Published online Nov 26, 2023. doi: 10.12998/wjcc.v11.i33.7972
Peer-review started: September 13, 2023
First decision: September 28, 2023
Revised: October 11, 2023
Accepted: October 30, 2023
Article in press: October 30, 2023
Published online: November 26, 2023
Acute myelitis (AM) can lead to sudden sensory, motor and autonomic nervous dysfunction, which negatively affects their daily activities and quality of life, so it is necessary to explore optimization from a therapeutic perspective to curb the progression of the disease.
To investigate the effect of ganglioside (GM) combined with methylprednisolone sodium succinate (MPSS) on the curative effect and neurological function of patients with AM.
First, we selected 108 AM patients visited between September 2019 and September 2022 and grouped them based on treatment modality, with 52 patients receiving gamma globulin (GG) + MPSS and 56 patients receiving GM + MPSS, assigned to the control group (Con) and observation group (Obs), respectively. The thera
The Obs had: (1) A significantly higher response rate of treatment than the Con; (2) Higher scores of sensory and motor functions after treatment that were higher than the baseline (before treatment) and higher than the Con levels; (3) Lower incidence rates of skin rash, gastrointestinal discomfort, dyslipidemia, osteoporosis and other AEs; (4) Faster posttreatment recovery of sphincter function, limb muscle strength and ambulation; and (5) Markedly lower posttreatment IL-6, CRP and TNF-α levels than the baseline and the Con levels.
From the above, it can be seen that GM + MPSS is highly effective in treating AM, with a favorable safety profile comparable to that of GG + MPSS. It can significantly improve patients’ neurological function, speed up their recovery and inhibit serum IFs.
Core Tip: Acute myelitis (AM) is an autoimmune demyelinating disease in which patients may experience clinical symptoms such as difficult defecation, nerve root pain, lower limb paralysis, low-grade fever, and other symptoms that lead to limitations in daily life. This study mainly verified the clinical advantages of ganglioside + methylprednisolone sodium succinate in the treatment of AM, so as to provide timely and effective treatment for patients with AM and improve the condition and prognosis of patients.
- Citation: Sun YF, Liu LL, Jiang SS, Zhang XJ, Liu FJ, Zhang WM. Influence of ganglioside combined with methylprednisolone sodium succinate on efficacy and neurological function in patients with acute myelitis. World J Clin Cases 2023; 11(33): 7972-7979
- URL: https://www.wjgnet.com/2307-8960/full/v11/i33/7972.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i33.7972
Acute myelitis (AM), also known as acute transverse myelitis, is an acute focal inflammatory disease occurring in the spinal cord (SC) that can cause sudden sensory, motor and autonomic dysfunction, reducing daily mobility and quality of life[1,2]. The nosogenesis of the disease is complex and diverse, as a variety of autoimmune reactions and infectious agents, such as herpesvirus, enterovirus, and varicella zoster virus, can be contributing factors[3]. According to epidemiological data, the disease mostly occurs in young and middle-aged groups, and its incidence is rising[4,5]. Prompt treatment of AM can help protect neurological function and prevent the disease from further progressing[6]. Our research mainly seeks effective treatment strategies for AM patients, which would carry great clinical implications for improving the condition of these patients and speeding up their recovery.
Ganglioside (GM) is mainly found in the brain tissues of all mammals and can help the molecular recognition of various glycan-binding proteins and mediate the activity of plasma membrane proteins through transverse binding, thus playing a regulatory role in the body’s neurodevelopment, differentiation and pathological changes[7,8]. In one animal experiment, intrathecal administration of GM showed significant therapeutic efficacy against bupivacaine-associated nerve injury and torsion dysfunction compared to the intravenously administered route[9]. Methylprednisolone sodium succinate (MPSS) is an anti-inflammatory corticosteroid that is beneficial to the recovery of damaged SCs[10] and can protect SC function by inhibiting lipid peroxidation and avoiding ischemia-induced tissue damage[11]. Research on the effect of GM + MPSS has been limited. This study mainly aimed to fill this research gap, seeking a new clinical exploration for the improvement of the condition and symptom recovery of patients with AM.
One hundred eight AM patients were selected the Affiliated Hospital of Qingdao University between September 2019 and September 2022 as the research participants, including 52 patients in the control group (the Con) and 56 patients in the observation group (the Obs), who were given gamma globulin (GG) + MPSS and GM + MPSS, respectively. The two patient cohorts showed no differences in age, sex, onset time or other general data (P > 0.05).
The eligible patients met all of the following criteria: AM as confirmed by spinal magnetic resonance imaging and cerebrospinal fluid examination[12]; no other treatment measures taken in the past six months; stable vital signs with clear consciousness; complete medical records; and willingness to cooperate with the research.
Patients were excluded if they met any of the following criteria: Heart, lung, or kidney dysfunction/disease; auto
The medication regimen GG + MPSS was given to the Con group. Patients received intravenous injections of 10 g of GG and 250 mL of 5% glucose once a day. MPSS (1000 mg) and 5% glucose injection (250 mL) were given intravenously for 4 wk. The medication regimen for the Obs was GM + MPSS. Patients were given an intravenous drip of monosialotetrahexosylganglioside sodium for injection (100 mg) and 5% glucose injection (250 mL) once a day; MPSS (1000 mg) and 5% glucose (250 mL) were injected intravenously once daily for 4 wk.
Curative effect: The clinical symptoms and recovery of the two groups of patients (before vs after treatment) were compared and analyzed, which were used as the evaluation criteria of the treatment effectiveness. Cured: The patients recovered nearly fully in terms of limb sensation and muscle strength and could take care of themselves with complications that disappeared; marked response: The patients had improved limb sensation, muscle strength and sphincter function and mostly controlled complications; response: Limb sensation and muscle strength improved, and the complications were gradually controlled; nonresponse: There was no change or even worsening of limb sensation and symptoms.
Neurological function: The recovery of patients’ nerve function was evaluated by referring to the International Standards for Neurological Classification of Spinal Cord Injury (SCI)[13], mainly by calculating the scores of sensory function and motor function, with scores ranging from 0 to 50 that were in direct proportion to the recovery degree of nerve function.
Occurrence of adverse events: The incidence of adverse events (AEs) was counted by observing and recording the cases of skin rash, gastrointestinal discomfort, dyslipidemia (DL), and osteoporosis (OS) in the two groups.
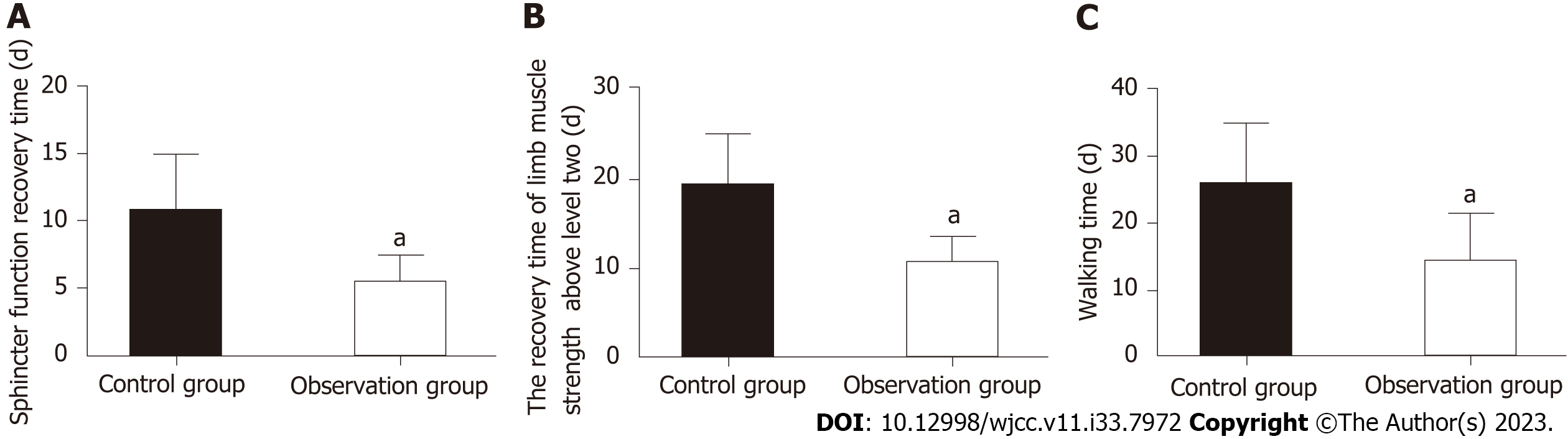
Recovery: Three clinical indices, namely, time to sphincter function recovery, time to recovery of limb muscle strength above grade 2, and time to ambulation, were recorded.
Inflammatory factors: On venous blood drawn from both cohorts before and after treatment, we performed enzyme-linked immunosorbent assay (ELISA)[14] to quantify the levels of inflammatory factors (IFs) such as interleukin (IL)-6, C-reactive protein (CRP) and tumor necrosis factor (TNF)-α.
The measurement data, statistically described by mean ± SEM, were compared between groups by the independent samples t test and within groups before and after treatment by the paired t test. The count data are denoted by n (%), and the comparison between the two groups of counting data was made by χ2-test. The collected experimental data were analyzed by SPSS 21.0, and the figures were made in GraphPad Prism 7.0. Differences were significant when P < 0.05.
Age, sex, onset time, lower limb muscle strength, marital status and other general data were similar between the two patient cohorts (P > 0.05; Table 1).
| Factors | Control group (n = 52) | Observation group (n = 56) | χ2/t | P value |
| Age (yr) | 43.85 ± 8.23 | 42.98 ± 8.30 | 0.546 | 0.586 |
| Sex (male/female) | 28/24 | 33/23 | 0.283 | 0.595 |
| Time of onset (d) | 3.06 ± 0.46 | 3.20 ± 0.62 | 1.324 | 0.188 |
| Lower limb muscle strength (grade 0-1/grade 2-3) | 26/26 | 30/26 | 0.138 | 0.711 |
| Marital status (married/single) | 35/17 | 29/27 | 2.691 | 0.101 |
The Obs had a higher overall response rate (ORR) (calculated as the percentage of the sum of cured, marked response and response in all cases) than the Con (89.29% vs 73.08%; P < 0.05; Table 2).
| Factors | Control group (n = 52) | Observation group (n = 56) | χ2 | P value |
| Cured | 17 (32.69) | 24 (42.86) | - | - |
| Marked response | 14 (26.92) | 20 (35.71) | - | - |
| Response | 7 (13.46) | 6 (10.71) | - | - |
| Nonresponse | 14 (26.92) | 6 (10.71) | - | - |
| Total | 38 (73.08) | 50 (89.29) | 4.695 | 0.030 |
By evaluating the scores of sensory and motor functions of AM patients in the two groups, we found that there was no significant difference between them before treatment (P > 0.05). Both scores increased in both groups after treatment, with significantly higher scores in the Obs (P < 0.05; Figure 1).
We observed and counted AEs such as rash, gastrointestinal discomfort, DL and OS and found that their total incidence was lower in the Obs group than in the Con group (7.14% vs 21.15%; P < 0.05; Table 3).
| Factors | Control group (n = 52) | Observation group (n = 56) | χ2 | P value |
| Rash | 2 (3.85) | 1 (1.79) | - | - |
| Gastrointestinal discomfort | 2 (3.85) | 1 (1.79) | - | - |
| Dyslipidemia | 4 (7.69) | 2 (3.57) | - | - |
| Osteoporosis | 3 (5.77) | 0 (0.00) | - | - |
| Total | 11 (21.15) | 4 (7.14) | 4.426 | 0.035 |
By evaluating the time to sphincter function recovery, time to limb muscle strength recovery above grade 2 and time to ambulation, we found that the recovery time of the above three aspects was significantly shorter in the Obs group than in the Con group (P < 0.05; Figure 2).
Three IFs, namely, IL-6, CRP, and TNF-α, were detected by ELISA in two groups of patients with AM. The three indices were not significantly different before treatment between groups (P > 0.05). Their posttreatment levels were markedly reduced in both cohorts, all three being significantly lower in the Obs (P < 0.05; Figure 3).
AM is a common and rapidly occurring neurological disorder that is essentially an autoimmune demyelinating condition[15]. It can lead to clinical symptoms such as difficulty urinating and defecating, nerve root pain, lower limb paralysis, and low fever, which seriously worsen patients’ everyday lives[16,17]. The early pathological changes of AM involve SC shock, increased muscle tone, active tendon reflex, etc. In severe cases, complications such as pressure sores and pulmonary and urinary tract infections may occur[18,19]. Therefore, providing timely and effective treatment to AM patients is of great significance to curb disease development and improve patient prognosis.
Our research data showed that the ORRs of Obs and Con were 89.29% and 73.08%, respectively. The significantly higher ORR in the Obs suggests that AM patients receiving GM + MPSS have obvious advantages in symptom recovery, self-care and complication control. GM is a structural component of the human nerve cell membrane that can not only mediate the growth, repair and reconstruction of damaged cranial nerves but also effectively modulate brain nerve conduction and the activities of various enzymes in cell membranes[20]. As a systemic immunosuppressive drug, MPSS can not only inhibit SCI-associated neuroinflammation through its immunomodulatory function but also avoid systemic immune responses, which may help explain its therapeutic mechanism in AM[21]. From the aspects of sensory and motor functions, the neurological function of the two groups was evaluated. The Obs were found to have obviously elevated sensory and motor function scores after treatment that were higher than the baseline and the Con, indicating that GM + MPSS used in the Obs was more beneficial to sensory and motor function recovery. In the study by Shen et al[22], the recovery of GM on neurons of SCI rats seemed to be linked to the increased secretion of GM in rat SC after CXCL14 silencing. A clinical study pointed out that MPSS can enhance the neurological function and activities of daily living of patients with acute SCI and cauda equina injury with sensory and motor dysfunction[23]. Our results of three recovery indices revealed that the times to sphincter function recovery, limb muscle strength recovery above grade 2 and ambulation were significantly shorter in the Obs group than in the Con group, suggesting that GM + MPSS was helpful in promoting the functional recovery of sphincters, limb muscle strength and ambulation in AM patients. Zhai et al[24] suggested that GM plus systematic rehabilitation training for SC patients is more conducive to limb function rehabilitation. In our safety evaluation, we found that the incidences of AEs such as rash, gastrointestinal discomfort, DL and OS in the Obs group were significantly lower than those in the Con group (7.14% vs 21.15%), indicating that GM + MPSS is safer for AM patients. Finally, we used ELISA to detect IFs. The posttreatment IL-6, CRP and TNF-α levels were markedly reduced in the Obs compared with the baseline and Con groups, demonstrating that GM + MPSS can inhibit excessive inflammation in AM patients. As reported by Hu et al[25], GM can significantly lower IL-1β, IL-6, TNF-α and other inflammatory proteins in the SC tissue of SCI rats, similar to our results. Consistent with these findings, Schmidt et al[26] found that MPSS can significantly inhibit the secretion of systemic inflammatory cytokines such as CRP and TNF-α in patients undergoing liver resection.
There are several areas in this study that need further improvement. First, since this is a small sample single-center analysis, it is necessary to expand the sample range and sample size in the future to improve the accuracy of the study results and to minimize or even avoid the bias of information collection. Second, the addition of follow-up analysis will enable in-depth evaluation of the long-term efficacy of GM + MPSS in the treatment of AM. Third, basic experiments related to GM + MPSS treatment of AM should be supplemented, which will be conducive to revealing the underlying mechanisms. In the future, we will make improvements based on the above points.
GM + MPSS can enhance the curative effect, neurological function, and functional recovery of patients’ perception, movement, sphincter function, limb muscle strength and ambulation, with favorable safety and anti-inflammatory action. These findings provide novel insight and clinical reference for the management and treatment of patients with AM.
Acute myelitis (AM) can cause sudden sensory, motor and autonomic nervous dysfunction in patients, which negatively affects their daily activities and quality of life. Therefore, it is necessary to optimize exploration from a therapeutic perspective to curb the progression of the disease.
It is necessary to optimize the therapeutic strategy to improve the clinical outcomes of AM patients.
In this research, the effect of ganglioside (GM) combined with methylprednisolone sodium succinate (MPSS) on the curative effect and neurological function of patients with AM was investigated.
Of the 108 AM patients selected, 52 cases were treated with gamma globulin plus MPSS (control group) and 56 cases were treated with GM plus MPSS (observation group). The two groups were then comparatively analyzed from the following perspectives: Efficacy, neurological function (sensory and motor function scores), occurrence of adverse events, recovery (time to sphincter function recovery, limb muscle strength recovery above grade 2, and ambulation), and inflammatory factors [interleukin-6 (IL-6); C-reactive protein (CRP); tumor necrosis factor-α (TNF-α)].
The treatment efficacy and sensory and motor function scores of the observation group were significantly higher than those of the control group, while the total incidence of adverse events such as rash, gastrointestinal discomfort, dyslipidemia and osteoporosis, as well as recovery indexes such as the time to sphincter function recovery, limb muscle strength recovery above grade 2, and ambulation was significantly lower. In addition, IL-6, CRP, and TNF-α levels reduced markedly in the observation group after treatment, significantly lower than the baseline and those of the control group.
GM combined with MPSS shows significant advantages in enhancing efficacy and nerve function in patients with AM, accelerating recovery, inhibiting serum inflammation, and improving safety.
Our findings may provide new insights and clinical references for the management and treatment of patients with AM.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Konstantinopoulos PA, United States; Kuroda K, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Zhang YL
| 1. | Vegezzi E, Ravaglia S, Buongarzone G, Bini P, Diamanti L, Gastaldi M, Prunetti P, Rognone E, Marchioni E. Acute myelitis and ChAdOx1 nCoV-19 vaccine: Casual or causal association? J Neuroimmunol. 2021;359:577686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Prete S, McShannic JD, Fertel BS, Simon EL. Acute transverse myelitis progressing to permanent quadriplegia following COVID-19 infection. Am J Emerg Med. 2022;56:391.e1-391.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Águila-Gordo D, Manuel Flores-Barragán J, Ferragut-Lloret F, Portela-Gutierrez J, LaRosa-Salas B, Porras-Leal L, Carlos Villa Guzmán J. Acute myelitis and SARS-CoV-2 infection. A new etiology of myelitis? J Clin Neurosci. 2020;80:280-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Hwang M, Flanagan A, Graf A, Kruger KM, Scullion N, Tayne S, Altiok H. Gait Characteristics in Youth With Transverse Myelitis. Top Spinal Cord Inj Rehabil. 2021;27:38-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Abbatemarco JR, Galli JR, Sweeney ML, Carlson NG, Samara VC, Davis H, Rodenbeck S, Wong KH, Paz Soldan MM, Greenlee JE, Rose JW, Delic A, Clardy SL. Modern Look at Transverse Myelitis and Inflammatory Myelopathy: Epidemiology of the National Veterans Health Administration Population. Neurol Neuroimmunol Neuroinflamm. 2021;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Gupta A, Kumar SN, Taly AB. Neurological and functional recovery in acute transverse myelitis patients with inpatient rehabilitation and magnetic resonance imaging correlates. Spinal Cord. 2016;54:804-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Schnaar RL. The Biology of Gangliosides. Adv Carbohydr Chem Biochem. 2019;76:113-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | Ohmi Y, Ohkawa Y, Tajima O, Sugiura Y, Furukawa K. Ganglioside deficiency causes inflammation and neurodegeneration via the activation of complement system in the spinal cord. J Neuroinflammation. 2014;11:61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Ji J, Yan X, Li Z, Lai Z, Liu J. Therapeutic effects of intrathecal versus intravenous monosialoganglioside against bupivacaine-induced spinal neurotoxicity in rats. Biomed Pharmacother. 2015;69:311-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Kamalov MI, Đặng T, Petrova NV, Laikov AV, Luong D, Akhmadishina RA, Lukashkin AN, Abdullin TI. Self-assembled nanoformulation of methylprednisolone succinate with carboxylated block copolymer for local glucocorticoid therapy. Colloids Surf B Biointerfaces. 2018;164:78-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Fehlings MG, Tetreault LA, Wilson JR, Kwon BK, Burns AS, Martin AR, Hawryluk G, Harrop JS. A Clinical Practice Guideline for the Management of Acute Spinal Cord Injury: Introduction, Rationale, and Scope. Global Spine J. 2017;7:84S-94S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 158] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 12. | Absoud M, Greenberg BM, Lim M, Lotze T, Thomas T, Deiva K. Pediatric transverse myelitis. Neurology. 2016;87:S46-S52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Kirshblum S, Snider B, Rupp R, Read MS; International Standards Committee of ASIA and ISCoS. Updates of the International Standards for Neurologic Classification of Spinal Cord Injury: 2015 and 2019. Phys Med Rehabil Clin N Am. 2020;31:319-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 14. | Konstantinou GN. Enzyme-Linked Immunosorbent Assay (ELISA). Methods Mol Biol. 2017;1592:79-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 15. | Rodríguez Y, Rojas M, Pacheco Y, Acosta-Ampudia Y, Ramírez-Santana C, Monsalve DM, Gershwin ME, Anaya JM. Guillain-Barré syndrome, transverse myelitis and infectious diseases. Cell Mol Immunol. 2018;15:547-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 16. | Fukuoka M, Kuki I, Kawawaki H, Kim K, Hattori Y, Tsuji H, Horino A, Nukui M, Okazaki S. A pediatric patient of hemorrhagic acute transverse myelitis. Brain Dev. 2017;39:252-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Merchan-Del Hierro X, Halalau A. Cytomegalovirus-related transverse myelitis in an immunocompetent host: a subacute onset of an immune-mediated disease? BMJ Case Rep. 2017;2017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | New PW, Astrakhantseva I. Rehabilitation outcomes following infections causing spinal cord myelopathy. Spinal Cord. 2014;52:444-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Tandon R, Kumar A. Long-Segment Myelitis, Meningoencephalitis, and Axonal Polyneuropathy in a Case of Scrub Typhus. Ann Indian Acad Neurol. 2019;22:237-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Wang J, Zhang Q, Lu Y, Dong Y, Dhandapani KM, Brann DW, Yu RK. Ganglioside GD3 is up-regulated in microglia and regulates phagocytosis following global cerebral ischemia. J Neurochem. 2021;158:737-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Chio JCT, Xu KJ, Popovich P, David S, Fehlings MG. Neuroimmunological therapies for treating spinal cord injury: Evidence and future perspectives. Exp Neurol. 2021;341:113704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Shen J, Gao F, Zhao L, Hao Q, Yang YL. MicroRNA-34c promotes neuronal recovery in rats with spinal cord injury through the C-X-C motif ligand 14/Janus kinase 2/signal transducer and activator of transcription-3 axis. Chin Med J (Engl). 2020;133:2177-2185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Zeng Y, Xiong M, Yu H, He N, Wang Z, Liu Z, Han H, Chen S. [Clinical effect of methylprednisolone sodium succinate and mouse nerve growth factor for injection in treating acute spinal cord injury and cauda equina injury]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2010;24:1208-1211. [PubMed] [Cited in This Article: ] |
| 24. | Zhai HW, Gong ZK, Sun J, Chen W, Zhang M, Zhou JJ, Zheng B. Ganglioside with nerve growth factor for the recovery of extremity function following spinal cord injury and somatosensory evoked potential. Eur Rev Med Pharmacol Sci. 2015;19:2282-2286. [PubMed] [Cited in This Article: ] |
| 25. | Hu H, Wang H, Liu W. Effect of ganglioside combined with Chip Jiaji electro-acupuncture on Nogo-NgR signal pathway in SCI rats. Saudi J Biol Sci. 2021;28:4132-4136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Schmidt SC, Hamann S, Langrehr JM, Höflich C, Mittler J, Jacob D, Neuhaus P. Preoperative high-dose steroid administration attenuates the surgical stress response following liver resection: results of a prospective randomized study. J Hepatobiliary Pancreat Surg. 2007;14:484-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |