Published online Mar 16, 2022. doi: 10.12998/wjcc.v10.i8.2382
Peer-review started: October 11, 2021
First decision: November 11, 2021
Revised: November 27, 2021
Accepted: January 29, 2022
Article in press: January 29, 2022
Published online: March 16, 2022
Pulmonary infections often lead to poor prognoses in patients with chronic obstructive pulmonary disease (COPD). Activin A and CD64 play crucial pathological roles in the development of COPD.
To explore the bacterial spectrum via analysis of activing A levels, CD64 index, and related mechanisms in COPD patients complicated with pulmonary infection.
Between March 2015 and January 2018, a total of 85 patients with COPD, who also suffered from pulmonary infections, were enrolled in this study as the pulmonary infection group. In addition, a total of 96 COPD patients, without pulmonary infection, were selected as the control group. Sputum samples of patients in the pulmonary infection group were cultivated for bacterial identification prior to administration of antibiotics. The neutrophil CD64 index was measured using flow cytometry, serum activin A levels were detected via an enzyme-linked immunosorbent assay, and activin A, Smad3, TLR4, MyD88, and NFκB protein expression was analyzed by Western blotting.
Gram-negative bacteria were identified in 57.65% of the sputum samples in the pulmonary infection group. The most prevalent Gram-negative species were Pseudomonas aeruginosa and Klebsiella pneumoniae. Conversely, Gram-positive bacteria were identified in 41.18% of the sputum samples in the pulmonary infection group. The most common Gram-positive species was Streptococcus pneumoniae. Fungi were identified in 1.17% of the sputum samples in the pulmonary infection group. The CD64 index was significantly higher in the pulmonary infection group (0.91 ± 0.38) than in the control group (0.23 ± 0.14, P < 0.001). The serum activin A levels were significantly higher in the pulmonary infection group (43.50 ± 5.22 ng/mL), compared to the control group (34.82 ± 4.16 ng/mL, P < 0.001). The relative expression levels of activin A, Smad3, TLR4, MyD88, and NFκB were all significantly higher in the pulmonary infection group, compared to the control group (all P < 0.001).
Pulmonary infections in COPD patients are mainly caused by Streptococcus pneumoniae, Pseudomonas aeruginosa, and Klebsiella pneumoniae. Pulmonary infections can significantly increase neutrophil CD64 index and serum levels of activin A, thereby activating the activin A/Smad3 signaling pathway, which may positively regulate the TLR4/MyD88/NFκB signaling pathway.
Core Tip: This study explores the bacterial spectrum via analysis of activin A levels, CD64 index, and related mechanisms in chronic obstructive pulmonary disease (COPD) patients complicated with pulmonary infection. Based on our analyses, pulmonary infections in COPD patients are mainly caused by Streptococcus pneumoniae, Pseudomonas aeruginosa, and Klebsiella pneumoniae. Pulmonary infections can significantly increase neutrophil CD64 index and serum activin A levels, thereby activating the activin A/Smad3 signaling pathway, which may positively regulate the TLR4/MyD88/NFκB signaling pathway.
- Citation: Fei ZY, Wang J, Liang J, Zhou X, Guo M. Analysis of bacterial spectrum, activin A, and CD64 in chronic obstructive pulmonary disease patients complicated with pulmonary infections. World J Clin Cases 2022; 10(8): 2382-2392
- URL: https://www.wjgnet.com/2307-8960/full/v10/i8/2382.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i8.2382
Chronic obstructive pulmonary disease (COPD) is a chronic respiratory disease, characterized by progressive and persistent airflow obstruction, along with high morbidity and mortality. COPD is often complicated by a collection of underlying diseases. This disease, coupled with poor nutrition and immunologic function, can often lead to a high incidence in pulmonary infections[1,2]. In turn, pulmonary infections can worsen COPD and promote acute exacerbations of COPD (AECOPD), which further aggravates patient prognosis. This is the main cause of death in COPD patients[1,3].
Under normal conditions, CD64 is scarcely expressed. Following infection, however, CD64 Levels rise due to the direct stimulation of pathogenic microorganisms or the indirect stimulation of inflammatory cytokines. Therefore, the neutrophil CD64 index can act as an early diagnostic marker for infection[4-6]. Moreover, multiple reports correlated the CD64 index with the severity associated with COPD and bacterial infections. Qian and Huang[7], for instance, observed that the CD64 index is higher in patients with AECOPD than those with stable COPD and healthy volunteers, and that the CD64 index is higher in AECOPD patients with positive bacterial sputum cultures than in those with negative cultures. This suggests that the CD64 index can be a guiding marker that offers better therapeutic implications, compared to conventional diagnosis, for the use of antibiotic treatments in AECOPD patients. Similarly, Titova et al[8] also demonstrated that the neutrophil CD64 index possesses approximately the same level of diagnostic accuracy as CRP in diagnosing pneumonia in patients hospitalized with AECOPD.
Activin A is a glycoprotein that promotes follicle-stimulating hormone secretion from pituitary gland. It is a member of the transforming growth factor beta superfamily and participates in the regulation of proliferation, chemotaxis, and apoptosis of neutrophils, macrophages, fibroblasts and other cells. Activin A also plays pathological roles in a series of respiratory diseases like COPD, asthma, and pulmonary fibrosis[9-11]. In 2014, Verhamme et al[12] first reported that activin A plays a key role in regulating inflammation in COPD patients and that the expression of activin A is significantly increased in the airway smooth muscle cells, bronchial epithelial cells, and alveolar macrophages of COPD patients. These conclusions were further confirmed in animal models whereby cigarette smoke exposure induced a significant increase in activin A levels in the lungs and bronchoalveolar lavage fluid of mice. Moreover, the cigarette smoke-exposed bronchial epithelial cells exhibited higher levels of activin A and lower levels of its endogenous inhibitor follistatin in vitro. Nevertheless, there are few reports on the effects of pulmonary infections on the CD64 index and activin A in patients with COPD, and the underlying mechanism remains unclear. Therefore, this study analyzed the bacterial spectrum and expressions of the CD64 index and activin A in COPD patients with pulmonary infection, and discussed the relevant mechanisms.
Between March 2015 and January 2018, a total of 85 patients with COPD, who also suffered from pulmonary infections, and a total of 96 COPD patients, without pulmonary infection, were enrolled from the First Affiliated Hospital of Chongqing Medical University, and were assigned to either the pulmonary infection or control group. Baseline characteristics, such as, age, gender, forced expiratory volume in the first second (FEV1), and FEV1/forced vital capacity (FVC) ratio were collected for comparisons. All participants signed informed consent before entry into the study, and all clinical practices were consistent with our institution’s code of ethics. This study conformed with the 2013 revised Helsinki Declaration and was approved by the Medical Ethics Committee of The First Affiliated Hospital of Chongqing Medical University.
Inclusion criteria: (1) COPD: Diagnostic criteria consistent with the Global Strategy for the Diagnosis, Management, and Prevention of COPD (2016 revised edition): Patients who had dyspnea, chronic cough or sputum production, and/or a history of exposure to risk factors, such as, smoking. The diagnosis was further confirmed via post-bronchodilator spirometry (FEV1/FVC ratio < 0.7)[13]; (2) AECOPD: Patients with COPD, who experienced a sustained increase in cough, shortness of breath, sputum production or purulence of sputum, and/or dyspnea; and (3) Pulmonary infections: COPD patients who experienced fever, produced abnormal sounds like crackles or rhonchi in lungs, and exhibited pulmonary infiltrates on chest X-ray. Pathogenic bacteria were isolated from the cultures of their sputum samples.
Exclusion criteria: (1) COPD patients who received antibiotics, hormones, or immunosuppressive drugs within 1 mo prior to admission; (2) COPD patients who suffered from other infections, such as abdominal, skin, soft tissue, bone, and cartilage infections; (3) COPD patients with pulmonary infection, but pathogenic bacteria could not be isolated from sputum culture; (4) COPD patients who also suffered from other respiratory diseases like asthma, bronchiectasis, pneumothorax, hemothorax, or comorbidities; (5) COPD patients who also suffered from tumors, autoimmune diseases, cardiovascular and cerebrovascular diseases, or renal and hepatic dysfunction; (6) COPD patients with other diseases that could lead to acute exacerbations, such as, heart failure, spontaneous pneumothorax, pulmonary embolism, or pleural effusion; and (7) COPD patients who died or suffered worsening of condition due to un-related diseases during hospitalization.
The sputum samples were collected from the lower airways of patients in the pulmonary infection group and were cultivated for bacterial identification before these patients were given antibiotics. Patients rinsed their mouths with normal saline before sputum collection to avoid contamination by oral flora. In case of difficulty in sputum collection, due to coughing, samples were taken via fiberoptic bronchoscopy. Gram staining was initially performed on the sputum samples. Sputum samples containing < 10 squamous epithelial cells and > 25 Leukocytes per low-power field (squamous epithelial cells/leukocytes < 1:2.5) were considered qualified, otherwise the sputum sample was re-collected. The qualified samples were then inoculated on blood agar and MacConkey agar plates, and cultured at 37 °C for 24 h before the fully automated VITEK 2 Compact bacterial identification system (BioMérieux) was applied for the identification of pathogenic bacteria. All samples were processed according to the National Guide to Clinical Laboratory Procedures[14].
Upon hospital admission, we collected 2 mL venous blood from the median cubital vein in the antecubital fossa of all patients and treated each sample with the anticoagulant agent, ethylenediaminetetraacetic acid. Subsequently, to each 50 μL of anti-coagulated blood, 5 μL of anti-CD64-PE (Invitrogen, MA5-16436) and 5 μL of anti-CD45-PerCP (Invitrogen, MHCD4531) were added, mixed thoroughly, and the mixture was incubated at room temperature in the dark for 30 min. This was followed by red cell lysis with 500 μL of hemolytic agent (Beijing Tongsheng Shidai Biotech Co., Ltd., Z6910001S) incubated at room temperature in the dark for an additional 15min. The test samples were then centrifuged at 3000 r/min for 5 min. Subsequently, the supernatants were removed and the cell pellets were resuspended in 300 μL PBS for flow cytometry analysis using instrument from Becton-Dickinson, FACS Calibur. Monocytes, lymphocytes, and neutrophils were identified by an established gate, based on the forward and side scatters, as well as CD45-PerCP. For each test sample, the fluorescence signals of 10000 cells were collected, and the average fluorescence intensities of sub-populations were measured. The lymphocyte CD64 Levels were used as the internal negative control (< 1.0), whereas the monocyte CD64 Levels were set as the internal positive control (> 8.0). The CD64 index was calculated as follows: CD64 index = (CD64 average fluorescence intensity on the neutrophil/CD64 average fluorescence intensity on the lymphocyte)/(CD64 average fluorescence intensity on the monocyte/CD64 average fluorescence intensity on the neutrophil).
Upon hospital admission, we collected 2 mL of venous blood from all patients. Following a 20 min incubation at room temperature, the blood samples were centrifuged at 6000 r/min for 15 min. Subsequently, the supernatants were collected and analyzed for serum activin A levels using the Elisa kit (Shanghai Renjie Biological Technology Co., Ltd. RJ12742), following manufacturer’s instructions.
Upon hospital admission, we collected 2 mL of heparinized venous blood from all patients. The blood samples were then centrifuged at 2000 r/min for 10 min to separate the blood components into three layers: Blood plasma, a buffy coat containing platelet cells, and red blood cells. The blood plasma was collected in sterile centrifuge tubes containing 1 mL of 1.090 g/mL Percoll solution (Solarbio, P8370) and an additional 1mL of 1.077 g/mL Percoll solution were successively added to the tubes. The buffy coat layer of centrifuged samples was next pipetted into a Percoll density gradient solution, and was followed by another centrifugation at 2000 r/min for 15 min, which separated the components into four layers: Blood plasma, Percoll solution, neutrophils, and Percoll solution. The neutrophils were then pipetted into a new centrifuge tube, mixed with blood plasma and centrifuged again at 1000 r/min for 10 min. Following this, the cells were rinsed three times, and re-suspended in appropriate amount of plasma. After the cell count, 106 cells were collected, completely lysed by adding 100 μL of lysis buffer (Beyotime Biotech, China, P0013), and centrifuged at 12000 r/min for 5 min. The supernatants were then collected for BCA protein quantification (Beyotime Biotech, China, P0012). For each test sample, 20 μg of total protein was obtained for polyacrylamide gel electrophoresis. The proteins were then transferred onto PVDF membranes, and blocked in 5% non-fat dry milk at room temperature for 2 h. After the blocking process, the membranes were incubated with primary antibodies for either anti-activator A (1:500, Abcam, ab89307), anti-Smad3 (1:500, Abcam, ab40854), anti-TLR4 (1:500, Abcam, ab13556), anti-MyD88 (1:500, Abcam, ab2064), anti-NFκB (1:1000, Abcam, ab32360), or anti-GAPDH (1:1000, Abcam, ab8245) at 4 °C overnight. The membranes were then rinsed three times and incubated with HRP-conjugated goat anti-rabbit IgG (Boster, BA1056, 1:2000) at room temperature for 1 h. The membranes were then rinsed three times, followed by incubation with the ECL substrate solution (Beyotime Biotech, China, P0018), and exposure and imaging using gel doc (Bio-Rad, GelDoc XR+). The protein band gray values were determined with the Image Pro Plus 6.0 software, and the intensity of the target protein band divided by the intensity of GAPDH in the control group was adjusted as 1[15].
All data were statistically processed using the SPSS 20.0 software. Data are expressed as mean ± SD. Inter-group comparisons were made with the independent-sample t-test. Enumeration data are expressed as cases/percentage (n/%). Inter-group comparisons were performed using the chi-squared test. P < 0.05 was considered statistically significant.
Baseline patient characteristics are summarized in Table 1, including gender, age, course of disease, smoking history, FEV1, and FEV1/FVC. A total of 181 cases met our inclusion criteria. Among these cases, 46 patients had pulmonary infection, and 96 patients were included in the control group. The gender, patient age, disease, and smoking history between the two groups were comparable (all P > 0.05). Patients in the pulmonary infection group presented with a lower level of FEV1 (%), compared to the control group (41.30 ± 9.91 vs 47.65 ± 10.07, P < 0.001). Similarly, the level of FEV1/FVC (%) was lower in the pulmonary infection group, compared to the control group (50.48 ± 9.13 vs 58.24 ± 8.62, P < 0.001).
| Baseline characteristics | The control group | The pulmonary infection group | χ2 | P value |
| Cases, n | 96 | 85 | ||
| Gender (male/female) | 59/37 | 50/35 | 0.131 | 0.718 |
| Age range (yr) | 67.44 ± 8.51 | 68.83 ± 8.90 | 1.073 | 0.285 |
| Course of disease (yr) | 16.35 ± 7.68 | 17.13 ± 8.06 | 0.666 | 0.506 |
| Smoking history, n (%) | 68 (70.83) | 65 (76.47) | 0.735 | 0.391 |
| FEV1 (%) | 47.65 ± 10.07 | 41.30 ± 9.91 | 4.266 | < 0.001 |
| FEV1/FVC (%) | 58.24 ± 8.62 | 50.48 ± 9.13 | 5.879 | < 0.001 |
The bacterial spectrum of patients in the pulmonary infection group is shown in Table 2. Among the 85 strains, 49 (57.65%) were gram-negative bacteria and 35 (41.18%) were gram-positive bacteria. The most prevalent gram-negative species were Pseudomonas aeruginosa (16, 18.82%), followed by Klebsiella pneumoniae (14, 16.47%), Haemophilus influenzae (7, 8.24%), and Haemophilus parainfluenzae (7, 8.24%). Among the gram-positive bacteria, 24 (28.24%) were streptococcus pneumoniae, and 11 (12.94%) were staphylococcus epidermidis. Apart from these, fungi were identified in 1.17% sputum samples.
| Bacteria | Strains | Proportion (%) |
| Gram-negative bacteria | 49 | 57.65 |
| Pseudomonas aeruginosa | 16 | 18.82 |
| Klebsiella pneumoniae | 14 | 16.47 |
| Haemophilus influenzae | 7 | 8.24 |
| Haemophilus parainfluenzae | 7 | 8.24 |
| Others | 5 | 5.88 |
| Gram-positive bacteria | 35 | 41.18 |
| Streptococcus pneumoniae | 24 | 28.24 |
| Staphylococcus epidermidis | 11 | 12.94 |
| Fungus | 1 | 1.17 |
| Total | 85 | 100.00 |
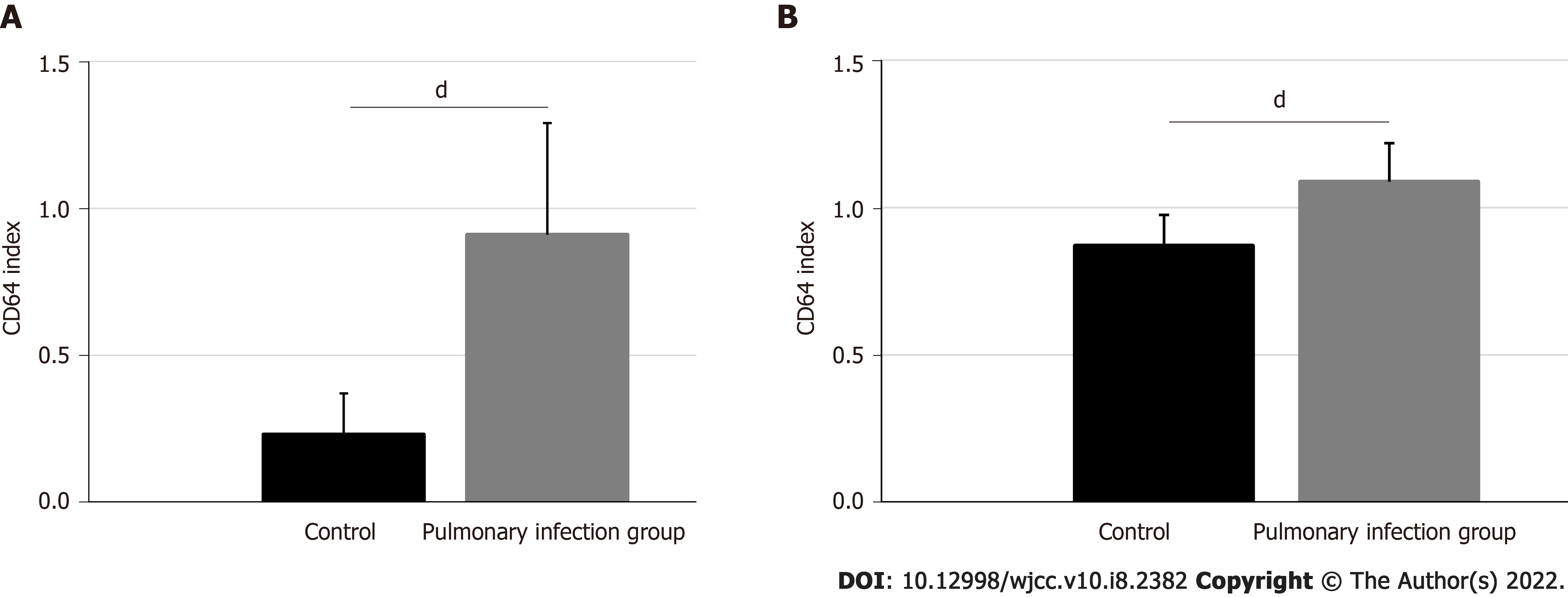
The CD64 index was 0.91 ± 0.38 in the pulmonary infection group and 0.23 ± 0.14 in the control group. Based on our statistical analyses, the pulmonary infection group had significantly lower CD64 index than the control group (P < 0.001) (Figure 1A).
Figure 1B illustrates the activin A levels in the pulmonary infection and control groups. The activin A levels were 43.50 ± 5.22 ng/mL in the pulmonary infection group, and 34.82 ± 4.16 ng/mL in the control group (P < 0.001). Hence, the pulmonary infection group had significantly higher activin A levels than the control group (P < 0.001).
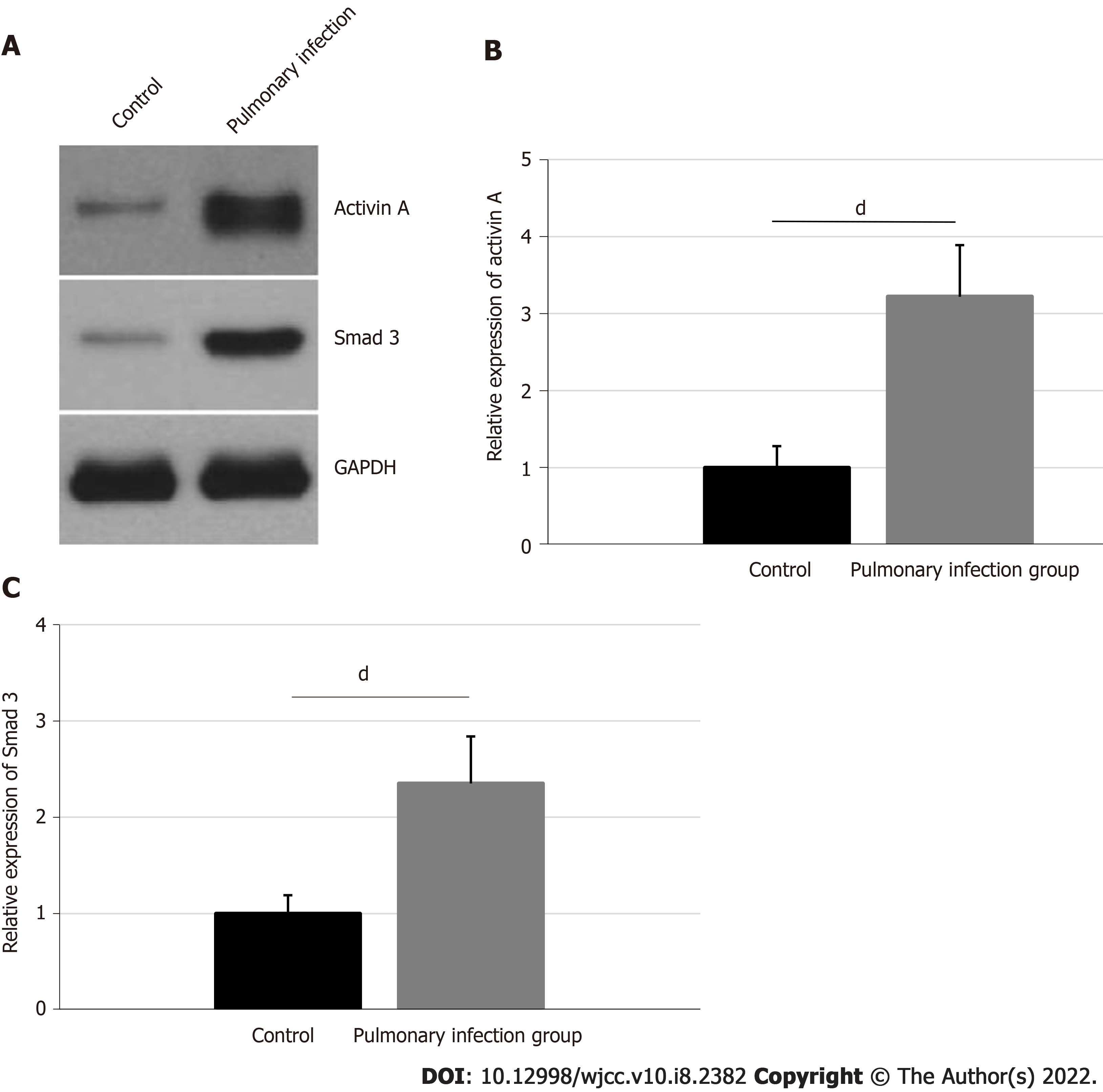
The activin A and neutrophil Smad3 protein expressions and their corresponding statistical analyses are shown in Figure 2. The activin A expression was 3.22 ± 0.67 in the pulmonary infection group, and 1.00 ± 0.28 in the control group. The expressions of neutrophil Smad3 in the pulmonary infection group was 2.35 ± 0.49, and in the control group was 1.00 ± 0.19. Hence, patients in the pulmonary infection group presented with higher levels of activin A and neutrophil Smad3, compared to the control group (P < 0.001).
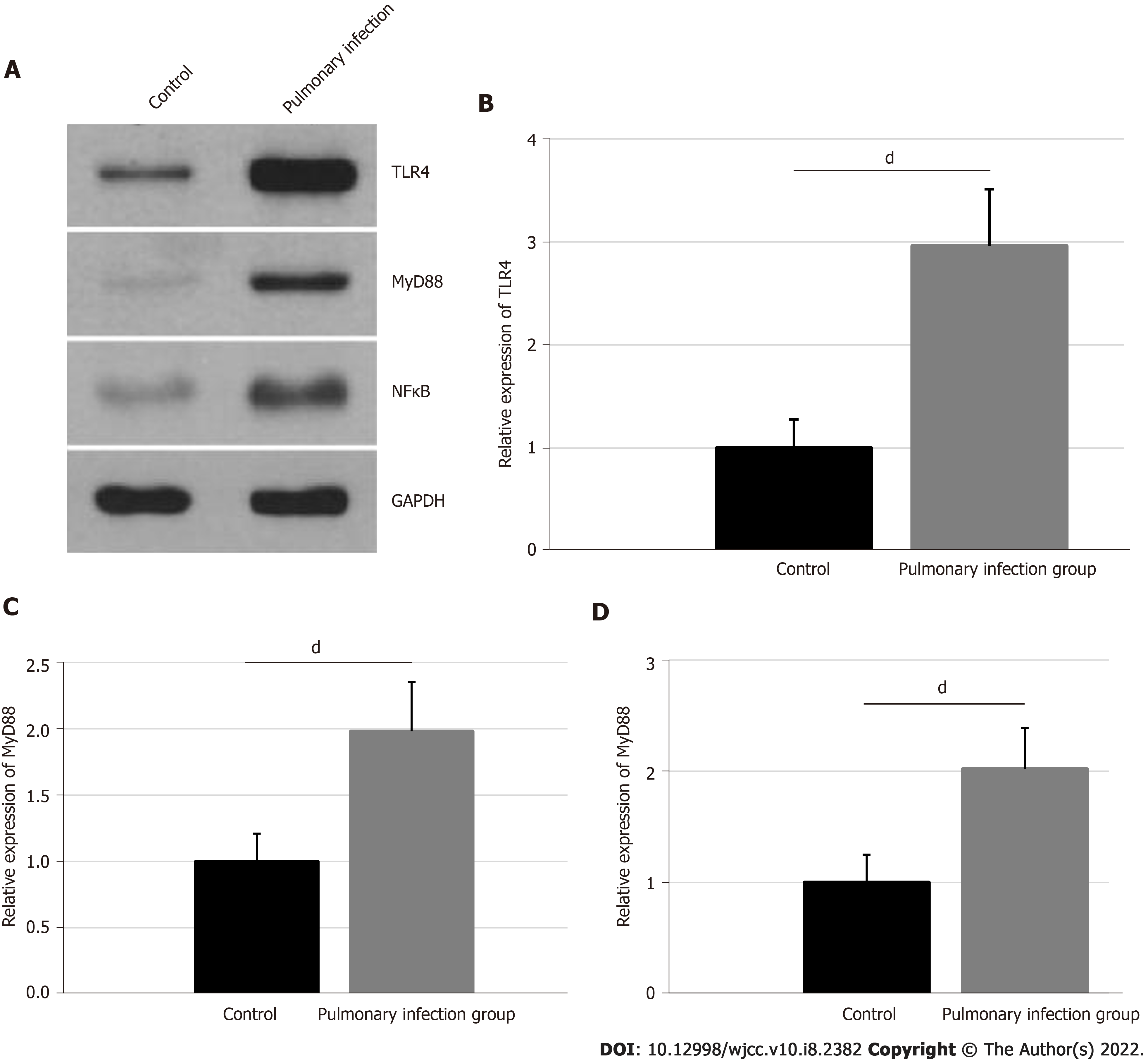
The relative expressions of neutrophil TLR4, MyD88, and NFκB were confirmed, using western blot, and are presented in Figure 3. The TLR4 expression was 2.96 ± 0.55 in the pulmonary infection group, and 1.00 ± 0.28 in the control group. The MyD88 expression was 1.98 ± 0.37 in the pulmonary infection group, and 1.00 ± 0.21 in the control group. Lastly, the NFκB expression was 2.02 ± 0.37 in the pulmonary infection group, and 1.00 ± 0.25 in the control group. Based on our statistical analyses, the levels of neutrophil TLR4, MyD88, and NFκB were significantly higher in the pulmonary infection group than the control group (all P < 0.001).
COPD often occurs in the elderly, due to multiple underlying diseases, weak cough reflex, and low immune function. This ultimately leads to high incidences of pulmonary infections[1,2]. This study found that pulmonary infections in COPD patients are mainly caused by Gram-negative bacteria like Klebsiella pneumoniae and Pseudomonas aeruginosa. Our conclusion is consistent with the results of the Qu et al[16] study. In another study, Zhou et al[17] reported that COPD patients with pulmonary infections are more likely to have diabetes, elevated risk of ventilator usage, and prolonged bed rest, compared to COPD patients without pulmonary infection. Moreover, the study demonstrated that pulmonary infections are mainly caused by Gram-negative bacteria including Escherichia coli, Klebsiella pneumoniae, and Acinetobacter baumannii. Nevertheless, our study revealed that the pulmonary infections in COPD patients are mainly caused by Gram-negative bacteria, such as, Pseudomonas aeruginosa and Klebsiella pneumoniae. This inconsistency may be related to the collection and treatment of samples and the isolation and identification of bacterial strains.
CD64 is usually expressed in low quantities in neutrophils, but a significant rise in expression occurs with the stimulation of pathological factors or inflammatory cytokines. Therefore, the neutrophil CD64 index is used as a diagnostic and prognostic biomarker in multiple diseases like systemic lupus erythematosus, neonatal sepsis, bacterial peritonitis, and inflammatory bowel diseases[4-6]. Previous studies found that CD64 is also an important diagnostic and prognostic biomarker in COPD patients[8,18]. In fact, Qian and Huang[7] found that the level of CD64 is significantly increased in patients with AECOPD or COPD with positive bacterial sputum culture. Our study also showed that the CD64 index is significantly higher in the pulmonary infection group than in the control group, which is consistent with prior publications.
Activin A is a member of the transforming growth factor beta superfamily that participates in multiple physiological and pathological processes like embryogenesis, neuroprotection, apoptosis, and fibrosis[9,19]. Several studies reported that activin A is significantly increased during infections and inflammatory diseases, including sepsis, inflammatory bowel diseases, and rheumatoid arthritis[10,11,20]. Moreover, activin A plays critical roles in regulating inflammation during COPD. Likewise, Verhamme et al[12] reported that the expression of activin A is significantly increased in the airway smooth muscle cells, bronchial epithelial cells, and alveolar macrophages of COPD patients. These conclusions were further confirmed in animal models. The administration of follistatin in cigarette smoke-exposed mice was shown to significantly decrease accumulation of monocytes, macrophages, neutrophils, as well as CD4+ and CD8+ T-lymphocytes. This suggests that the significant increase in activin-A is not caused by pulmonary inflammation in COPD models, but is, in fact, a mediator of COPD development. Likewise, the results of our study demonstrated that the level of serum activin A is significantly higher in the pulmonary infection group than in the control group. This indicates that pulmonary infections are consistent with other infections or inflammatory diseases in stimulating a significant increase of activin A levels in COPD patients.
Smad3 is a downstream key effector of transforming growth factor-β1 and it plays a significant role in COPD patients, particularly, in terms of regulating inflammation, airway remodeling, and fibrosis[21,22]. Mahmood et al[23] observed that the activation of the Smad3 signaling pathway is linked to the epithelial mesenchymal transition and loss of lung function. Furthermore, a recent study reported that the Smad3 signaling pathway is also a major effector of the activin A biological activity[24]. In our research, we employed neutrophils protein expression analysis to reveal that pulmonary infection significantly promotes expressions of activin A and Smad3 in neutrophils, which indicates that the activin A/Smad3 signaling pathway is strongly activated during this time. In another study, the TLR4/MyD88/NFκB signaling pathway activation in neutrophils was shown to be an important contributor to the significant activin A secretion during the pathophysiology of endotoxemia[25]. In our study, we also demonstrated that pulmonary infection can significantly promote expressions of TLR4, MyD88, and NFκB in neutrophils, which indicates that the elevated serum activin A levels and activation of the activin A/Smad3 signaling pathway may be strongly related to the activation of the TLR4/MyD88/NFκB signaling pathway.
This study focused on the bacterial spectrum, and expressions of the CD64 index and activin A in patients with COPD, complicated with pulmonary infections. But, there are still many scientific aspects that have not been discussed. First, this article only discussed the effects of pulmonary infections in COPD patients, however it remains unclear whether infections caused by other types of bacteria produces similar or different results. Second, previous studies reported that the activin A antagonist follistatin effectively alleviates pathological conditions associated with pulmonary fibrosis. But, the role of follistatin in patients with COPD, complicated with pulmonary infections, remains unclear[26-28]. Third, this study explored alterations within the TLR4/MyD88/NFκB signaling pathway after separation of the patients’ neutrophils. However, the relationship between this signaling pathway and activin A expression still lacks strong evidence. Therefore, in vitro cytological experiments are warranted for verification of this correlation via targeted inhibition and/or overexpression investigations.
In conclusion, pulmonary infections in COPD patients are mainly caused by Streptococcus pneumoniae, Pseudomonas aeruginosa, and Klebsiella pneumoniae. Pulmonary infections can result in a significant increase in the neutrophil CD64 index and serum levels of activin A, and, in turn, activate the activin A/Smad3 signaling pathway, which may be positively regulate the TLR4/MyD88/NFκB signaling pathway.
A sharp exacerbation of chronic obstructive pulmonary disease (COPD) is often triggered by a lung infection and often has a poor prognosis.
Since COPD induces complex inflammatory events, Activin A and CD64 may collectively contribute to the development and progression of this disorder.
To analyze the bacterial profile of COPD patients with pulmonary infections and to assess activin A levels, CD64 index, and the underlying mechanisms involved in disease development.
The whole data set consisted of 85 COPD patients with pulmonary infection and 96 COPD patients without pulmonary infection. Sputum samples were obtained from patients with pulmonary infections for further bacterial culture. The levels of CD64 index, activin A, Smad3, TLR4, MyD88, and NFκB proteins were assessed and compared between 85 COPD patients with pulmonary infections and 96 COPD patients without pulmonary infections.
In the pulmonary infection group sputum samples, the Gram-negative bacteria, Gram-positive bacteria, and Fungi were 57.65%, 41.18%, and 1.17%, respectively. In addition, the relative CD64 index, and levels of activin A, Smad3, TLR4, MyD88, and NFκB proteins were all significantly higher in the pulmonary infection group, compared to the control group (all P < 0.001).
Pulmonary infections in COPD patients may be caused by a variety of pathogens. In COPD patients, the CD64 index and serum activin A levels were significantly increased in patients with lung infection, compared to those without. This may have a positive regulatory effect on the downstream activin A/Smad3 and TLR4/MyD88/NFκB signaling pathways.
Together, our findings provide a novel mechanism underlying pulmonary infection in COPD patients, and offer a potential therapeutic target for an enhanced and effective therapy against COPD.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Respiratory system
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gounis MJ, Volovici V S-Editor: Xing YX L-Editor: Filipodia P-Editor: Xing YX
| 1. | Ghanem M, Zein A, Makhlouf H, Farghaly S, El-Gezawy E, Mohrram A. Role of Comorbidities in Acquiring Pulmonary Fungal Infection in COPD Patients. Chest. 2016;150:915A. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Song Y, Chen R, Zhan Q, Chen S, Luo Z, Ou J, Wang C. The optimum timing to wean invasive ventilation for patients with AECOPD or COPD with pulmonary infection. Int J Chron Obstruct Pulmon Dis. 2016;11:535-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Eltom S, Dale N, Raemdonck KR, Stevenson CS, Snelgrove RJ, Sacitharan PK, Recchi C, Wavre-Shapton S, McAuley DF, O'Kane C, Belvisi MG, Birrell MA. Respiratory infections cause the release of extracellular vesicles: implications in exacerbation of asthma/COPD. PLoS One. 2014;9:e101087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Liu C, Zhao D. Correlation between CD64 and PCT levels in cerebrospinal fluid and degree of hearing impairment sequelae in neonates with purulent meningitis. Exp Ther Med. 2017;14:5997-6001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 5. | Liu M, Weng X, Gong S, Chen H, Ding J, Guo M, Hu X, Wang J, Yang J, Tang G. Flow cytometric analysis of CD64 expression pattern and density in the diagnosis of acute promyelocytic leukemia: a multi-center study in Shanghai, China. Oncotarget. 2017;8:80625-80637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Minar P, Jackson K, Tsai YT, Sucharew H, Rosen MJ, Denson LA. Validation of Neutrophil CD64 Blood Biomarkers to Detect Mucosal Inflammation in Pediatric Crohn's Disease. Inflamm Bowel Dis. 2017;24:198-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Qian W, Huang GZ. Neutrophil CD64 as a Marker of Bacterial Infection in Acute Exacerbations of Chronic Obstructive Pulmonary Disease. Immunol Invest. 2016;45:490-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Titova E, Aune MW, Fonn K, Henriksen AH, Åsberg A. Neutrophil CD64 Expression as a Diagnostic Marker in Patients Hospitalized with Exacerbations of COPD: A Prospective Observational Study. Lung. 2015;193:717-724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Kuo CS, Lu YW, Hsu CY, Chang CC, Chou RH, Liu LK, Chen LK, Huang PH, Chen JW, Lin SJ. Increased activin A levels in prediabetes and association with carotid intima-media thickness: a cross-sectional analysis from I-Lan Longitudinal Aging Study. Sci Rep. 2018;8:9957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Protic O, Islam MS, Greco S, Giannubilo SR, Lamanna P, Petraglia F, Ciavattini A, Castellucci M, Hinz B, Ciarmela P. Activin A in Inflammation, Tissue Repair, and Fibrosis: Possible Role as Inflammatory and Fibrotic Mediator of Uterine Fibroid Development and Growth. Semin Reprod Med. 2017;35:499-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Takahashi S, Nakasatomi M, Takei Y, Ikeuchi H, Sakairi T, Kaneko Y, Hiromura K, Nojima Y, Maeshima A. Identification of Urinary Activin A as a Novel Biomarker Reflecting the Severity of Acute Kidney Injury. Sci Rep. 2018;8:5176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Verhamme FM, Bracke KR, Amatngalim GD, Verleden GM, Van Pottelberge GR, Hiemstra PS, Joos GF, Brusselle GG. Role of activin-A in cigarette smoke-induced inflammation and COPD. Eur Respir J. 2014;43:1028-1041. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Vestbo J, Hurd SS, Rodriguez-Roisin R. The 2011 revision of the global strategy for the diagnosis, management and prevention of COPD (GOLD)--why and what? Clin Respir J. 2012;6:208-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 14. | Shang H, Wang YS, Shen ZY. National Guide to Clinical Laboratory Procedures. People's Medical Publishing House, 2015. [Cited in This Article: ] |
| 15. | Yang Y, Shi C, Hou X, Zhao Y, Chen B, Tan B, Deng Z, Li Q, Liu J, Xiao Z, Miao Q, Dai J. Modified VEGF targets the ischemic myocardium and promotes functional recovery after myocardial infarction. J Control Release. 2015;213:27-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Qu MJ, Cao QF, Wang DP. Distribution of pathogens causing pulmonary infections in elderly patients with chronic obstructive pulmonary disease. Zhonghua Yiyuanganranxue Zazhi. 2014;17:4200-4202. [Cited in This Article: ] |
| 17. | Zhou M, Yu ZL, Wang MF, Zhu LY, Luo JJ. Clinical characteristics and distribution of pathogenic bacteria in chronic obstructive pulmonary disease patients with pulmonary infections. Zhonghua Yiyuanganranxue Zazhi. 2017;14:3158-3160, 3175. [Cited in This Article: ] |
| 18. | Xu N, Chen J, Chang X, Zhang J, Liu Q, Li A, Lin D. nCD64 index as a prognostic biomarker for mortality in acute exacerbation of chronic obstructive pulmonary disease. Ann Saudi Med. 2016;36:37-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Diesselberg C, Ribes S, Seele J, Kaufmann A, Redlich S, Bunkowski S, Hanisch UK, Michel U, Nau R, Schütze S. Activin A increases phagocytosis of Escherichia coli K1 by primary murine microglial cells activated by toll-like receptor agonists. J Neuroinflammation. 2018;15:175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Zhang Y, Qi Y, Zhao Y, Sun H, Ge J, Liu Z. Activin A induces apoptosis of mouse myeloma cells via the mitochondrial pathway. Oncol Lett. 2018;15:2590-2594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Zandvoort A, Postma DS, Jonker MR, Noordhoek JA, Vos JT, van der Geld YM, Timens W. Altered expression of the Smad signalling pathway: implications for COPD pathogenesis. Eur Respir J. 2006;28:533-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Zandvoort A, Postma DS, Jonker MR, Noordhoek JA, Vos JT, Timens W. Smad gene expression in pulmonary fibroblasts: indications for defective ECM repair in COPD. Respir Res. 2008;9:83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Mahmood MQ, Reid D, Ward C, Muller HK, Knight DA, Sohal SS, Walters EH. Transforming growth factor (TGF) β1 and Smad signalling pathways: A likely key to EMT-associated COPD pathogenesis. Respirology. 2017;22:133-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 24. | Qi Y, Ge J, Ma C, Wu N, Cui X, Liu Z. Activin A regulates activation of mouse neutrophils by Smad3 signalling. Open Biol. 2017;7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Jones KL, Mansell A, Patella S, Scott BJ, Hedger MP, de Kretser DM, Phillips DJ. Activin A is a critical component of the inflammatory response, and its binding protein, follistatin, reduces mortality in endotoxemia. Proc Natl Acad Sci U S A. 2007;104:16239-16244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 212] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 26. | Dong Y, Geng Y, Li L, Li X, Yan X, Fang Y, Dong S, Liu X, Yang X, Zheng X, Xie T, Liang J, Dai H, Yin Z, Noble PW, Jiang D, Ning W. Blocking follistatin-like 1 attenuates bleomycin-induced pulmonary fibrosis in mice. J Exp Med. 2015;212:235-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 27. | Forrester HB, de Kretser DM, Leong T, Hagekyriakou J, Sprung CN. Follistatin attenuates radiation-induced fibrosis in a murine model. PLoS One. 2017;12:e0173788. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Seachrist DD, Sizemore ST, Johnson E, Abdul-Karim FW, Weber Bonk KL, Keri RA. Follistatin is a metastasis suppressor in a mouse model of HER2-positive breast cancer. Breast Cancer Res. 2017;19:66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |