Published online Jan 6, 2023. doi: 10.12998/wjcc.v11.i1.150
Peer-review started: September 1, 2022
First decision: October 24, 2022
Revised: October 27, 2022
Accepted: December 21, 2022
Article in press: December 21, 2022
Published online: January 6, 2023
Nesidioblastosis usually refers to a series of clinical manifestations caused by the proliferation of β-cells in pancreatic islets, and these clinical manifestations are hyperinsulinemia and persistent hypoglycemia. According to the size of the lesion, nesidioblastosis is divided into focal nesidioblastosis, diffuse nesidioblastosis and atypical nesidioblastosis, and its pathogenesis is still unclear. Nesidioblastosis is mainly seen in infants and rarely reported in adults, especially focal nesidioblastosis, which is difficult to distinguish from insulinoma.
We report a case of adult focal β-cell nesidioblastosis in which the preoperative diagnosis was insulinoma. The patient was a 48-year-old male who suffered from repeated morning and fasting palpitations, sweating, and severe disturbance of consciousness for 5 years. His blood glucose was found to be as low as 1.79 mmol/L during an attack. However, abdominal computed tomography showed no abnormalities. Magnetic resonance imaging and endoscopic ultrasonography demonstrated a nodular mass in the head of the pancreas, combined with hyperinsulinemia and high serum C-peptide. The patient was diagnosed with insulinoma and underwent Beger surgery; however, the postoperative pathological results showed nesidioblastosis.
Although surgical resection is the preferred option for nesidioblastosis, some cases can be treated non-surgically. In order to increase clinicians' understanding of nesidioblastosis, it is necessary to review the pathogenesis, diagnosis and treatment of this disease.
Core Tip: Focal nesidioblastosis is a neuroendocrine disease which is rarely reported in adults and easily confused with insulinoma, and its pathogenesis is still unclear. We report a rare case of adult focal β-cell nesidioblastosis, which was diagnosed as insulinoma before surgery. Increased glucose level after resection of the lesion may assist in the diagnosis of focal β-cell nesidioblastosis, due to intra-pancreatic regulation in focal nesidioblastosis. We also review the related literature. This case report may enhance clinicians' understanding of this disease.
- Citation: Tu K, Zhao LJ, Gu J. Adult focal β-cell nesidioblastosis: A case report. World J Clin Cases 2023; 11(1): 150-156
- URL: https://www.wjgnet.com/2307-8960/full/v11/i1/150.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i1.150
Nesidioblastosis usually refers to a series of clinical manifestations caused by the proliferation of α cells or β cells in pancreatic islets. Pathologically, islet structure is normal, but islet cells show hypertrophy, with pleomorphic changes in the nucleus, an increased and transparent cytoplasm, and some pancreatic endocrine cells sprout on pancreatic microducts[1]. The clinical manifestations of β-cell nesidioblastosis are hyperinsulinemia and persistent hypoglycemia. According to the size of the lesion, β-cell nesidioblastosis is divided into focal nesidioblastosis, diffuse nesidioblastosis and atypical nesidioblastosis[2]. We report a case of adult focal β-cell nesidioblastosis in which the preoperative diagnosis was insulinoma, and review the possible pathogenesis, imaging manifestations, treatment and progression of β-cell nesidioblastosis.
A 48-year-old male patient suffered from repeated morning and fasting palpitations, sweating, and severe disturbance of consciousness for 5 years.
Five years ago, the patient suffered from palpitation and sweating in the morning or after starvation, which were relieved after food eating.
During the 5 years, these symptoms occurred repeatedly, with an increasing frequency year by year. More than 10 attacks and two episodes of comas occurred last year. After glucose injection, his symptoms were alleviated. During the attacks, the patient had no abdominal pain, distension and other discomfort. The patient's weight increased by about 10 kg over the past 5 years, and there was no obvious abnormality in urine and stool tests.
There was no specific history.
All family members were healthy and denied any history of genetic disease and genetic predisposition.
Nervous system, cardiopulmonary and abdominal examinations showed no positive signs.
Blood glucose was measured to be as low as 1.79 mmol/L during an attack, which was relieved after oral food or glucose supplementation. Blood pressure was 160/80 mmHg after admission, and the patient’s body mass index was 27.064 kg/m2. Blood tests showed that there were no obvious abnormalities in relation to liver function and kidney function. Fasting serum insulin was 219.3 μIU/mL (normal value 2.6-24.9 μIU/mL), and fasting C-peptide was 6440 pmol/L (normal value 370-1470 pmol/L).
