修回日期: 2022-08-07
接受日期: 2023-02-13
在线出版日期: 2023-02-28
结直肠癌(colorectal cancer, CRC)在世界各国都是高发性疾病. 肿瘤相关成纤维细胞(cancer-associated fibroblasts, CAFs)是肿瘤微环境(tumor microen-vironment, TME)中的重要成分, 在CRC的发生发展过程中起了重要作用. CAFs通过分泌多种细胞因子及外泌体、激活多种相关信号通路促进CRC的侵袭、转移、代谢和耐药、免疫抑制等, 也可作为CRC的预后标志物及治疗靶点. 因此, 研究CAFs在CRC中所扮演的角色及具体的作用机制有重要意义, 以期为CRC的治疗提供新的方向. 本文就CAFs在CRC发生发展中的多种调控机制及治疗进行综述, 介绍CAFs在调节结直肠癌细胞的侵袭、转移、代谢和耐药、免疫抑制中的作用.
核心提要: 肿瘤相关成纤维细胞是肿瘤微环境的重要组成部分, 该细胞的激活与细胞增殖及肿瘤进展密切相关, 本文综述了肿瘤相关成纤维细胞对结直肠癌血管生成、侵袭和转移、代谢、耐药及放疗抵抗、免疫抑制的影响以及治疗的进展, 为结直肠癌的诊治提供新的思路.
引文著录: 梁俏, 周喜汉. 肿瘤相关成纤维细胞在结直肠癌中的研究进展. 世界华人消化杂志 2023; 31(4): 129-137
Revised: August 7, 2022
Accepted: February 13, 2023
Published online: February 28, 2023
Colorectal cancer (CRC) is a malignancy that has a high incidence in all countries around the world. Cancer-associated fibroblasts (CAFs) are a vital component of the tumor microenvironment (TME), playing an important role in the development of CRC. CAFs can release multiple cytokines and exosomes, activating a variety of related signaling pathways and boosting the processes of the invasion, metastasis, metabolism, drug resistance, and immunosuppression in CRC. Thus, CAFs are a prognostic marker and therapeutic target for CRC. Understanding the role and mechanism of CAFs can provide new insights for the treatment of CRC.
- Citation: Liang Q, Zhou XH. Role of cancer-associated fibroblasts in colorectal cancer. Shijie Huaren Xiaohua Zazhi 2023; 31(4): 129-137
- URL: https://www.wjgnet.com/1009-3079/full/v31/i4/129.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v31.i4.129
结直肠癌(colorectal cancer, CRC)是发病率高及致死率高的癌症, 2020年数据显示, 全球CRC患者估计有190万例及约90万例CRC患者死亡[1]. 肿瘤微环境(tumor microenvironment, TME)是肿瘤生存的细胞环境, 包括癌细胞、基质细胞、免疫细胞、细胞因子以及细胞外基质等[2]. 肿瘤相关成纤维细胞cancer-associated fibroblasts, CAFs)是在肿瘤中发生的成纤维细胞群体, 具有表型及功能异质性, 与肿瘤的发生、发展和转移有关[3,4]. 通过多种途径, 活化的CAFs可以促进肿瘤增殖、侵袭和转移, 血管生成, 以及通过细胞外基质重塑、化学耐药等促进肿瘤的发展[5,6]. 近年来CAFs对CRC的发生发展可能发挥的作用有了更深的研究. 本文从CRC激活CAFs, CAFs促进CRC血管生成、侵袭和转移、代谢、耐药及放疗抵抗、免疫抑制等方面综述CAFs在CRC中的作用.
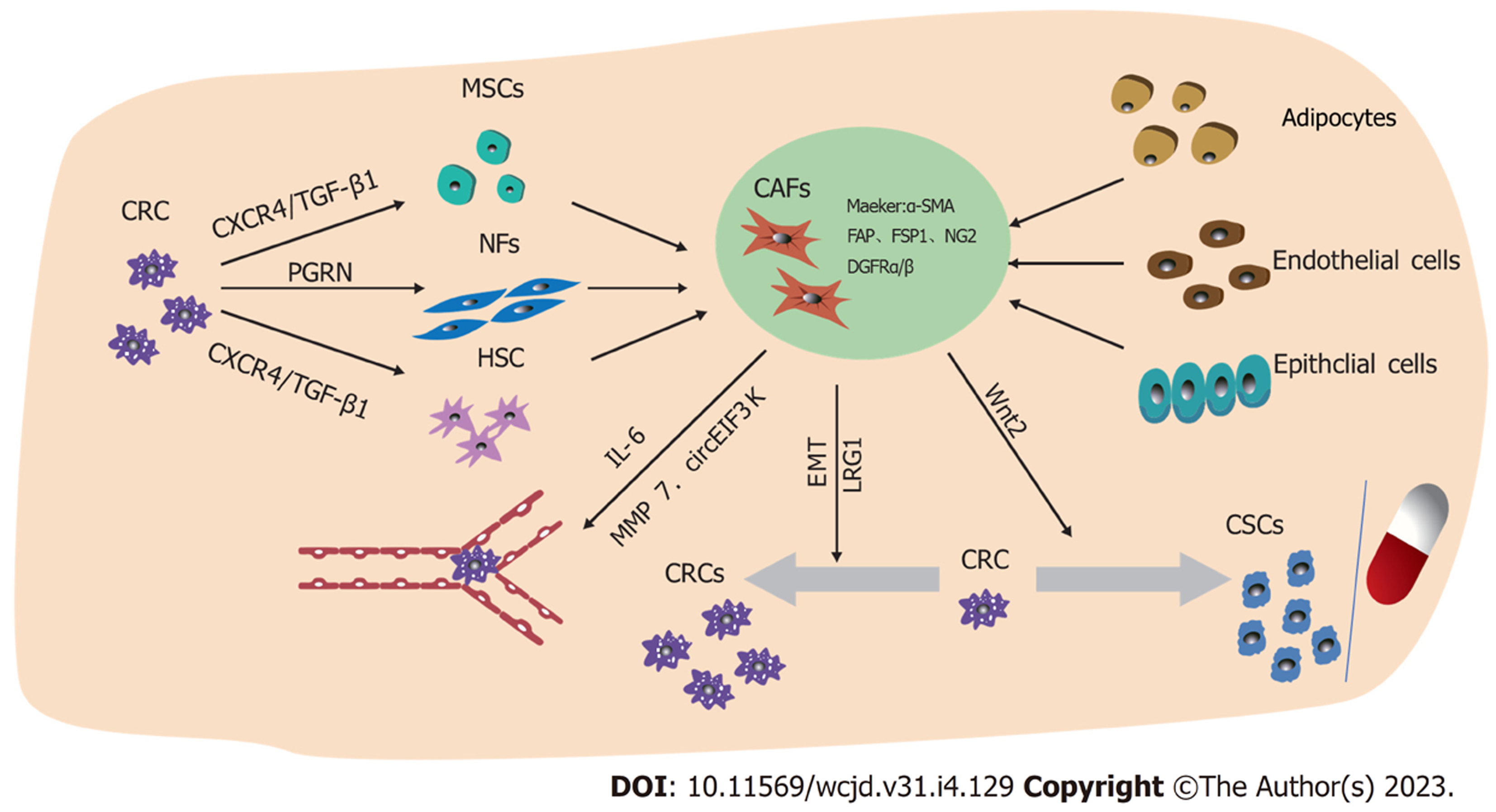
名称"CAFs"经常被用作一个总称来定义一个复杂的动态异质性间充质细胞群体. CAFs可由正常成纤维细胞(normal fibroblasts, NFs)或其他前体细胞的活化而来, 包括骨髓来源的间充质干细胞(mesenchymal stem cells, MSC)、上皮细胞、癌细胞、内皮细胞、平滑肌细胞、脂肪细胞、纤维细胞、胰腺和肝脏中的星状细胞, 乳腺中的肌上皮细胞和胃肠道的围隐肌成纤维细胞等[7]. CAFs可以通过增殖速度及表型来识别, 增殖速度快及以α-平滑肌肌动蛋白(α-smooth muscle actin, α-SMA)为代表的标志物的表达是CAFs的突出特征. 