Published online Apr 6, 2022. doi: 10.12998/wjcc.v10.i10.3121
Peer-review started: September 27, 2021
First decision: December 2, 2021
Revised: December 12, 2021
Accepted: February 20, 2022
Article in press: February 20, 2022
Published online: April 6, 2022
Vascular variations are frequently encountered during surgery. Approximately thirty percent of these variations are aberrant left hepatic arteries originating from the left gastric artery.
To summarize the safety and feasibility of aberrant left hepatic arteries (ALHA) ligation in gastric cancer patients who underwent laparoscopic-assisted gastrectomy (LAG).
The literature search was systematically performed on databases including PubMed, Embase, and Cochrane Library. The publishing date of eligible studies was from inception to June 2021.
A total of nine studies were included according to the inclusion and exclusion criteria in this review. The variation rate of ALHA ranged from 7.00% to 20.70%, and four studies compared the differences between the ALHA ligation group and the preservation group. Only one study showed worse postoperative outcomes in the ALHA ligation group. In all the included studies, a significant difference was found between the ALHA ligation group and the preservation group in terms of postoperative liver enzymes after LAG. However, there was no significant difference in the number of retrieved lymph nodes between the two groups.
In conclusion, it is not always safe and feasible for surgeons to ligate the ALHA during LAG surgery, and it is necessary for gastric cancer patients to undergo preoperative examination to clarify the ALHA subtypes, measure the diameter of the ALHA, and determine whether the patients have chronic liver disease.
Core Tip: Vascular variations are frequently encountered during surgery. Approximately thirty percent of these variations are aberrant left hepatic arteries (ALHAs) originating from the left gastric artery. And liver dysfunction occurs more frequently after laparoscopic-assisted gastrectomy (LAG). The purpose of this systematic review is to summarize the safety and feasibility of ALHA ligation in gastric cancer patients who underwent LAG.
- Citation: Tao W, Peng D, Cheng YX, Zhang W. Clinical significance of aberrant left hepatic artery during gastrectomy: A systematic review. World J Clin Cases 2022; 10(10): 3121-3130
- URL: https://www.wjgnet.com/2307-8960/full/v10/i10/3121.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i10.3121
Gastric cancer (GC) is the second leading cause of cancer-related death worldwide, with 78000 patients dying every year[1-2]. Although multidisciplinary treatment, including chemotherapy, targeted therapy, and immunotherapy, has been recommended in many countries, radical gastrectomy remains the main or even the only curative therapy for GC. Radical gastrectomy is also beneficial to long-term survival[3-4].
Laparoscopic-assisted gastrectomy (LAG) has become the main surgical method for early-stage gastric cancer[5]. Although LAG has more advantages than open gastrectomy (OG), including less estimated blood loss, shorter hospital stays and fewer complications[6-7], liver dysfunction occurs more frequently after LAG. Several studies have reported that a long carbon dioxide (CO2) exposure time caused by a difficult LAG could result in liver function alterations[8]. However, a previous study, comparing LAG with laparoscopy-assisted colectomy, found that liver dysfunction might not be caused by CO2 pneumoperitoneum[9]. Vascular variations are frequently encountered during surgery. Variations in the anatomy of the hepatic artery are most frequently found, and approximately thirty percent of these variations are aberrant left hepatic arteries (ALHAs) originating from the left gastric artery (LGA)[10-12]. There are two main subtypes of ALHA variations: accessory LHA (acLHA) and replaced LHA type (RLHA)[10]. Radical LAG for GC is required to sever the LGA at the root level to ensure lymph node dissection, and the existing ALHA will inevitably be severed[13]. However, the ligation of ALHA may cause liver ischemia and severe postoperative complications, such as left hepatic lobe necrosis and liver dysfunction, because parts of the liver may be supplied by this artery[14-15]. There was no significant difference in surgical outcomes between the ALHA ligation group and the ALHA preservation group according to a previous study[16].
According to existing studies, there is still controversy regarding whether ALHA should be ligated during LAG surgery, and whether liver dysfunction occurs when ALHA is ligated. Therefore, the purpose of this systematic review is to summarize and assess the safety and feasibility of ALHA ligation in GC patients who underwent LAG surgery.
This systematic review was designed and performed based on the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) Statement[17]. This study is a systematic review of literature related to the change in postoperative liver function after gastrectomy with ligation of the ALHA. The literature search was systematically performed on databases including PubMed, Embase, and Cochrane Library. The publishing date of eligible studies was from inception to June 2021. We searched databases using the following keywords: “left hepatic artery”, “LHA”, “stomach tumor”, “stomach neoplasm”, “stomach cancer”, “cancer of stomach”, “gastric cancer” and “gastrectomy” (Table 1).
| Search strategy |
| Search Databases: PubMed, Embase, Cochrane Library |
| Date: Up to June 3, 2021 |
| Strategy: #1 AND #2 |
| #1 Left hepatic artery [Title/Abstract] or LHA[Title/Abstract] |
| #2 (((((stomach tumor [Title/Abstract]) OR (stomach neoplasm [Title/Abstract])) OR (stomach cancer [Title/Abstract])) OR (cancer of the stomach [Title/Abstract])) OR (gastric neoplasm [Title/Abstract])) OR (gastric cancer [Title/Abstract]) AND gastrectomy [Title/Abstract] |
The title and abstract of all searched articles were independently screened by two authors. All articles conforming to the inclusion and exclusion criteria were eventually included in the study. The Inclusion criteria were as follows: (1) Study type: cohort studies, case-control studies, and retrospective studies on humans; (2) English language articles published in scientific journals; (3) Full-text articles available; and (4) Articles related to this research. The exclusion criteria were as follows: review, case reports, commentaries, randomized clinical trials and conference abstracts.
The full text of all included articles was independently screened by two researchers. Perioperative information, including the first author, publication year, country, study date, surgical procedure, simple size, vascular variation rate and main outcome, was extracted.
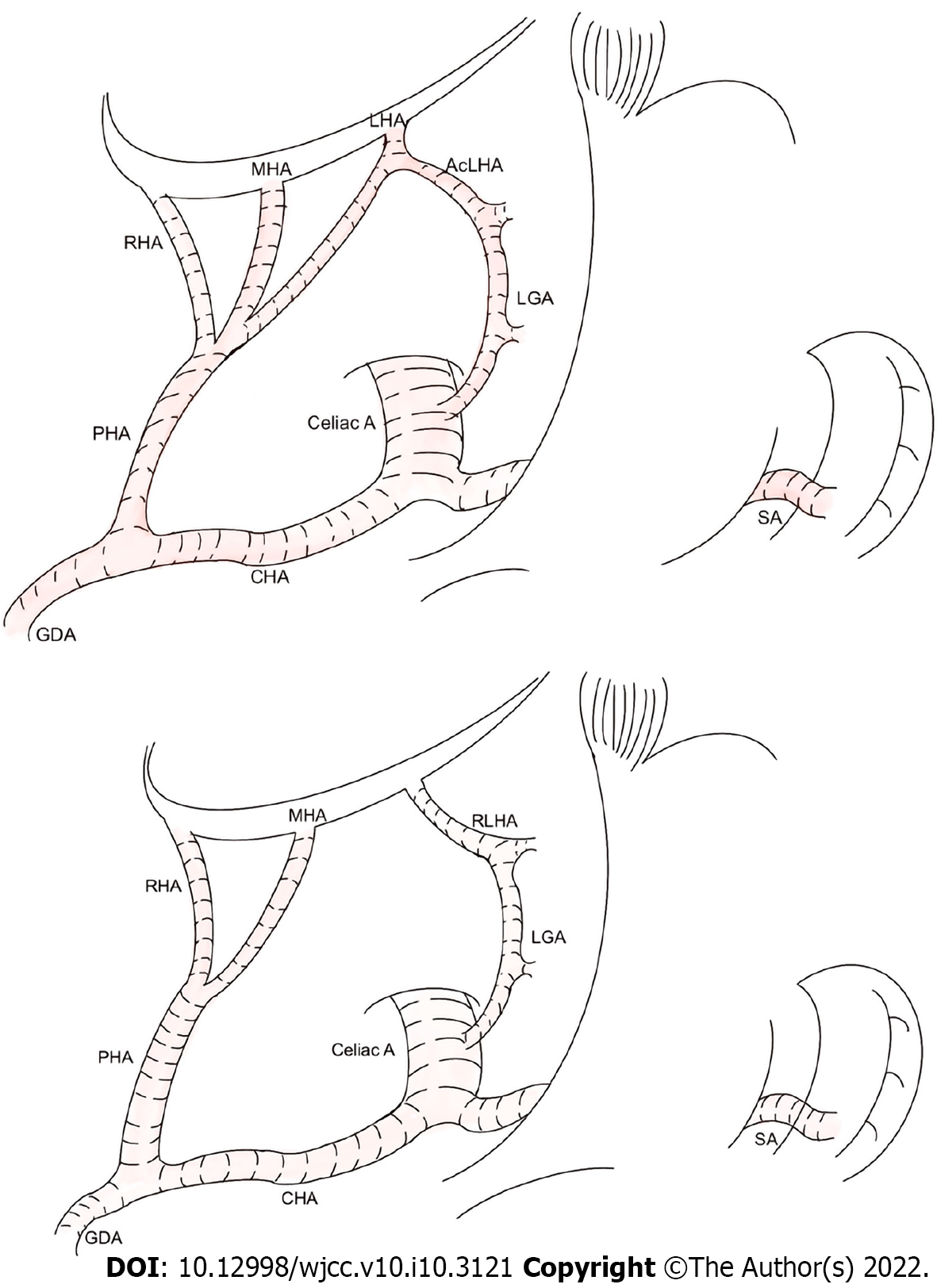
The ALHA has two primary subtypes. The first originates from the LGA in the extrahepatic area and communicates with the LHA via the accessory LHA (acLHA). In the second subtype, there is no LHA in the extrahepatic area, and an artery that emanates from the LGA communicates with the hepatic vasculature to replace the function of the LHA. This artery is referred to as the replaced LHA (RLHA). Figure 1 shows the two subtypes of ALHA in detail.
The quality of the nine included studies was assessed by the Newcastle-Ottawa Scale[18], and the results are reported in Table 2.
| Ref. | Country | Study design | Study date | Surgical procedure | Simple size | Vascular variation rate | Main results | NOS |
| Waki et al[25], 2020 | Japan | RS | 2012-2018 | LDG | 106 | 20.70% | Preserve. Surgeons should confirm the RLHA preoperatively and preserve it, because the preservation of RLHA could reduced postoperative transaminase elevation and hepatic infraction | 8 |
| Okano et al[26], 1993 | Japan | RS | 1985-1991 | LG | 28 | 19.90% | Possible preserve. For patients with preoperative liver dysfunction or a large LHLG, the LHLG diameter should be estimated, as it can help with the decision of whether to preserve it | 7 |
| Ang et al[29], 2020 | Korea | RS | 2012-2016 | LG | 204 | 8.20% | Possible preserve. When ligating ALHA > 1.5 mm in diameter regardless of subtype, a transient rise would be seen in postoperative SGOT and SGPT levels, and liver enzymes should be monitored postoperatively | 8 |
| Shinohara et al[17], 2007 | Japan | RS | 1997-2001 | Gastrectomy | 50 | 7.00% | Preserve. Routine division of the ALHA does not be required as long as it is not directly involved by the tumour | 7 |
| Huang et al[24], 2013 | China | RS | 2007-2012 | LG | 135 | 11.50% | Possible preserve. ALHA is a common anomaly that was found in 11.5% of patients. It can be safely severed during radical gastrectomy in patients without CLD, but should be left intact in patients with CLD to prevent liver dysfunction | 7 |
| Jeong et al[10], 2011 | Korea | RS | 2006-2007 | Gastrectomy | 215 | N/A | Preserve. Patients who underwent a gastrectomy showed significantly increased hepatic enzyme levels on POD1, regardless of the surgical technique, which returned to normal on POD5. This study concludes that the liver function alteration after LAG may have been caused by direct liver manipulation or aberrant hepatic artery ligation rather than the CO2 pneumoperitoneum | 8 |
| Kim et al[30], 2016 | Korea | RS | 2009-2014 | LDG | 150 | 12.50% | Preserve. Preservation of an ALHA during laparoscopic gastrectomy is feasible. This study suggests preserving ALHA which arises from a large LGA, diameter larger than 5 mm, during laparoscopic gastrectomy to prevent immediate postoperative hepatic dysfunction | 8 |
| Sano et al[27], 2021 | Japan | RS | 2013-2019 | LG | 54 | 35.30% | Preserve. Liver retraction using the NLR and ligation of an ALHA were recognized as independent risk factors for PLEE after LG for gastric cancer. ALHA preservation may contribute to avoiding postoperative liver dysfunction | 7 |
| Lee et al[28], 2021 | Korea | RS | 2015-2019 | Gastrectomy | 160 | 17.60% | Possible preserve. 8.6% patients with a ligated ALHA presented with MS liver enzyme elevation. These patients showed poorer short-term postoperative outcomes, in terms of the length of hospital stay and the incidence and severity of postoperative complications, than patients with NM liver enzyme elevation | 8 |
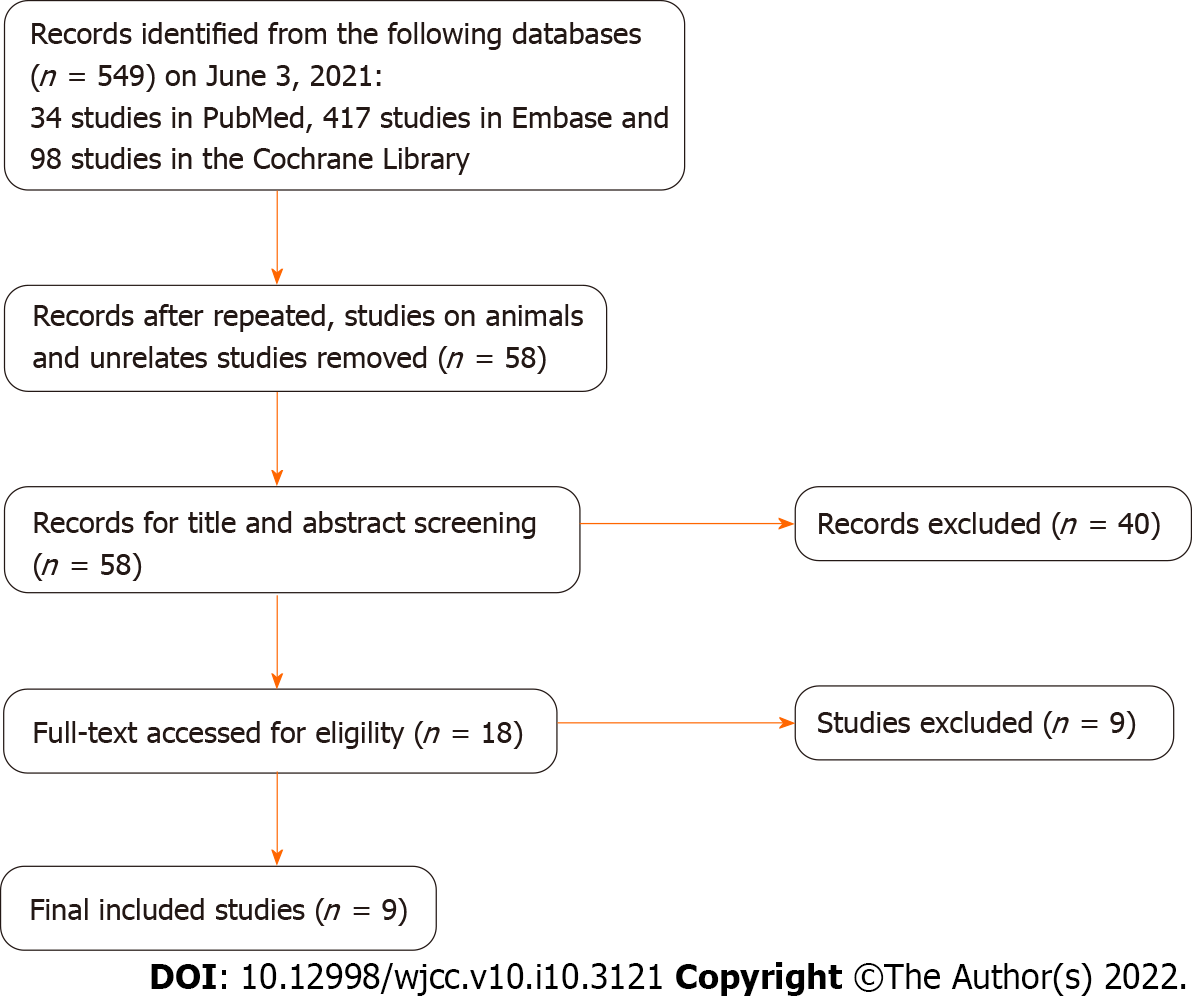
A total of 549 studies were screened from the databases using the keywords. Then, duplicate studies, animal related studies and unrelated studies were excluded, and according to the inclusion criteria, 58 studies were left for further review. Next, the title and abstract from all selected studies were examined for final decisions, and 40 studies were excluded. Finally, eighteen full-text were thoroughly assessed for eligibility, and nine studies remained. According to the PRISMA statement, a flowchart was made for the detailed screening process of the study (Figure 2).
The nine included studies all originated in Asian countries, including 4 studies in Japan, 4 studies in Korea and 1 study in China. The year of publication ranged from 1993 to 2021. The sample size for these studies ranged from 28 to 215, with an average sample size of 135. Six studies were conducted to evaluate the safety of severing ALHA during LAG, and OG and LAG were discussed in the other three studies. According to the population characteristics of the included studies, 8 studies calculated the vascular variation rate of the simple set, and the rate ranged from 7.00% to 20.70%.
We summarized the main results of nine studies and divided their conclusions on the preservation of ALHA into two degrees of suggestions, of which the “possible preserve” was weak one, and the “preserve” was strong. Five studies suggested that it is of great necessity to preserve the ALHA to prevent postoperative liver dysfunction. However, the conclusions of other studies were that the ALHA could be ligated in appropriate situations, such as patients without chronic liver disease, an ALHA diameter less than 1.5 mm, and when more precise examinations are needed (Table 2).
We extracted operative outcome data from four studies that compared the surgical outcomes of the ALHA ligation group and the ALHA preservation group. The surgical outcome reported by Shinohara et al[16] indicated that the operation time was significantly longer, and the estimated blood loss and the number of retrieved lymph nodes were significantly higher in the ALHA-divided group than in the ALHA-preserved group (P = 0.014, P = 0.005 and P = 0.018, respectively). However, the surgical outcomes were not significantly different between the two groups in the other three studies. Furthermore, the surgical complications between the two groups were not significantly different in the four studies. According to these four studies, the postoperative variation of liver enzymes was used to reflect the changes in liver function, of which the variation of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were used as the main postoperative indicators. There were statistically significant differences in postoperative changes in liver enzymes between the ALHA-ligated group and the ALHA-preserved group on postoperative day (POD) 1, POD2, POD3, and POD5 (Table 3).
| ALHAWaki et al[25], ALHA | Ang et al[29], RLHA | AcLHA
| Shinohara et al[17], ALHA | Kim et al[30], ALHA | |||||||||||
| Divided | Preserved | P value | Divided | Preserved | P value | Divided | Preserved | P value | Divided | Preserved | P value | Divided | Preserved | P value | |
| Operation time (min) | 285 (171-490) | 301 (173-476) | 0.36 | 222 ± 55 | 243 ± 73 | 0.158 | 216 ± 49 | 221 ± 59 | 0.727 | 293 ± 19 | 223 ± 18 | 0.0141 | 151.5 (84-315) | 177.5 (118-329) | 0.084 |
| EBL (mL) | 10 (0-155) | 18 (0-308) | 0.427 | 102 ± 93 | 134 ± 126 | 0.316 | 108 ± 93 | 129 ± 126 | 0.429 | 450 ± 44 | 269 ± 43 | 0.0051 | 100 (20-1000) | 100 (30-200) | 0.791 |
| RLS (n) | 59 (34-64) | 36.5 (21-53) | 0.152 | 54 ± 5.7 | 38 ± 3.5 | 0.0181 | 37 (16-87) | 33 (16-66) | 0.207 | ||||||
| PHS (d) | 10 (7-38) | 9 (7-21) | 0.113 | 11.8 ± 8.0 | 9.7 ± 7.5 | 0.295 | 10.9 ± 16.7 | 11.9 ± 9.2 | 0.804 | - | - | - | - | - | - |
| Complications, n (%) | 6 (33.3%) | 6 (16.2%) | 0.177 | 3 (17.6%) | 16 (14%) | 0.713 | 8 (15.4%) | 6 (28.6%) | 0.207 | - | - | - | - | - | - |
| PLECT | POD1, POD3 | < 0.0011 | AST POD2, ALT (POD2, POD5) | < 0.0011, (< 0.0011, 0.0461) | - | - | POD1, POD3 | < 0.011 | AST POD1; ALT (POD1, POD5) | 0.0091; (0.0031, 0.0071) | |||||
In this study, we systematically reviewed nine published scientific studies that focused on the safety and clinical significance of ligating the ALHA during LAG.
To our knowledge, this is the first systematic review to summarize the clinical significance of preserving the ALHA during LAG in GC patients. This is the first systematic review only on GC patients to summarize the clinical significance of ligating the ALHA during LAG by comparing the postoperative complications and the number of retrieved lymph nodes in the ALHA preserved group and ALHA ligated group. In this systematic review, we found that in five studies, the researchers strongly recommended that preserving ALHA could reduce postoperative complications. In the other four studies, the researchers reported that there was no significant difference in the changes in liver function between the ALHA ligation group and the preservation group. Only in special situations should postoperative liver function be monitored, such as in patients with chronic liver disease or those with an ALHA diameter > 1.5 mm.
ALHA variations were first reported in 1764. According to a previous study that had 57 studies related to ALHA variation, the ALHA prevalence rate reached 13.52%[19]. For a clear understanding of vascular anatomic variation, patients were asked to undergo preoperative examination before surgery. The difference in preoperative examination led to various results, with prevalence of 11.16%, 15.63%, and 13.1% for abdomen computed tomography (CT), angio-CT and visceral angiography respectively[20]. Previous studies suggested that using three-dimensional CT angiography before surgery was more beneficial than using multidetector-row CT to recognize ALHA[21]. The ALHA has been categorized into ten types based on Michel’s classification according to Cirocchi et al[20]. The ALHA is primarily composed of acLHA and RLHA. It is necessary to recognize the existence of ALHA by preoperative examination before LAG surgery, because it is difficult to determine whether the anatomic structure of ALHA is normal during LGA surgery. Also, the intraoperative ligation of ALHA may lead to increased postoperative complications and prolonged hospitalization.
Based on the five types of vascular variations reported by Cirocchi et al[20], the ALHA originates from the left gastric artery to supply the left hepatic lobe directly or indirectly. For GC patients, lymph node metastasis is commonly diagnosed, and LAG is recommended as a curative treatment, according to the Japanese Classification of Gastric Carcinoma[13]. Thus, it is necessary to dissect the metastasizing lymph nodes around the tumor completely during LAG, including lymph node Stations 1, 3, and 7[22]. However, the LGA should be severed at its root level to achieve radical cure, and the ALHA, including acLHA and RLHA, should also be ligated.
When the ALHA was ligated during surgery, severe complications, such as liver ischemia and liver failure, were reported by previous studies[16,23]. In contrast, other studies indicated that preserving ALHA could increase the operation time and estimated blood loss; however, it could not increase complications[24]. In this systematic review, four studies[16,24,28-29] that compared the ALHA preservation group with the ALHA ligation group were assessed to investigate the outcomes of GC patients with ALHA during LAG. In one of the four studies, Shinohara et al[16] reported that the ALHA ligation group had a longer operation time, higher estimated blood loss, more retrieved lymph nodes and a longer postoperative hospital stay. However, the other three studies reported negative outcomes. Furthermore, the rest five studies[9,23,25-27] used different methods to determine whether to ligate ALHA. Okano et al[25] reported that ligation of ALHA would cause transient liver enzyme changes on POD1, and the surgical outcome might be related to the ALHA-fed area and the diameter of the ALHA. Huang et al[23] showed that patients with chronic liver disease (CLD) were more susceptible to tissue damage when they encountered ischemia and hypoxia than patients without CLD. Jeong GA et al[10] indicated that liver tissue primarily produces ALT, and the ALT level should be considered the gold standard for reflecting liver function changes. It was found that the ALT level increased when the ALHA was severed, but the clinical consequences of this were not significantly different. Sano et al[26] reported that ALHA ligation was an independent risk factor (P = 0.042) for increased postoperative liver enzyme in multivariate analysis, and it was feasible to preserve ALHA when dissected the lymph nodes as standard requested. Lee et al[27], in their analysis, demonstrated that 8.6% of their included patients with ALHA ligated have longer hospital stay and higher incidence of postoperative complications.
Postoperative liver enzyme variations were reported by all nine included studies. A significant transient elevation in ALT and AST enzymes was observed within POD5. In accordance with anatomic differences in the ALHA[19], the two types of ALHA can induce various degrees of liver dysfunction. The RLHA can directly provide a larger blood supply for part of the liver tissue than acLHA, which means that ischemia and hypoxia of liver tissue may be more serious in RLHA ligation than acLHA ligation. Therefore, Waki et al[24] and Ang et al[28] recommended that liver function variations should be monitored in patients, especially those with the RLHA type. Furthermore, three studies showed that regardless of the ALHA anatomic subtypes, when the ALHA has been severed in surgery, the diameter of the ALHA was related to postoperative liver enzymes elevation. Among these studies, two studies believed that when the diameter of ALHA was greater than 1.5 mm, postoperative liver damage should be monitored carefully[24,28]. The third study believed that an ALHA diameter larger than 0.5 mm should be preserved[29]. However, the nine included studies only reported that liver enzymes were elevated in the short- term after LAG, and long- term liver function changes were not reported, because liver dysfunction gradually recovered to normal levels after POD5. Although a few studies have shown that serious complications, such as liver failure, may occur after ALHA ligation[30], most studies reported transient postoperative liver enzyme elevation, which has no obvious influence on the recovery of most patients after surgery. Surgeons should pay more attention to patients with CLD because preserving ALHA in these patients could prevent postoperative liver function deterioration.
In this systematic review, although the ALHA may influence the decision of surgeons to complete a full lymph node dissection, there was no significant difference in the number of retrieved lymph nodes between the ALHA preservation and ALHA ligation groups. Therefore, it is not necessary for the surgeon to divide ALHA for the purpose of lymph node dissection in LAG unless there are obvious signs of tumor metastasis.
This current review had some limitations. First, this systematic review included nine retrospective studies with languages limited to English, and only 4 of these studies compared the ALHA-divided group with the ALHA-preserved group to analyze the relationship between ALHA variations and liver function changes in GC patients. Second, the sample size of the included studies was relatively small. Third, these nine studies lacked pliable data to implement a meta-analysis to support this research. Forth, between the initiations of this study, the included studies lacked western studies because of none of western studies could be searched based on this search strategy. Therefore, more large-scale studies and randomized controlled trials clarifying the relationship between ALHA ligation and LAG surgery in postoperative liver function variations in GC patients are needed in the future.
In conclusion, it is not always safe and feasible for surgeons to ligate the ALHA during LAG surgery. Also, it is necessary for gastric cancer patients to undergo preoperative examination, to clarify the ALHA subtypes, measure the diameter of ALHA, and determine whether the patients have CLD.
Vascular variations are frequently encountered during surgery. Approximately thirty percent of these variations are aberrant left hepatic arteries (ALHAs) originating from the left gastric artery (LGA).
A previous study, comparing LAG with laparoscopy-assisted colectomy, found that liver dysfunction might not be caused by carbon dioxide pneumoperitoneum. According to existing studies, there is still controversy regarding whether ALHA should be ligated during LAG surgery, and whether liver dysfunction occurs when ALHA is ligated.
The purpose of this systematic review is to summarize and assess the safety and feasibility of ALHA ligation in GC patients who underwent LAG surgery.
The literature search was systematically performed on databases including PubMed, Embase, and Cochrane Library. The publishing date of eligible studies was from inception to June 2021.
A total of nine studies were included in this review. In all the included studies, a significant difference was found between the ALHA ligation group and the preservation group in terms of postoperative liver enzymes after LAG. However, there was no significant difference in the number of retrieved lymph nodes between the two groups.
It is not always safe and feasible for surgeons to ligate the ALHA during LAG surgery, and it is necessary for gastric cancer patients to undergo preoperative examination to clarify the ALHA subtypes, measure the diameter of the ALHA, and determine whether the patients have chronic liver disease.
More large-scale studies and randomized controlled trials clarifying the relationship between ALHA ligation and LAG surgery in postoperative liver function variations in GC patients are needed in the future.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Nuclear science and technology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Garbarino GM, Mishra TS, Nur Azlina M S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53206] [Cited by in F6Publishing: 51030] [Article Influence: 8505.0] [Reference Citation Analysis (122)] |
| 2. | Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14:26-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 578] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 3. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1575] [Cited by in F6Publishing: 1800] [Article Influence: 257.1] [Reference Citation Analysis (0)] |
| 4. | Peng D, Cheng YX, Liao G. Effect of endoscopic resection on short-term surgical outcomes of subsequent laparoscopic gastrectomy: a meta-analysis. World J Surg Oncol. 2021;19:119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Jiao J, Liu S, Chen C, Maimaiti A, He Q, Hu S, Yu W. Comparative study of laparoscopic radical gastrectomy and open radical gastrectomy. J Minim Access Surg 2020; 16: 41-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N; Japanese Laparoscopic Surgery Study Group. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007;245:68-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 519] [Cited by in F6Publishing: 509] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 7. | Huang X, Du H, Aihemaiti M, Liu T, Chen N, Yu W, Hu S, Liu S. Laparoscopic-assisted Versus Open D2 Gastrectomy for Advanced Gastric Cancer in Highly Selective Patients: Short-term Surgical and Chemotherapy Outcomes of a Prospective Cohort Study. Am J Clin Oncol. 2019;42:459-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Etoh T, Shiraishi N, Tajima M, Shiromizu A, Yasuda K, Inomata M, Kitano S. Transient liver dysfunction after laparoscopic gastrectomy for gastric cancer patients. World J Surg. 2007;31:1115-1120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Jeong GA, Cho GS, Shin EJ, Lee MS, Kim HC, Song OP. Liver function alterations after laparoscopy-assisted gastrectomy for gastric cancer and its clinical significance. World J Gastroenterol. 2011;17:372-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. 1966;112:337-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 579] [Cited by in F6Publishing: 516] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 11. | Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg. 1994;220:50-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 545] [Cited by in F6Publishing: 464] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 12. | Tiwari S, Roopashree R, Padmavathi G, Varalakshmi KL, Sangeeta M. Study of aberrant lef hepatic artery from lef gastric artery and its clinical importance. IJCRR. 2014;6:25-28. [DOI] [Cited in This Article: ] |
| 13. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2390] [Cited by in F6Publishing: 2656] [Article Influence: 204.3] [Reference Citation Analysis (0)] |
| 14. | Yamamoto M, Zaima M, Yamamoto H, Harada H, Kawamura J, Yamada M, Yazawa T, Kawasoe J. Liver necrosis shortly after pancreaticoduodenectomy with resection of the replaced left hepatic artery. World J Surg Oncol. 2017;15:77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | FRIESEN SR. The significance of the anomalous origin of the left hepatic artery from the left gastric artery in operations upon the stomach and esophagus. Am Surg. 1957;23:1103-1108. [PubMed] [Cited in This Article: ] |
| 16. | Shinohara T, Ohyama S, Muto T, Yanaga K, Yamaguchi T. The significance of the aberrant left hepatic artery arising from the left gastric artery at curative gastrectomy for gastric cancer. Eur J Surg Oncol. 2007;33:967-971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7463] [Cited by in F6Publishing: 7256] [Article Influence: 806.2] [Reference Citation Analysis (0)] |
| 18. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8858] [Cited by in F6Publishing: 10693] [Article Influence: 763.8] [Reference Citation Analysis (0)] |
| 19. | Haller AV. Elementa phynologiae corporis humani. Neapoli, Apud Vincentium Ursinum, Bern. 1764 VIeVII. [DOI] [Cited in This Article: ] |
| 20. | Cirocchi R, D'Andrea V, Amato B, Renzi C, Henry BM, Tomaszewski KA, Gioia S, Lancia M, Artico M, Randolph J. Aberrant left hepatic arteries arising from left gastric arteries and their clinical importance. Surgeon. 2020;18:100-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 21. | Yamashita K, Sakuramoto S, Mieno H, Shibata T, Nemoto M, Katada N, Kikuchi S, Watanabe M. Preoperative dual-phase 3D CT angiography assessment of the right hepatic artery before gastrectomy. Surg Today. 2014;44:1912-1919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Maruyama K, Gunvén P, Okabayashi K, Sasako M, Kinoshita T. Lymph node metastases of gastric cancer. General pattern in 1931 patients. Ann Surg. 1989;210:596-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 324] [Cited by in F6Publishing: 306] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | Huang CM, Chen QY, Lin JX, Zheng CH, Li P, Xie JW, Wang JB, Lu J. Short-term clinical implications of the accessory left hepatic artery in patients undergoing radical gastrectomy for gastric cancer. PLoS One. 2013;8:e64300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Waki Y, Kamiya S, Li Y, Hikage M, Tanizawa Y, Bando E, Terashima M. Preserving a Replaced Left Hepatic Artery Arising from the Left Gastric Artery During Laparoscopic Distal Gastrectomy for Gastric Cancer. World J Surg. 2021;45:543-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Okano S, Sawai K, Taniguchi H, Takahashi T. Aberrant left hepatic artery arising from the left gastric artery and liver function after radical gastrectomy for gastric cancer. World J Surg. 1993;17:70-3; discussion 74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Sano A, Saito K, Kuriyama K, Nakazawa N, Ubukata Y, Hara K, Sakai M, Ogata K, Fukasawa T, Sohda M, Fukuchi M, Naitoh H, Shirabe K, Saeki H. Risk Factors for Postoperative Liver Enzyme Elevation After Laparoscopic Gastrectomy for Gastric Cancer. In Vivo. 2021;35:1227-1234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Lee S, Son T, Song JH, Choi S, Cho M, Kim YM, Kim HI, Hyung WJ. Adverse Effects of Ligation of an Aberrant Left Hepatic Artery Arising from the Left Gastric Artery during Radical Gastrectomy for Gastric Cancer: a Propensity Score Matching Analysis. J Gastric Cancer. 2021;21:74-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Ang RRG, Lee HJ, Bae JS, Zhu CC, Berlth F, Kim TH, Park SH, Suh YS, Kong SH, Kim SH, Yang HK. Safety of Ligation of Aberrant Left Hepatic Artery Originating from Left Gastric Artery in Laparoscopic Gastrectomy for Gastric Cancer. Sci Rep. 2020;10:5856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Kim J, Kim SM, Seo JE, Ha MH, An JY, Choi MG, Lee JH, Bae JM, Kim S, Jeong WK, Sohn TS. Should an Aberrant Left Hepatic Artery Arising from the Left Gastric Artery Be Preserved during Laparoscopic Gastrectomy for Early Gastric Cancer Treatment? J Gastric Cancer. 2016;16:72-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Mays ET, Wheeler CS. Demonstration of collateral arterial flow after interruption of hepatic arteries in man. N Engl J Med. 1974;290:993-996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 156] [Article Influence: 3.1] [Reference Citation Analysis (0)] |