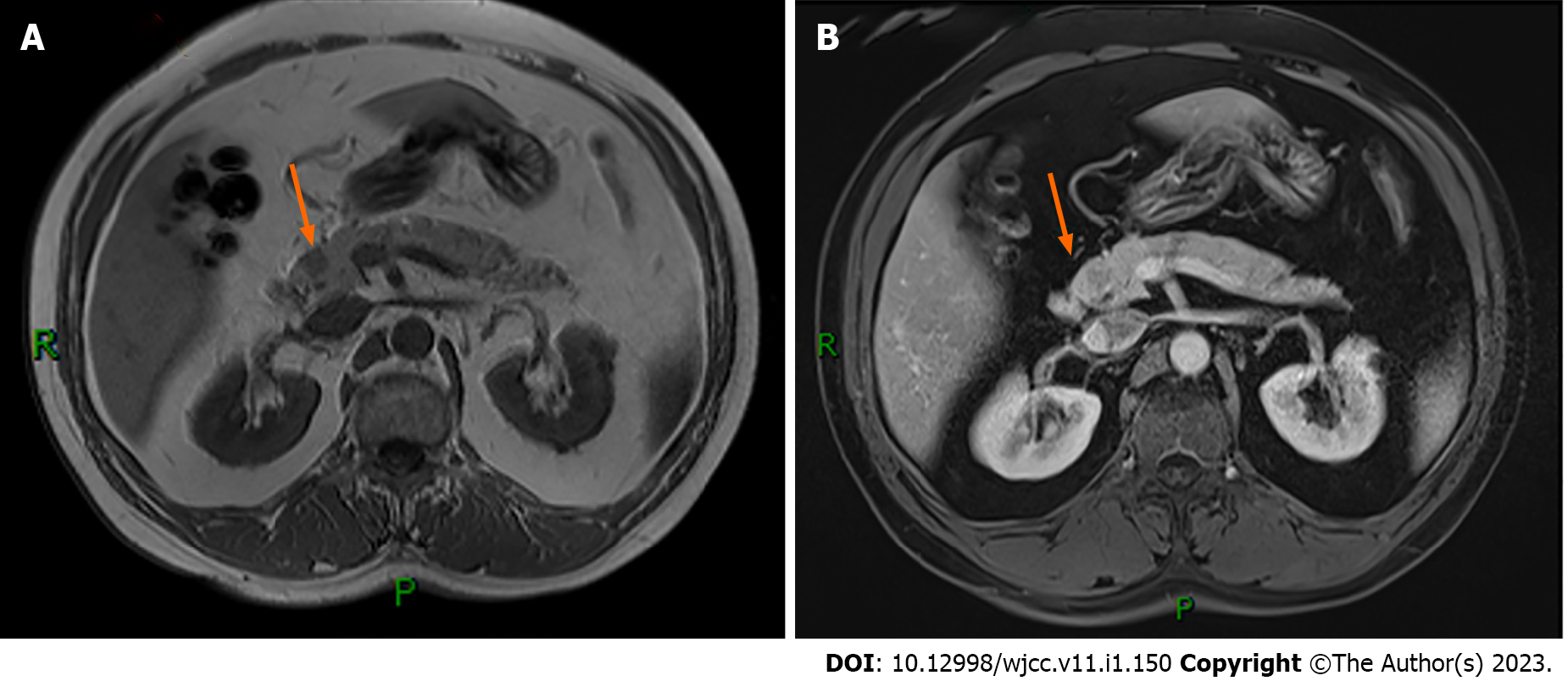
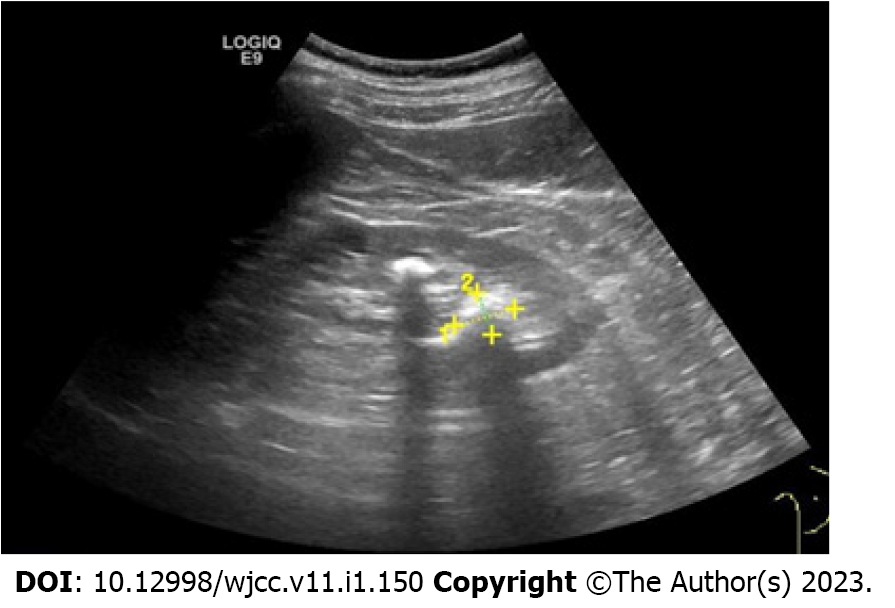
Abdominal computed tomography (CT) showed no abnormalities. Abdominal magnetic resonance imaging (MRI) revealed a small, slightly long T1 signal nodule in the head of the pancreas, about 10 mm × 12 mm in size, with a clear boundary and mild enhancement on the enhanced scan, suggesting a tumor (Figure 1). Endoscopic ultrasonography suggested a hypoechoic lesion in the head of the pancreas, with a hyperechoic nodule about 1 cm × 1.4 cm in size, which was thought to be insulinoma (Figure 2). Positron emission tomography (PET)-CT showed that 68GA-DOTA-TATE was increased in the head of the pancreas, suggesting a possible neuroendocrine tumor.
Based on the above clinical data of the Whipple triad, significantly increased insulin and C-peptide, and imaging suggesting pancreatic head occupation, the patient was considered to have an insulinoma.
After obtaining consent from the patient and his family, he underwent Beger surgery (duodenum preserving resection of the head of the pancreas).
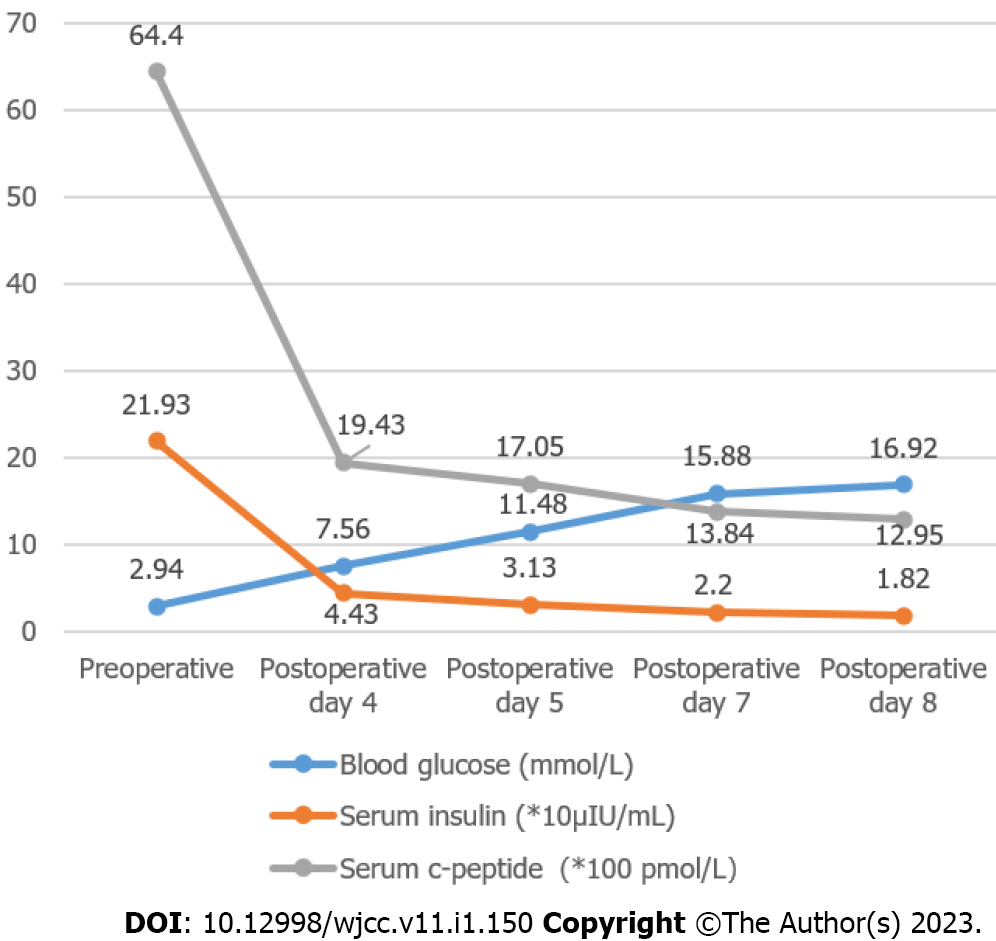
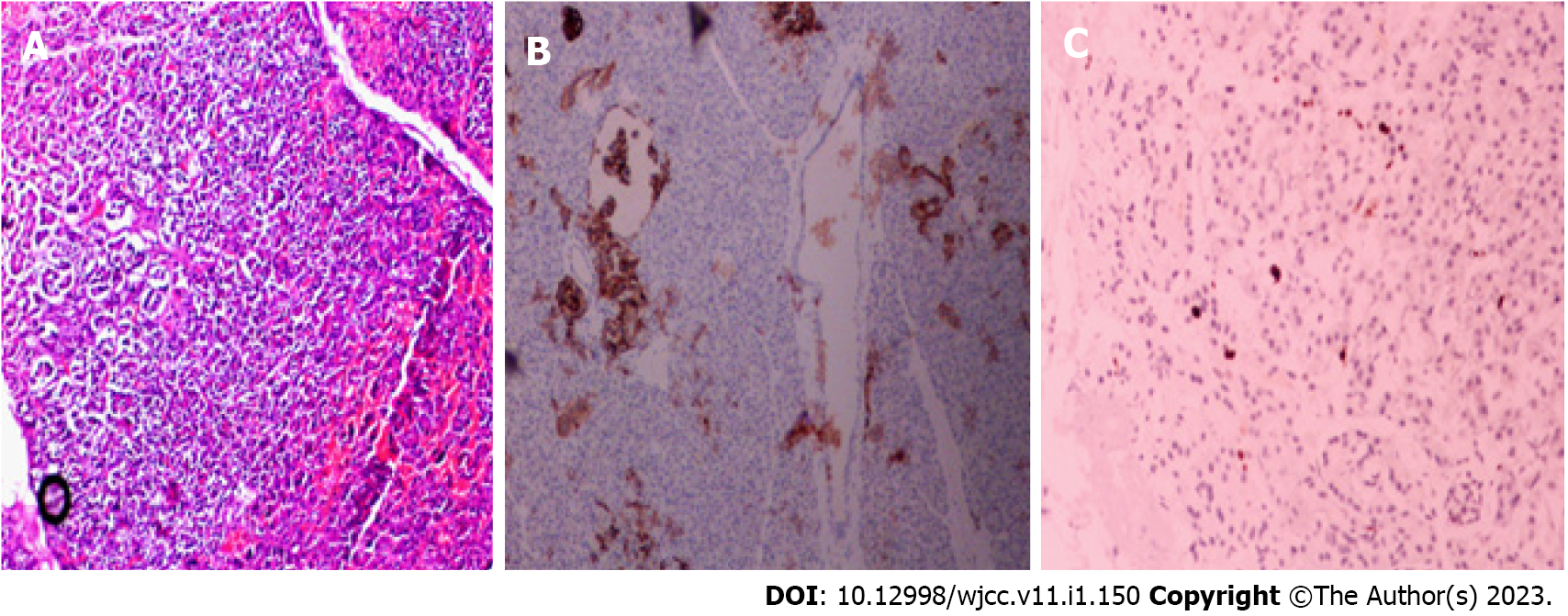
Two weeks after surgery, the patient did not have hypoglycemia, and serum insulin and C-peptide gradually returned to normal, no significant change in weight. Postoperative changes in blood glucose, insulin and C-peptide are shown in Figure 3; however, the postoperative pathological results suggested nesidioblastosis, and immunohistochemistry showed CK (+), Syn (+), MGMT (+), CD56 (-), CgA (-), Insulin (-), Ki-67 approximately 2% (+), and SSTR2 (0) (Figure 4). Unfortunately, after the patient left the hospital two weeks after surgery, he could not be contacted again.
Nesidioblastosis was coined by Laidlaw in 1938, its pathological characteristics are islet hyperplasia, endocrine cell dysplasia, islet cell adenomatosis, and ductoinsular proliferation[2]. It is mainly seen in infants with an incidence of between approximately 1 in 25000 to 50000, and focal nesidioblastosis accounts for 30%-40%[3]. It has rarely been reported in adults, especially focal nesidioblastosis, but precise figures do not exist.
In infant nesidioblastosis, mutations in gene ABCC8 coding for the sulfonylurea receptor and gene KCNJ11 coding for inward rectifying K+ channels are thought to be the main causes of the disease, and these mutations disable the function of the β-cell membrane ATP-sensitive K+ (KATP) channels which results in channel depolarization and inappropriate insulin secretion[4]. The main cause of focal nesidioblastosis is the mutation of paternally inherited ABCC8 and the somatic loss of the maternal 11p allele involving the ABCC8 and KCNJ11 region[5]. Through the detection of proinsulin mRNA and proinsulin in focal nesidioblastosis lesions, it was found that proinsulin mRNA was similar to that in adjacent islets but the level of proinsulin was higher than that in adjacent islets, which indicated that the mutation of these genes effect the level of translation and not transcription[6]. Other metabolic disorders can also result in nesidioblastosis, such as glycogen storage disease, by activating mutations of the mono
Nesidioblastosis is a rare disease, the clinical presentation is similar to that of insulinoma, and both cause persistent hypoglycemia and the Whipple triad. Therefore, it is difficult to diagnose nesidioblastosis in adults on the basis of clinical features, and the final diagnosis relies on pathologic analysis of the pancreatic tissue. Conventional imaging has limited sensitivity, especially for diffuse nesidioblastosis in which histological structure is similar to normal pancreatic tissue and lesions spread throughout the pancreas. For focal nesidioblastosis, conventional imaging sensitivity may be similar to the sensitivity to insulinoma: arterial calcium stimulation and venous sampling (ASVS) (85%), endoscopic ultrasonography (EUS) (76%), MRI (58%) and CT (54%)[10]. Despite the high positive rate of ASVS, its limited localization and invasiveness has restricted its clinical application. For some types of nesidioblastosis which highly express glucagon-like peptide-1 receptors (GLP-1R), they can be visualized by PET/CT with GLP-1R radiolabeled analogue (68Ga-DOTA-exendin-4) (Exendin). Victor Kalff et al[11] in a retrospective study found that Exendin correctly identified diffuse nesidioblastosis in 2/3 cases requiring partial pancreatectomy for hypoglycemia control. Some studies have even suggested that the sensitivity of 68 Ga-DOTA-exendin-4 PET/CT was higher than 97%[7], and considerably higher than SPECT/CT (67.5%) and MRI (67.6%)[10]. However, there were a number of false-negative readings, and nesidioblastosis needs to be distinguished from peripancreatic uptake and insulinoma. Despite the high accuracy, this method is expensive and injection of Exendin can cause severe hypoglycemia and nausea[12]; therefore, the first step in diagnosing the disease is a comprehensive analysis of the clinical history and full biochemical characterization, when 68 Ga-DOTA-exendin-4 PET/CT is positive, endoscopic ultrasound and MRI can be used to confirm the location.
At present, the most effective treatment for adult nesidioblastosis is total or partial surgical resection of the pancreas. Some patients can achieve clinical cure, especially those with focal nesidioblastosis, but partial pancreatectomy is only beneficial in 50% of nesidioblastosis patients and carries a high risk of complications, such as pancreatic fistula, postoperative bleeding, infection, and diabetes. According to the 2016 International Study Group on Pancreatic Surgery data, the dreaded complication of pancreatic fistula still ranges between 3%-45% following pancreatic surgery at high volume centers[13] and a study reported that 40% of patients developed insulin-dependent diabetes after near-total pancreatectomy[14]. It is not possible to predict recurrence of hypoglycemia in adult patients with nesidioblastosis. Medications are also used in the treatment of nesidioblastosis, and the first-line drug is diazoxide. A study researching diazoxide concentrations in maternal and infant blood showed that diazoxide had no harmful effects in infants[15], but its side effects and ineffectiveness in some forms have limited its use in clinical application[16]. The second-line drugs are somatostatin analogues, including octreotide and lanreotide. Kato et al[17] reported a case of adult-onset nesidioblastosis which was successfully treated with pancreaticoduodenectomy and octreotide, but their poor affinity to somatostatin receptors resulting in reduced efficiency. Pasireotide is another somatostatin analogue with a higher affinity to somatostatin receptors, and its long-acting release has been proved effective in isolated nesidioblastosis[18]. Other medications include glucocorticoids and calcium channel-blocking agents. Medication to treat nesidioblastosis has varying efficacy and causes side effects. A novel treatment method for nesidioblastosis has been reported, where exendin-4-IRDye700DX, targeting the GLP-1R, was shown to kill β-cells highly selectively; thus, this could in the future provide a new, minimally invasive and highly specific treatment for nesidioblastosis[19]. Treatment of nesidioblastosis is not always simple, it is not only dependent on medication and surgery, but also requires dietary control, dividing food intake into five to six daily meals, slowing gastric emptying, using fructose and avoiding stress at meals. Uncooked cornstarch is a source of slow release carbohydrates, it can produce low glucose peaks and maintains a stable blood glucose level during fasting; therefore, uncooked cornstarch can be considered in patients with persistent hypoglycemia particularly when surgery is impracticable/unaccepted and pharmacologic therapy is ineffective[20].
The preoperative differentiation of adult nesidioblastosis from an insulinoma is very difficult. The pathogenesis of nesidioblastosis requires further study and it can be cured by partial or subtotal pancreatectomy. However, in some cases, a combination of medication and dietary control may be useful and surgery can be avoided. It can be concluded from the present case, that focal nesidioblastosis can also occur in adults, although it is extremely rare. The diagnosis of hyperinsulinemia and persistent hypoglycemia in patients with an isolated lesion in the pancreas should not be limited to insulinoma. Increased glucose level beyond the normal range after resection of the lesion may assist in the diagnosis of focal β-cell nesidioblastosis, due to intra-pancreatic regulation in focal nesidioblastosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dragonieri S, Italy; Klinnontov VV, Russia S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Myint KS, Greenfield JR, Farooqi IS, Henning E, Holst JJ, Finer N. Prolonged successful therapy for hyperinsulinaemic hypoglycaemia after gastric bypass: the pathophysiological role of GLP1 and its response to a somatostatin analogue. Eur J Endocrinol. 2012;166:951-955. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Kowalewski AM, Szylberg Ł, Kasperska A, Marszałek A. The diagnosis and management of congenital and adult-onset hyperinsulinism (nesidioblastosis) - literature review. Pol J Pathol. 2017;68:97-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Stanley CA. Perspective on the Genetics and Diagnosis of Congenital Hyperinsulinism Disorders. J Clin Endocrinol Metab. 2016;101:815-826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 169] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 4. | Dunne MJ, Kane C, Shepherd RM, Sanchez JA, James RF, Johnson PR, Aynsley-Green A, Lu S, Clement JP 4th, Lindley KJ, Seino S, Aguilar-Bryan L. Familial persistent hyperinsulinemic hypoglycemia of infancy and mutations in the sulfonylurea receptor. N Engl J Med. 1997;336:703-706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 148] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Fournet JC, Mayaud C, de Lonlay P, Gross-Morand MS, Verkarre V, Castanet M, Devillers M, Rahier J, Brunelle F, Robert JJ, Nihoul-Fékété C, Saudubray JM, Junien C. Unbalanced expression of 11p15 imprinted genes in focal forms of congenital hyperinsulinism: association with a reduction to homozygosity of a mutation in ABCC8 or KCNJ11. Am J Pathol. 2001;158:2177-2184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Sempoux C, Guiot Y, Dahan K, Moulin P, Stevens M, Lambot V, de Lonlay P, Fournet JC, Junien C, Jaubert F, Nihoul-Fekete C, Saudubray JM, Rahier J. The focal form of persistent hyperinsulinemic hypoglycemia of infancy: morphological and molecular studies show structural and functional differences with insulinoma. Diabetes. 2003;52:784-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Lopes AA, Miranda AC, Maior MS, de Mello RV, Bandeira FA. Nesidioblastosis Associated with Pancreatic Heterotopia as a Differential Diagnosis of Hypoglycemia: A Literature Review and Case Report. Am J Case Rep. 2020;21:e922778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Liou AP, Paziuk M, Luevano JM Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5:178ra41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 643] [Cited by in F6Publishing: 671] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 9. | Christ E, Wild D, Antwi K, Waser B, Fani M, Schwanda S, Heye T, Schmid C, Baer HU, Perren A, Reubi JC. Preoperative localization of adult nesidioblastosis using ⁶⁸Ga-DOTA-exendin-4-PET/CT. Endocrine. 2015;50:821-823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Antwi K, Fani M, Heye T, Nicolas G, Rottenburger C, Kaul F, Merkle E, Zech CJ, Boll D, Vogt DR, Gloor B, Christ E, Wild D. Comparison of glucagon-like peptide-1 receptor (GLP-1R) PET/CT, SPECT/CT and 3T MRI for the localisation of occult insulinomas: evaluation of diagnostic accuracy in a prospective crossover imaging study. Eur J Nucl Med Mol Imaging. 2018;45:2318-2327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 11. | Kalff V, Iravani A, Akhurst T, Pattison DA, Eu P, Hofman MS, Hicks RJ. Utility of (68) Ga-DOTA-Exendin-4 positron emission tomography-computed tomography imaging in distinguishing between insulinoma and nesidioblastosis in patients with confirmed endogenous hyperinsulinaemic hypoglycaemia. Intern Med J. 2021;51:1657-1664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Sowa-Staszczak A, Pach D, Mikołajczak R, Mäcke H, Jabrocka-Hybel A, Stefańska A, Tomaszuk M, Janota B, Gilis-Januszewska A, Małecki M, Kamiński G, Kowalska A, Kulig J, Matyja A, Osuch C, Hubalewska-Dydejczyk A. Glucagon-like peptide-1 receptor imaging with [Lys40(Ahx-HYNIC- 99mTc/EDDA)NH2]-exendin-4 for the detection of insulinoma. Eur J Nucl Med Mol Imaging. 2013;40:524-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M; International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2435] [Cited by in F6Publishing: 2375] [Article Influence: 339.3] [Reference Citation Analysis (1)] |
| 14. | Dravecka I, Lazurova I. Nesidioblastosis in adults. Neoplasma. 2014;61:252-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Saito J, Kawasaki H, Adachi N, Sasaki A, Yakuwa N, Suzuki T, Sago H, Yamatani A, Horikawa R, Murashima A. Diazoxide during pregnancy and lactation: drug levels in maternal serum, cord blood, breast milk, and infant serum: a case report. Gynecol Endocrinol. 2022;38:528-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 16. | Snider KE, Becker S, Boyajian L, Shyng SL, MacMullen C, Hughes N, Ganapathy K, Bhatti T, Stanley CA, Ganguly A. Genotype and phenotype correlations in 417 children with congenital hyperinsulinism. J Clin Endocrinol Metab. 2013;98:E355-E363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 203] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 17. | Kato R, Nishimura A, Matsumura K, Kikuno S, Nagasawa K, Mori Y. Successful treatment of adult-onset nesidioblastosis by continuous subcutaneous octreotide infusion in a patient on hemodialysis. Clin Case Rep. 2021;9:278-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 18. | Rouland A, Bouillet B, Legris P, Simoneau I, Petit JM, Vergès B. Successful Control of Hypoglycemia with Pasireotide LAR in a Patient with Inappropriate Insulin Secretion. Clin Pharmacol. 2021;13:33-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Boss M, Bos D, Frielink C, Sandker G, Bronkhorst P, van Lith SAM, Brom M, Buitinga M, Gotthardt M. Receptor-Targeted Photodynamic Therapy of Glucagon-Like Peptide 1 Receptor-Positive Lesions. J Nucl Med. 2020;61:1588-1593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Dardano A, Daniele G, Lupi R, Napoli N, Campani D, Boggi U, Del Prato S, Miccoli R. Nesidioblastosis and Insulinoma: A Rare Coexistence and a Therapeutic Challenge. Front Endocrinol (Lausanne). 2020;11:10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |