular Imaging and Interventional Radiology. ACR Appropriateness Criteria(®) Chylothorax Treatment Planning. J Am Coll Radiol. 2017;14:S118-S126. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | An R, Xia S, Sun Y, Chang K, Li Y, Shen W. New application of direct lymphangiography in the diagnosis and treatment of chylothorax after lung cancer surgery: a case series. Ann Palliat Med. 2021;10:4768-4776. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Pan F, Loos M, Do TD, Richter GM, Kauczor HU, Hackert T, Sommer CM. The roles of iodized oil-based lymphangiography and post-lymphangiographic computed tomography for specific lymphatic intervention planning in patients with postoperative lymphatic fistula: a literature review and case series. CVIR Endovasc. 2020;3:79. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Kortes N, Radeleff B, Sommer CM, Bellemann N, Ott K, Richter GM, Kauczor HU, Stampfl U. Therapeutic lymphangiography and CT-guided sclerotherapy for the treatment of refractory lymphatic leakage. J Vasc Interv Radiol. 2014;25:127-132. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Jin D, Sun X, Shen W, Zhao Q, Wang R. Diagnosis of Lymphangiomatosis: A Study Based on CT Lymphangiography. Acad Radiol. 2020;27:219-226. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Dong J, Xin J, Shen W, Chen X, Wen T, Zhang C, Wang R. Unipedal Diagnostic Lymphangiography Followed by Sequential CT Examinations in Patients With Idiopathic Chyluria: A Retrospective Study. AJR Am J Roentgenol. 2018;210:792-798. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Patel S, Hur S, Khaddash T, Simpson S, Itkin M. Intranodal CT Lymphangiography with Water-soluble Iodinated Contrast Medium for Imaging of the Central Lymphatic System. Radiology. 2022;302:228-233. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Doerr CH, Allen MS, Nichols FC 3rd, Ryu JH. Etiology of chylothorax in 203 patients. Mayo Clin Proc. 2005;80:867-870. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Kesim S, Ozguven S, Oksuzoglu K, Erdil TY. Detection of Chyle Leakage Site With Lymphoscintigraphy SPECT/CT in a Patient With Chylothorax Secondary to T-Cell Lymphoblastic Leukemia. Clin Nucl Med. 2022;47:e59-e60. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Patel N, Patel M, Inja R, Krvavac A, Lechner AJ. Plastic Bronchitis in Adult and Pediatric Patients: A Review of its Presentation, Diagnosis, and Treatment. Mo Med. 2021;118:363-373. [PubMed] [Cited in This Article: ] |
| 21. | Soyer T, Yalcin Ş, Emiralioğlu N, Yilmaz EA, Soyer O, Orhan D, Doğru D, Sekerel BE, Tanyel FC. Use of serial rigid bronchoscopy in the treatment of plastic bronchitis in children. J Pediatr Surg. 2016;51:1640-1643. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | McGrath EE, Blades Z, Anderson PB. Chylothorax: aetiology, diagnosis and therapeutic options. Respir Med. 2010;104:1-8. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | O'Leary CN, Khaddash T, Nadolski G, Itkin M. Abnormal Pulmonary Lymphatic Flow on Novel Lymphangiographic Imaging Supports a Common Etiology of Lymphatic Plastic Bronchitis and Nontraumatic Chylothorax. Lymphat Res Biol. 2022;20:153-159. [PubMed] [DOI] [Cited in This Article: ] |