与NFs相比, 成纤维活化蛋白(fibroblast activation protein, FAP)、成纤维特异性细胞蛋白1(fibroblast-specific protein-1)、骨质肌群素、desmin、血小板衍生生长因子受体α或β(platelet derived growth factor receptor, PDGFRα/β)、神经元神经胶质抗原-2(neuroglia antigen 2, NG2)、骨桥蛋白(osteopontin, OPN)、骨膜素、鬼臼素、tenascin-C或含盘状蛋白结构域的受体2和间充质细胞标志物vimentin在CAFs高表达, 也被认为是CAFs的标志物[7-9]. 然而, 这些标志物并不具有特异性, 其它正常细胞也表达[10]. CAFs通过分泌转化生长因子β1 (transforming growth factor-beta 1, TGF-β1)、CXC趋化因子配体(C-X-C chemokine ligand, CXCL)-12、白细胞介素(interleukin, IL)-6、肝细胞生长因子(hepatocyte growth factor, HGF)等细胞因子及趋化因子积极与肿瘤沟通并促进肿瘤进展[11,12]. NFs可以通过TGF-β1和基质细胞衍生因子-1(stromal cell-derived factor-1, SDF-1)以自分泌方式刺激, 从而分化成CAFs[13]. 在多种类型的癌症中, 上皮-间充质转化(epithelial-mesenchymal transition, EMT)是上皮细胞转化为CAFs的主要机制. 研究发现肾成纤维细胞来自上皮细胞通过局部EMT转变为富含成FSP1阳性成纤维细胞[14]. 此外, 众所周知的胰腺星状细胞是胰腺中的常驻成纤维细胞, 可以从大鼠胰腺中分离出来并在体外培养, 在与肿瘤相互作用时, 这些细胞失去维生素A表达, 随后通过激活有丝分裂原活化蛋白激酶(mitotic activation protein kinase, MAPK)途径呈现分泌表型, 从而促进肿瘤存活[15]. 大量研究表明[16], 癌细胞分泌的可溶性因子重新编程脂肪细胞以分泌生长因子, 细胞因子和细胞外基质(extracellular matrix, ECM)重塑蛋白, 将这些细胞转化为CAFs. 但CAFs转化的分子机制尚不完全清楚. 见图1.
越来越清楚的是, 由于存在不同功能状态的CAF亚群, 从而引发了CAF异质性的问题. 证据表明, CAF的异质性包括不同的器官、组织、来源、功能、分泌类型等[17]. Li等[18]使用单细胞转录组学测序(scRNA-seq)确定了结直肠癌肿瘤中的两种CAFs亚群: 表达金属蛋白酶(matrix metalloproteinase, MMP)-2的CAFs-A和表达α-SMA的CAFs-B, CAFs-A是NFs和CAFs-B的中间状态或是独立的亚型, 其两者在生物学功能方面的研究尚不清楚. 另外, 由早期癌症外泌体(SW480-Exos)激活的成纤维细胞具有高度的促增殖和促血管生成, 并且显示出促血管生成和促增殖蛋白的表达升高. 相比之下, 由晚期癌症外泌体(SW620-Exos)激活的成纤维细胞通过上调膜突起的促侵入性调节因子和基质重塑蛋白, 显示出通过细胞外基质侵入的惊人能力[19].
研究发现, 结肠NFs与结肠CAFs形态结构上存在差异, 结肠NFs形态呈大小一致、规则, 排列规整, 无重叠生长的多胞突的扁平星状, 而结肠CAFs形态则为大小不一、重叠生长、生长密集、排列无规则的少胞突的纺锤形或长梭形[20]. 从CRC患者的结肠组织中分离出CAF和正常的成纤维细胞并对其进行了表征, 发现CAFs显示分解素和金属蛋白酶(A disintegrin and metalloproteinase, ADAMs)物种的表达增加, 如ADAM9、ADAM10、ADAM12、ADAM17等, 并且在细胞外基质表达进一步增加[21]. 从结直肠癌患者中成功分离出CAFs、癌周成纤维细胞(pericarcinoma fibroblasts, PFs)和NFs, 发现与PFs和NFs相比, CAFs显示α-SMA、PFA的表达增高[22]. 对结肠NFs和结肠CAFs的公开转录数据进行整合分析, 发现结肠粘膜下NFs中相对于非胃肠道成纤维细胞表达更高的基因在结肠CAFs中低于NFs[23,24].
CRC细胞可通过多种途径激活其他细胞活化为CAFs. 例如, CRC细胞可以通过分泌外泌体使成纤维细胞的α-SMA表达及基质重构能力增强, 激活成为CAFs[19,25]. CRC细胞来源的原粒蛋白(progranulin, PGRN)可能会使NFs活化成CAF, 部分通过肿瘤坏死因子受体2(tumor necrosis factor receptor-2, TNFR-2)/蛋白激酶B(protein kinase B, PKB)又称AKT和细胞外信号调控激酶(endocytosis pathways, extracellular signal-regulated kinases, ERK)信号通路诱导Ki67、FAP和α-SMA分子的表达, 最终导致CAFs的增殖[26]. 通过对CRC间质中CAFs与MSCs的分泌组差异分析显示, 两个样本中检测到52.5%的相同蛋白质, 推测部分CAFs是由MSCs衍化而来的, CRC细胞还可能是通过分泌C-X-C基序趋化因子配体4(C-X-C motif chemokine receptor 4, CXCR4)、TGF-β1介导MSCs自分化为CAFs[27,28]. 肝星状细胞(hepatic stellate cell, HSC)和CRC细胞相互作用, 通过CRC细胞中的CXCR4/TGF-β1轴使HSC中α-SMA表达增加, 从而使HSC分化为CAFs[29].
CAFs分泌的FGF-1/-3激活成纤维细胞生长因子受体(fibroblast growth factor receptor, FGFR4), 进而通过Mek/Erk的激活和MMP7表达的调节促进癌细胞生长和血管生成[22]. FAP-α可能通过Akt和ERK信号通路有效促进CRC中的血管生成[30]. WNT在胎盘血管形成中作为促血管生成因子, 其在CAFs中选择性表达升高, 有研究表明, 来自基质CRC-CAFs的Wnt2通过增加EC迁移和侵袭及促进CAFs分泌促血管生成因子IL-6、中粒细胞集落刺激因子(granulocyte colony-stimulating factor, G-CSF)和胎盘生长因子(placental growth factor, PGF)来增强血管生成[31]. 程序性死亡配体-1(programmed death ligand-1, PD-L1)在许多肿瘤中过表达, 在CRC中, CAFs在缺氧条件下分泌的外泌体circEIF3K被证明通过调节miR-214/PD-L1轴导致癌细胞增殖、侵袭和血管形成[32]. CAFs还可以通过miR-135b-5p下调叉头盒蛋白O1(forkhead box protein O1, FOXO1)抑制硫氧还蛋白相互作用蛋白(thioredoxin-interacting protein, TXNIP)促进CRC细胞生长和血管生成[33,34].
侵袭及转移是导致癌症相关死亡的重要原因. 由Lenos等[35]人进行的小鼠异种移植物中结直肠肿瘤干细胞(cancer stem cells, CSCs)的谱系追踪进一步强调了CAFs在将干细胞功能传达给肿瘤侵袭边缘的邻近细胞, 其分泌因子OPN可使CRC细胞重新编程为具有转移潜力的CSCs. 细胞外信号调节激酶5(extracellular signal-regulated kinase 5, ERK5)是MAPKs家族成员中独特的激酶, CAFs通过激活结直肠癌中的ERK5/PD-L1信号轴来促进细胞生长[36]. IL-6通过结合IL-6R并激活Janus激酶(janus kinase, JAK)和下游途径调节消化系统癌症中CAFs的多种促恶性功能, CAFs衍生的IL-6支持癌细胞中的EMT和STAT信号传导上调富含亮氨酸的α-2-糖蛋白1(leucine rich alpha-2-glycoprotein 1, LRG1)促进CRC的转移[37]. Todaro等人[38]研究发现CAFs分泌的生长因子OPN、HGF和SDF1激活Wnt/β-连环蛋白途径使CRC细胞获得CD44v6表型以及CSC表型, 随后发现表达CD44v6的CRC干细胞迁徙和侵袭能力增强. 通过对临床标本和原位肝转移模型的观察发现, CAFs通过HGF/MET/AKT信号通路上调伴有分化簇44(cluster of differentiation 44, CD44)增加了癌细胞对内皮细胞的黏附及肝转移中癌细胞的迁移[39]. CAFs外泌体miR-92a-3p靶向CRC细胞中的FBXW7和MOAP1抑制线粒体细胞凋亡, 促进迁移、侵袭[40]. LINC00659作为一种新型癌基因在CRC中显著表达, 来自CAFs的外泌体可以将LINC00659转移到CRC细胞, 上调LINC00659直接与miR-342-3P相互作用, 并增加CRC细胞中膜联蛋白A2(annexin A2, ANXA2)的表达, 后者促进CRC中细胞的侵袭、转移[41]. 另外, 来自CAFs的Wnt2蛋白、外泌体miR224-5p也可以诱导CRC细胞的增殖、侵袭和迁移[42,43].
代谢重编程是癌症的新兴标志之一. Warburg等人[44]报告说, 即使在存在足够氧气的情况下, 肿瘤细胞与正常组织相比, 肿瘤细胞的葡萄糖代谢也显着增强, 这一过程称为"Warburg效应", 是癌症的主要和代谢特征, 与癌细胞的代谢重编程有关. CAFs通过自我代谢重编程促进肿瘤生长: CAFs在肿瘤微环境中的糖酵解增加, CAFs产生的乳酸盐被肿瘤吸收和利用; CAFs通过三羧酸循环合成氨基酸, 氨基酸被肿瘤用于生物合成; 在CAFs中, 脂质代谢被重新编程, LPC被分泌到微环境中以促进肿瘤生长[45]. 例如: Sun等[46]报道, 缺氧诱导的氧化性共济失调-毛细血管扩张突变蛋白激酶(ataxia-telangiectasia mutated protein kinase, ATM)通过在S490位置磷酸化葡萄糖转运蛋白1(glucose transporter 1, GLUT1)并增加丙酮酸激酶M2(pyruvate kinase M2, PKM2)的表达来促进乳腺癌相关成纤维细胞中的糖酵解活性, 缺氧CAF衍生的乳酸盐提供CAF和乳腺癌细胞之间的代谢偶联, 通过激活TGF-β1/p38 MAPK/MMP2/9信号传导轴并促进癌细胞中的线粒体活性来促进乳腺癌细胞的侵袭. 人体皮肤鳞癌细胞和CAFs之间存在氨基酸代谢串扰, 天冬氨酸和谷氨酸通过天冬氨酸/谷氨酸转运蛋白在皮肤鳞癌细胞和CAF之间交换, 谷氨酸通过三羧酸循环产生天冬氨酸, 随后被CAF用于生物合成和维持细胞增殖, 或者谷氨酸在CAFs中形成谷胱甘肽以平衡细胞的氧化还原状态并促进ECM重塑[47].
CAFs-癌细胞代谢网络可能促进肿瘤生长, 基于使用代谢抑制剂的代谢治疗在治疗CRC方面取得一定进展[48]. CAFs通过刺激糖酵解、磷酸戊糖途径、谷氨酰胺溶解以及抑制三羧酸循环, 使CRC能够加速糖酵解及乳酸的生成加剧Warburg效应[49]. CRC中的CAFs诱导氧化应激, 经过糖酵解酶的增加、自噬等代谢改变, 为癌细胞增殖提供丙酮酸、乳酸及防止氧化损伤的保护促进CRC的存活[50]. Gong等[51]发现来自CRC组织的CAFs经历脂质组学重编程, 比NF排泄更多的脂肪酰基和磷脂, 通过使用CD36抑制剂在体内、体外证明CAFs分泌的脂肪酸被CRC吸收用于合成其他脂质促进CRC的迁移. CRC分泌的外泌体HSPC111改变CAFs的脂质代谢, 上调了乙酰辅酶A使CXCL5的表达增多, 促进CRC的肝转移[52]. CAFs还可通过上调肉碱棕榈酰转移酶IA(carnitine palmitoyl transferase IA, CPT1A)增强脂肪酸分解代谢以及上调磷脂酰胆碱中的不饱和酰基链来增加细胞膜流动性促进CRC细胞的迁徙和腹膜内传播[53,54].
耐药及放疗抵抗是CRC治疗失败的重要原因. 耐药性是癌细胞及肿瘤微环境共同作用的结果, CAFs通过为癌细胞提供保护性生态位从而抵抗抗癌药物[55,56].
2.5.1 CAFS通过促进癌症干细胞质介导耐药性: CSC被认为是造成CRC治疗耐药的重要原因. 研究发现[57,58], Wnt信号传导对于在癌症进展期建立耐药性至关重要, CAFs分泌的Wnt刺激已分化的CRC细胞恢复CSC特征, CSCs对化疗具有固有抵抗力, 同时外泌体Wnts导致CSCs产生更多耐药性. H19在结肠炎相关癌症小鼠肿瘤组织中与在正常结肠组织中表达相比高度表达, CAFs通过转移外泌体H19充当miR-141的竞争性内源性RNA海绵来激活β-连环蛋白途径, 而miR-141抑制CRC细胞的干细胞, 从而促进CRC的干性和化学抗性[59].
2.5.2 CAF分泌因子介导的耐药性: 源自CAFs的结直肠癌相关lncRNA(CCAL)通过与mRNA稳定蛋白HuR相互作用上调β-连环蛋白的表达, 后者促进CRC的奥沙利铂(oxaliplatin, L-OHP)耐药性[60]. 成纤维细胞是IL-6的重要来源, 有数据表明[61], IL-6/JAK2信号传导介导的BECN1磷酸化有助于CRC的化疗耐药性. 李明等[62]发现CAFs可能通过上调LoVo细胞环氧合酶-2(cyclooxygenase-2, COX-2)表达和前列腺素E2(prostaglandin E2, PGE2)合成及诱导EMT导致癌细胞对L-OHP的敏感性降低. CAFs分泌的外泌体miR-92a-3p通过直接抑制FBXW7和MOAP1来激活Wnt/β-catenin途径并抑制线粒体凋亡, 有助于CRC细胞对5-氟尿嘧啶(5-fluorouracil, 5-FU)/L-OHP抗性[40]. 伊立替康(irinotecan, CTP-11)是喜树碱的半合成类似物, CPT-11被用作晚期或复发性结直肠癌的一线和二线化疗, 有研究证明了CAFs衍生的HGF通过c-MET信号传导, 促进CRC细胞对CPT-11耐药性[63]. CAFs通过富含亮氨酸重复的G蛋白偶联受体5(leucine-rich repeat-containing G-protein-coupled receptor 5, LGR5), β-连环蛋白和雷帕霉素(mammalian target of rapamycin, mTOR)信号传导的表达增加使DLD1和HCT116细胞明显更具肿瘤性, 导致5-FU耐药性和肿瘤球形成能力增强[64]. CAF来源的外泌体miR-24-3p通过下调尾部相关同源盒2(caudal-related homeobox 2, Cdx2)/肝素(hephaestin, HEPH)轴加速了CRC细胞对甲氨蝶呤的抵抗力[65]. 也有学者证明了CAFs衍生的外泌体通过METTL3/miR-181d-5p轴抑制CRC对5-FU敏感性[66].
2.5.3 CAF分泌因子介导的放疗抵抗: CAFs的外泌体通过激活TGF-β信号通路促进CRC细胞的干性, 从而增加了抗辐射性[67]. CAFs还可通过外泌体miR-93-5p促进细胞增殖和集落形成及通过下调FOXA1表达抑制CRC凋亡促进其放疗抵抗[68]. 研究发现CAFs分泌的miR-31的抑制上调了结直肠CAFs中的自噬, 促进了CRC细胞的增殖、侵袭和转移, 此外还增加了CRC细胞的放射敏感性[69].
综上, CAFs可通过多种途径导致耐药及放疗抵抗.
多数研究表明CAFs浸润提示预后较差. 通过对375名CRC患者的组织芯片定量了CAFs中STAT3(pSTAT3)的磷酸化表达, 显示基质pSTAT3表达增加与CRC生存率呈负相关[70]. 也有学者通过免疫组织化学评估了CRC揭示了CAFs高表达CD70的CRC的生存率更差[71]. FAP是一个独立的阴性预后因素, PAF高表达的CRC患者的预后较差[72]. Wnt5a是CAF中CRC进展的调节因子, CAF中的Wnt5a状态与肿瘤大小、浸润深度、淋巴和血管浸润、淋巴结转移和复发显著相关[73]. Du等[74]发现在CRC患者中vimentin表达预测的总生存率和无病生存率较差, 上调的vimentin可能意味着预后不良. Zhu等人[75]通过CRC标本的CAFs和邻近的NFs进行免疫组化显示, C-型凝集素域家族3成员B(C-type lectin domain family 3 member B, CLEC3B)表达与CRC的血清浸润有关, CLEC3B和α-SMA共表达的患者比仅表达CLEC3B或α-SMA的患者具有更差的生存结局. 有研究证明, ADAM12与CRC患者生存期相关, ADAM12低表达者中位生存期为25.3 mo, 而ADAM12高表达的CRC患者中位生存期仅为17.1 mo[76]. 进一步研究CAFs相关分泌物、蛋白等在临床上的应用是今后研究的方向.
CAFs通过上调趋化因子CC基序配体2[Chemokine(C-C motif)ligand 2, CCL2]分泌来招募骨髓细胞, 促进CRC免疫微环境中的免疫抑制[77]. Zhang等[78]发现CRC中的CAFs产生IL-8吸引单核细胞并产生IL-6促进血管细胞粘附分子-1(vascular cell adhesion molecule-1, VCAM-1)在CRC中表达并增强单核细胞黏附, 同时促进巨噬细胞M2极化, 与CAFs协同抑制自然杀伤细胞的功能. CRC中CAF表达的黑色素瘤细胞黏附分子与IL-1受体相互作用, 增强κB-IL34/CCL8信号传导, 导致肿瘤相关巨噬细胞趋化性[79]. 另外, CAFs衍生的CXCL5通过激活磷脂酰肌醇-3-激酶(phosphoinositide 3-kinase, PI3K)/AKT信号传导促进小鼠肿瘤细胞中PD-L1表达, 保护了免疫抑制微环境[80].
以CAFs为靶点的疗法目前是一个深入研究的领域. 在Fourniols等人[81]的一项研究中, 紫杉醇(paclitaxel, PTC)和吖啶黄素(acriflavine, ACF)作为CAF发展潜在抑制剂的疗效被测试, 使用PTX和ACF的脂质纳米胶囊(lipid nanocapsules, LNC)制剂证明了LNC-ACF对CAF的抑制和LNC-PTX对CRC的抑制. CAFs衍生的外泌体circSLC7A6可作为CRC细胞增殖和侵袭的启动子, Gu等人[82]发现苦参碱通过阻断circSLC7A6外泌体的分泌, 从而抑制CRC的增殖. 研究证明α5整合素亚基只要位于结直肠肿瘤成纤维细胞中, α5整合素亚基消耗显著抑制了异种移植裸鼠中CAF诱导的肿瘤生长, 并抑制了CAF在体外诱导的癌细胞迁移和侵袭, 表明CAF表达的α5整合素亚基可能作为结直肠腺癌的预后治疗靶点[83].
然而, 许多针对CAF或相关基质成分的治疗策略未能显著改善临床结果, 识别和精确表征不同CAF人群的肿瘤促进和肿瘤抑制功能可能为开发新的诊断和治疗方法提供机会[84].
作为肿瘤免疫微环境的重要组成部分, CAFs对于CRC的调节作用已被广泛认可, 但其具体作用机制尚未完全阐明. 虽然多数研究指出CAFs与CRC预后不佳相关, 但也有相关研究指出CAFs具有肿瘤抑制作用. 通过对文献的梳理发现,CRC与CAFs相互作用调控CRC细胞的增殖、侵袭与转移、代谢、耐药; CAFs有望成为CRC治疗的靶点, 但目前针CAFs靶点的治疗药物仍需进行更深入的研究. 总的来说, 我们仍需要继续研究获得更精细的CAFs分型及功能性质, 进一步揭示CAFs在CRC的作用机制及预后的预测价值, 明确靶向CAFs治疗的适应证和潜在获益人群, 从而成为临床治疗靶点, 以期改善CRC患者的生活质量.
学科分类: 胃肠病学和肝病学
手稿来源地: 广西壮族自治区
同行评议报告学术质量分类
A级 (优秀): 0
B级 (非常好): B, B
C级 (良好): C
D级 (一般): 0
E级 (差): 0
科学编辑:张砚梁 制作编辑:张砚梁
| 1. | Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14:101174. [PubMed] [DOI] |
| 2. | Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017;387:61-68. [PubMed] [DOI] |
| 3. | Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582-598. [PubMed] [DOI] |
| 4. | Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR, Hunter T, Hynes RO, Jain RK, Janowitz T, Jorgensen C, Kimmelman AC, Kolonin MG, Maki RG, Powers RS, Puré E, Ramirez DC, Scherz-Shouval R, Sherman MH, Stewart S, Tlsty TD, Tuveson DA, Watt FM, Weaver V, Weeraratna AT, Werb Z. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020;20:174-186. [PubMed] [DOI] |
| 5. | Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, Zhang B, Meng Q, Yu X, Shi S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021;20:131. [PubMed] [DOI] |
| 6. | An Y, Liu F, Chen Y, Yang Q. Crosstalk between cancer-associated fibroblasts and immune cells in cancer. J Cell Mol Med. 2020;24:13-24. [PubMed] [DOI] |
| 7. | Santi A, Kugeratski FG, Zanivan S. Cancer Associated Fibroblasts: The Architects of Stroma Remodeling. Proteomics. 2018;18:e1700167. [PubMed] [DOI] |
| 8. | Ziani L, Chouaib S, Thiery J. Alteration of the Antitumor Immune Response by Cancer-Associated Fibroblasts. Front Immunol. 2018;9:414. [PubMed] [DOI] |
| 9. | Biffi G, Tuveson DA. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol Rev. 2021;101:147-176. [PubMed] [DOI] |
| 10. | Peltanova B, Raudenska M, Masarik M. Effect of tumor microen-vironment on pathogenesis of the head and neck squamous cell carcinoma: a systematic review. Mol Cancer. 2019;18:63. [PubMed] [DOI] |
| 11. | Nagasaki T, Hara M, Nakanishi H, Takahashi H, Sato M, Takeyama H. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br J Cancer. 2014;110:469-478. [PubMed] [DOI] |
| 12. | Ding X, Ji J, Jiang J, Cai Q, Wang C, Shi M, Yu Y, Zhu Z, Zhang J. HGF-mediated crosstalk between cancer-associated fibroblasts and MET-unamplified gastric cancer cells activates coordinated tumorigenesis and metastasis. Cell Death Dis. 2018;9:867. [PubMed] [DOI] |
| 13. | Mezawa Y, Daigo Y, Takano A, Miyagi Y, Yokose T, Yamashita T, Morimoto C, Hino O, Orimo A. CD26 expression is attenuated by TGF-β and SDF-1 autocrine signaling on stromal myofibroblasts in human breast cancers. Cancer Med. 2019;8:3936-3948. [PubMed] [DOI] |
| 14. | Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341-350. [PubMed] [DOI] |
| 15. | Ferdek PE, Jakubowska MA. Biology of pancreatic stellate cells-more than just pancreatic cancer. Pflugers Arch. 2017;469:1039-1050. [PubMed] [DOI] |
| 16. | Strong AL, Pei DT, Hurst CG, Gimble JM, Burow ME, Bunnell BA. Obesity Enhances the Conversion of Adipose-Derived Stromal/Stem Cells into Carcinoma-Associated Fibroblast Leading to Cancer Cell Proliferation and Progression to an Invasive Phenotype. Stem Cells Int. 2017;2017:9216502. [PubMed] [DOI] |
| 17. | LeBleu VS, Neilson EG. Origin and functional heterogeneity of fibroblasts. FASEB J. 2020;34:3519-3536. [PubMed] [DOI] |
| 18. | Li H, Courtois ET, Sengupta D, Tan Y, Chen KH, Goh JJL, Kong SL, Chua C, Hon LK, Tan WS, Wong M, Choi PJ, Wee LJK, Hillmer AM, Tan IB, Robson P, Prabhakar S. Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat Genet. 2017;49:708-718. [PubMed] [DOI] |
| 19. | Rai A, Greening DW, Chen M, Xu R, Ji H, Simpson RJ. Exosomes Derived from Human Primary and Metastatic Colorectal Cancer Cells Contribute to Functional Heterogeneity of Activated Fibroblasts by Reprogramming Their Proteome. Proteomics. 2019;19:e1800148. [PubMed] [DOI] |
| 20. | 王 甜甜, 陆 建波, 普 苹. 癌相关成纤维细胞对结肠癌细胞增殖侵袭能力的影响. 临床与实验病理学杂志. 2013;5. |
| 21. | Mochizuki S, Ao T, Sugiura T, Yonemura K, Shiraishi T, Kajiwara Y, Okamoto K, Shinto E, Okada Y, Ueno H. Expression and Function of a Disintegrin and Metalloproteinases in Cancer-Associated Fibroblasts of Colorectal Cancer. Digestion. 2020;101:18-24. [PubMed] [DOI] |
| 22. | Bai YP, Shang K, Chen H, Ding F, Wang Z, Liang C, Xu Y, Sun MH, Li YY. FGF-1/-3/FGFR4 signaling in cancer-associated fibroblasts promotes tumor progression in colon cancer through Erk and MMP-7. Cancer Sci. 2015;106:1278-1287. [PubMed] [DOI] |
| 23. | Higuchi Y, Kojima M, Ishii G, Aoyagi K, Sasaki H, Ochiai A. Gastrointestinal Fibroblasts Have Specialized, Diverse Transcriptional Phenotypes: A Comprehensive Gene Expression Analysis of Human Fibroblasts. PLoS One. 2015;10:e0129241. [PubMed] [DOI] |
| 24. | Ferrer-Mayorga G, Gómez-López G, Barbáchano A, Fernández-Barral A, Peña C, Pisano DG, Cantero R, Rojo F, Muñoz A, Larriba MJ. Vitamin D receptor expression and associated gene signature in tumour stromal fibroblasts predict clinical outcome in colorectal cancer. Gut. 2017;66:1449-1462. [PubMed] [DOI] |
| 25. | Rai A, Greening DW, Xu R, Suwakulsiri W, Simpson RJ. Exosomes Derived from the Human Primary Colorectal Cancer Cell Line SW480 Orchestrate Fibroblast-Led Cancer Invasion. Proteomics. 2020;20:e2000016. [PubMed] [DOI] |
| 26. | Wang L, Yang D, Tian J, Gao A, Shen Y, Ren X, Li X, Jiang G, Dong T. Tumor necrosis factor receptor 2/AKT and ERK signaling pathways contribute to the switch from fibroblasts to CAFs by progranulin in microenvironment of colorectal cancer. Oncotarget. 2017;8:26323-26333. [PubMed] [DOI] |
| 27. | Tan HX, Xiao ZG, Huang T, Fang ZX, Liu Y, Huang ZC. CXCR4/TGF-β1 mediated self-differentiation of human mesenchymal stem cells to carcinoma-associated fibroblasts and promoted colorectal carcinoma development. Cancer Biol Ther. 2020;21:248-257. [PubMed] [DOI] |
| 28. | Harper J, Sainson RC. Regulation of the anti-tumour immune response by cancer-associated fibroblasts. Semin Cancer Biol. 2014;25:69-77. [PubMed] [DOI] |
| 29. | Tan HX, Gong WZ, Zhou K, Xiao ZG, Hou FT, Huang T, Zhang L, Dong HY, Zhang WL, Liu Y, Huang ZC. CXCR4/TGF-β1 mediated hepatic stellate cells differentiation into carcinoma-associated fibroblasts and promoted liver metastasis of colon cancer. Cancer Biol Ther. 2020;21:258-268. [PubMed] [DOI] |
| 30. | Cao F, Wang S, Wang H, Tang W. Fibroblast activation protein-α in tumor cells promotes colorectal cancer angiogenesis via the Akt and ERK signaling pathways. Mol Med Rep. 2018;17:2593-2599. [PubMed] [DOI] |
| 31. | Unterleuthner D, Neuhold P, Schwarz K, Janker L, Neuditschko B, Nivarthi H, Crncec I, Kramer N, Unger C, Hengstschläger M, Eferl R, Moriggl R, Sommergruber W, Gerner C, Dolznig H. Cancer-associated fibroblast-derived WNT2 increases tumor angiogenesis in colon cancer. Angiogenesis. 2020;23:159-177. [PubMed] [DOI] |
| 32. | Yang K, Zhang J, Bao C. Exosomal circEIF3K from cancer-associated fibroblast promotes colorectal cancer (CRC) progression via miR-214/PD-L1 axis. BMC Cancer. 2021;21:933. [PubMed] [DOI] |
| 33. | Dai X, Xie Y, Dong M. Cancer-associated fibroblasts derived extracellular vesicles promote angiogenesis of colorectal adenocarcinoma cells through miR-135b-5p/FOXO1 axis. Cancer Biol Ther. 2022;23:76-88. [PubMed] [DOI] |
| 34. | Yin H, Yu S, Xie Y, Dai X, Dong M, Sheng C, Hu J. Cancer-associated fibroblasts-derived exosomes upregulate microRNA-135b-5p to promote colorectal cancer cell growth and angiogenesis by inhibiting thioredoxin-interacting protein. Cell Signal. 2021;84:110029. [PubMed] [DOI] |
| 35. | Lenos KJ, Miedema DM, Lodestijn SC, Nijman LE, van den Bosch T, Romero Ros X, Lourenço FC, Lecca MC, van der Heijden M, van Neerven SM, van Oort A, Leveille N, Adam RS, de Sousa E Melo F, Otten J, Veerman P, Hypolite G, Koens L, Lyons SK, Stassi G, Winton DJ, Medema JP, Morrissey E, Bijlsma MF, Vermeulen L. Stem cell functionality is microenvironmentally defined during tumour expansion and therapy response in colon cancer. Nat Cell Biol. 2018;20:1193-1202. [PubMed] [DOI] |
| 36. | Zhang M, Shi R, Guo Z, He J. Cancer-associated fibroblasts promote cell growth by activating ERK5/PD-L1 signaling axis in colorectal cancer. Pathol Res Pract. 2020;216:152884. [PubMed] [DOI] |
| 37. | Zhong B, Cheng B, Huang X, Xiao Q, Niu Z, Chen YF, Yu Q, Wang W, Wu XJ. Colorectal cancer-associated fibroblasts promote metastasis by up-regulating LRG1 through stromal IL-6/STAT3 signaling. Cell Death Dis. 2021;13:16. [PubMed] [DOI] |
| 38. | Todaro M, Gaggianesi M, Catalano V, Benfante A, Iovino F, Biffoni M, Apuzzo T, Sperduti I, Volpe S, Cocorullo G, Gulotta G, Dieli F, De Maria R, Stassi G. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell. 2014;14:342-356. [PubMed] [DOI] |
| 39. | Zhang R, Qi F, Shao S, Li G, Feng Y. Human colorectal cancer-derived carcinoma associated fibroblasts promote CD44-mediated adhesion of colorectal cancer cells to endothelial cells by secretion of HGF. Cancer Cell Int. 2019;19:192. [PubMed] [DOI] |
| 40. | Hu JL, Wang W, Lan XL, Zeng ZC, Liang YS, Yan YR, Song FY, Wang FF, Zhu XH, Liao WJ, Liao WT, Ding YQ, Liang L. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol Cancer. 2019;18:91. [PubMed] [DOI] |
| 41. | Zhou L, Li J, Tang Y, Yang M. Exosomal LncRNA LINC00659 transferred from cancer-associated fibroblasts promotes colorectal cancer cell progression via miR-342-3p/ANXA2 axis. J Transl Med. 2021;19:8. [PubMed] [DOI] |
| 42. | Aizawa T, Karasawa H, Funayama R, Shirota M, Suzuki T, Maeda S, Suzuki H, Yamamura A, Naitoh T, Nakayama K, Unno M. Cancer-associated fibroblasts secrete Wnt2 to promote cancer progression in colorectal cancer. Cancer Med. 2019;8:6370-6382. [PubMed] [DOI] |
| 43. | Zheng Y, Zeng J, Lin D, Xia H, Wang X, Chen L, Chen H, Huang L, Zeng C. Extracellular vesicles derived from cancer-associated fibroblast carries miR-224-5p targeting SLC4A4 to promote the proliferation, invasion and migration of colorectal cancer cells. Carcinogenesis. 2021;42:1143-1153. [PubMed] [DOI] |
| 44. | Warburg O, Wind F, Negelein E. THE METABOLISM OF TUMORS IN THE BODY. J Gen Physiol. 1927;8:519-530. [PubMed] [DOI] |
| 45. | Li Z, Sun C, Qin Z. Metabolic reprogramming of cancer-associated fibroblasts and its effect on cancer cell reprogramming. Theranostics. 2021;11:8322-8336. [PubMed] [DOI] |
| 46. | Sun K, Tang S, Hou Y, Xi L, Chen Y, Yin J, Peng M, Zhao M, Cui X, Liu M. Oxidized ATM-mediated glycolysis enhancement in breast cancer-associated fibroblasts contributes to tumor invasion through lactate as metabolic coupling. EBioMedicine. 2019;41:370-383. [PubMed] [DOI] |
| 47. | Bertero T, Oldham WM, Grasset EM, Bourget I, Boulter E, Pisano S, Hofman P, Bellvert F, Meneguzzi G, Bulavin DV, Estrach S, Feral CC, Chan SY, Bozec A, Gaggioli C. Tumor-Stroma Mechanics Coordinate Amino Acid Availability to Sustain Tumor Growth and Malignancy. Cell Metab. 2019;29:124-140.e10. [PubMed] [DOI] |
| 48. | Nenkov M, Ma Y, Gaßler N, Chen Y. Metabolic Reprogramming of Colorectal Cancer Cells and the Microenvironment: Implication for Therapy. Int J Mol Sci. 2021;22. [PubMed] [DOI] |
| 49. | Wang J, Delfarah A, Gelbach PE, Fong E, Macklin P, Mumenthaler SM, Graham NA, Finley SD. Elucidating tumor-stromal metabolic crosstalk in colorectal cancer through integration of constraint-based models and LC-MS metabolomics. Metab Eng. 2022;69:175-187. [PubMed] [DOI] |
| 50. | Zhou W, Xu G, Wang Y, Xu Z, Liu X, Xu X, Ren G, Tian K. Oxidative stress induced autophagy in cancer associated fibroblast enhances proliferation and metabolism of colorectal cancer cells. Cell Cycle. 2017;16:73-81. [PubMed] [DOI] |
| 51. | Gong J, Lin Y, Zhang H, Liu C, Cheng Z, Yang X, Zhang J, Xiao Y, Sang N, Qian X, Wang L, Cen X, Du X, Zhao Y. Reprogramming of lipid metabolism in cancer-associated fibroblasts potentiates migration of colorectal cancer cells. Cell Death Dis. 2020;11:267. [PubMed] [DOI] |
| 52. | Zhang C, Wang XY, Zhang P, He TC, Han JH, Zhang R, Lin J, Fan J, Lu L, Zhu WW, Jia HL, Zhang JB, Chen JH. Cancer-derived exosomal HSPC111 promotes colorectal cancer liver metastasis by reprogramming lipid metabolism in cancer-associated fibroblasts. Cell Death Dis. 2022;13:57. [PubMed] [DOI] |
| 53. | Peng S, Chen D, Cai J, Yuan Z, Huang B, Li Y, Wang H, Luo Q, Kuang Y, Liang W, Liu Z, Wang Q, Cui Y, Wang H, Liu X. Enhancing cancer-associated fibroblast fatty acid catabolism within a metabolically challenging tumor microenvironment drives colon cancer peritoneal metastasis. Mol Oncol. 2021;15:1391-1411. [PubMed] [DOI] |
| 54. | Peng S, Li Y, Huang M, Tang G, Xie Y, Chen D, Hu Y, Yu T, Cai J, Yuan Z, Wang H, Wang H, Luo Y, Liu X. Metabolomics reveals that CAF-derived lipids promote colorectal cancer peritoneal metastasis by enhancing membrane fluidity. Int J Biol Sci. 2022;18:1912-1932. [PubMed] [DOI] |
| 55. | Jena BC, Das CK, Bharadwaj D, Mandal M. Cancer associated fibroblast mediated chemoresistance: A paradigm shift in understanding the mechanism of tumor progression. Biochim Biophys Acta Rev Cancer. 2020;1874:188416. [PubMed] [DOI] |
| 56. | Bu L, Baba H, Yasuda T, Uchihara T, Ishimoto T. Functional diversity of cancer-associated fibroblasts in modulating drug resistance. Cancer Sci. 2020;111:3468-3477. [PubMed] [DOI] |
| 57. | Hu Y, Yan C, Mu L, Huang K, Li X, Tao D, Wu Y, Qin J. Fibroblast-Derived Exosomes Contribute to Chemoresistance through Priming Cancer Stem Cells in Colorectal Cancer. PLoS One. 2015;10:e0125625. [PubMed] [DOI] |
| 58. | Hu YB, Yan C, Mu L, Mi YL, Zhao H, Hu H, Li XL, Tao DD, Wu YQ, Gong JP, Qin JC. Exosomal Wnt-induced dedifferentiation of colorectal cancer cells contributes to chemotherapy resistance. Oncogene. 2019;38:1951-1965. [PubMed] [DOI] |
| 59. | Ren J, Ding L, Zhang D, Shi G, Xu Q, Shen S, Wang Y, Wang T, Hou Y. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics. 2018;8:3932-3948. [PubMed] [DOI] |
| 60. | Deng X, Ruan H, Zhang X, Xu X, Zhu Y, Peng H, Zhang X, Kong F, Guan M. Long noncoding RNA CCAL transferred from fibroblasts by exosomes promotes chemoresistance of colorectal cancer cells. Int J Cancer. 2020;146:1700-1716. [PubMed] [DOI] |
| 61. | Hu F, Song D, Yan Y, Huang C, Shen C, Lan J, Chen Y, Liu A, Wu Q, Sun L, Xu F, Hu F, Chen L, Luo X, Feng Y, Huang S, Hu J, Wang G. IL-6 regulates autophagy and chemotherapy resistance by promoting BECN1 phosphorylation. Nat Commun. 2021;12:3651. [PubMed] [DOI] |
| 63. | Woo JK, Kang JH, Kim B, Park BH, Shin KJ, Song SW, Kim JJ, Kim HM, Lee SJ, Oh SH. Humanized anti-hepatocyte growth factor (HGF) antibody suppresses innate irinotecan (CPT-11) resistance induced by fibroblast-derived HGF. Oncotarget. 2015;6:24047-24060. [PubMed] [DOI] |
| 64. | Yadav VK, Huang YJ, George TA, Wei PL, Sumitra MR, Ho CL, Chang TH, Wu ATH, Huang HS. Preclinical Evaluation of the Novel Small-Molecule MSI-N1014 for Treating Drug-Resistant Colon Cancer via the LGR5/β-catenin/miR-142-3p Network and Reducing Cancer-Associated Fibroblast Transformation. Cancers (Basel). 2020;12. [PubMed] [DOI] |
| 65. | Zhang HW, Shi Y, Liu JB, Wang HM, Wang PY, Wu ZJ, Li L, Gu LP, Cao PS, Wang GR, Ma YS, Fu D. Cancer-associated fibroblast-derived exosomal microRNA-24-3p enhances colon cancer cell resistance to MTX by down-regulating CDX2/HEPH axis. J Cell Mol Med. 2021;25:3699-3713. [PubMed] [DOI] |
| 66. | Pan S, Deng Y, Fu J, Zhang Y, Zhang Z, Qin X. N6methyla-denosine upregulates miR181d5p in exosomes derived from cancerassociated fibroblasts to inhibit 5FU sensitivity by targeting NCALD in colorectal cancer. Int J Oncol. 2022;60. [PubMed] [DOI] |
| 67. | Liu L, Zhang Z, Zhou L, Hu L, Yin C, Qing D, Huang S, Cai X, Chen Y. Cancer associated fibroblasts-derived exosomes contribute to radioresistance through promoting colorectal cancer stem cells phenotype. Exp Cell Res. 2020;391:111956. [PubMed] [DOI] |
| 68. | Chen X, Liu J, Zhang Q, Liu B, Cheng Y, Zhang Y, Sun Y, Ge H, Liu Y. Exosome-mediated transfer of miR-93-5p from cancer-associated fibroblasts confer radioresistance in colorectal cancer cells by downregulating FOXA1 and upregulating TGFB3. J Exp Clin Cancer Res. 2020;39:65. [PubMed] [DOI] |
| 69. | Yang X, Xu X, Zhu J, Zhang S, Wu Y, Wu Y, Zhao K, Xing C, Cao J, Zhu H, Li M, Ye Z, Peng W. miR-31 affects colorectal cancer cells by inhibiting autophagy in cancer-associated fibroblasts. Oncotarget. 2016;7:79617-79628. [PubMed] [DOI] |
| 70. | Heichler C, Scheibe K, Schmied A, Geppert CI, Schmid B, Wirtz S, Thoma OM, Kramer V, Waldner MJ, Büttner C, Farin HF, Pešić M, Knieling F, Merkel S, Grüneboom A, Gunzer M, Grützmann R, Rose-John S, Koralov SB, Kollias G, Vieth M, Hartmann A, Greten FR, Neurath MF, Neufert C. STAT3 activation through IL-6/IL-11 in cancer-associated fibroblasts promotes colorectal tumour development and correlates with poor prognosis. Gut. 2020;69:1269-1282. [PubMed] [DOI] |
| 71. | Inoue S, Ito H, Tsunoda T, Murakami H, Ebi M, Ogasawara N, Kasugai K, Kasai K, Ikeda H, Inaguma S. CD70 expression in tumor-associated fibroblasts predicts worse survival in colorectal cancer patients. Virchows Arch. 2019;475:425-434. [PubMed] [DOI] |
| 72. | Wikberg ML, Edin S, Lundberg IV, Van Guelpen B, Dahlin AM, Rutegård J, Stenling R, Oberg A, Palmqvist R. High intratumoral expression of fibroblast activation protein (FAP) in colon cancer is associated with poorer patient prognosis. Tumour Biol. 2013;34:1013-1020. [PubMed] [DOI] |
| 73. | Hirashima T, Karasawa H, Aizawa T, Suzuki T, Yamamura A, Suzuki H, Kajiwara T, Musha H, Funayama R, Shirota M, Ohnuma S, Nakayama K, Unno M. Wnt5a in cancer-associated fibroblasts promotes colorectal cancer progression. Biochem Biophys Res Commun. 2021;568:37-42. [PubMed] [DOI] |
| 74. | Du L, Li J, Lei L, He H, Chen E, Dong J, Yang J. High Vimentin Expression Predicts a Poor Prognosis and Progression in Colorectal Cancer: A Study with Meta-Analysis and TCGA Database. Biomed Res Int. 2018;2018:6387810. [PubMed] [DOI] |
| 75. | Zhu HF, Zhang XH, Gu CS, Zhong Y, Long T, Ma YD, Hu ZY, Li ZG, Wang XY. Cancer-associated fibroblasts promote colorectal cancer progression by secreting CLEC3B. Cancer Biol Ther. 2019;20:967-978. [PubMed] [DOI] |
| 76. | Ten Hoorn S, Waasdorp C, van Oijen MGH, Damhofer H, Trinh A, Zhao L, Smits LJH, Bootsma S, van Pelt GW, Mesker WE, Mol L, Goey KKH, Koopman M, Medema JP, Tuynman JB, Zlobec I, Punt CJA, Vermeulen L, Bijlsma MF. Serum-based measurements of stromal activation through ADAM12 associate with poor prognosis in colorectal cancer. BMC Cancer. 2022;22:394. [PubMed] [DOI] |
| 77. | Chen L, Qiu X, Wang X, He J. FAP positive fibroblasts induce immune checkpoint blockade resistance in colorectal cancer via promoting immunosuppression. Biochem Biophys Res Commun. 2017;487:8-14. [PubMed] [DOI] |
| 78. | Zhang R, Qi F, Zhao F, Li G, Shao S, Zhang X, Yuan L, Feng Y. Cancer-associated fibroblasts enhance tumor-associated macrophages enrichment and suppress NK cells function in colorectal cancer. Cell Death Dis. 2019;10:273. [PubMed] [DOI] |
| 79. | Kobayashi H, Gieniec KA, Lannagan TRM, Wang T, Asai N, Mizutani Y, Iida T, Ando R, Thomas EM, Sakai A, Suzuki N, Ichinose M, Wright JA, Vrbanac L, Ng JQ, Goyne J, Radford G, Lawrence MJ, Sammour T, Hayakawa Y, Klebe S, Shin AE, Asfaha S, Bettington ML, Rieder F, Arpaia N, Danino T, Butler LM, Burt AD, Leedham SJ, Rustgi AK, Mukherjee S, Takahashi M, Wang TC, Enomoto A, Woods SL, Worthley DL. The Origin and Contribution of Cancer-Associated Fibroblasts in Colorectal Carcinogenesis. Gastroenterology. 2022;162:890-906. [PubMed] [DOI] |
| 80. | Li Z, Zhou J, Zhang J, Li S, Wang H, Du J. Cancer-associated fibroblasts promote PD-L1 expression in mice cancer cells via secreting CXCL5. Int J Cancer. 2019;145:1946-1957. [PubMed] [DOI] |
| 81. | Fourniols T, Bastien E, Canevat A, Feron O, Préat V. Inhibition of colorectal cancer-associated fibroblasts by lipid nanocapsules loaded with acriflavine or paclitaxel. Int J Pharm. 2020;584:119337. [PubMed] [DOI] |
| 82. | Gu C, Lu H, Qian Z. Matrine reduces the secretion of exosomal circSLC7A6 from cancer-associated fibroblast to inhibit tumorigenesis of colorectal cancer by regulating CXCR5. Biochem Biophys Res Commun. 2020;527:638-645. [PubMed] [DOI] |
| 83. | Lu L, Xie R, Wei R, Cai C, Bi D, Yin D, Liu H, Zheng J, Zhang Y, Song F, Gao Y, Tan L, Wei Q, Qin H. Integrin α5 subunit is required for the tumor supportive role of fibroblasts in colorectal adenocarcinoma and serves as a potential stroma prognostic marker. Mol Oncol. 2019;13:2697-2714. [PubMed] [DOI] |
| 84. | Chen Y, McAndrews KM, Kalluri R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol. 2021;18:792-804. [PubMed] [DOI] |