Abstract
A study on 51 SNPs belonging to 29 genes related to heat stress was carried out in 720 sheep from 17 different breeds adapted to different climates from Hungary, Bosnia and Herzegovina, Morocco and Romania, using Kompetitive Allele-Specific Polymerase Chain Reaction. Genotype frequency and the Hardy–Weinberg equilibrium were calculated, followed by a clustering using the Principal Component Analysis. We analyzed the polymorphisms in the following genes analyzed: HSPA12A, HSP90AA1, IL33, DIO2, BTNL2, CSN2, ABCG1, CSN1S1, GHR, HSPA8, STAT3, and HCRT. We emphasized on HSPA12A and HSPA8 genes as they were successfully genotyped in all studied flocks in which genotype frequency patterns were identified. Contrary to previous findings, the A allele for HSPA8 SNP was not observed in the heat tolerant breeds, being found exclusively in cold-tolerant breeds. The principal component analysis could not clearly differentiate the breeds, while plot concentration was slightly varied among the three groups, with HSP90AA1 and IL33 SNPs’ loading values significantly contributing to PC1 and PC2. We confirmed previous works that the HSPA12A, HSPA8, HSP90AA1 and IL33 SNPs are potential candidate markers for thermotolerance adaptation in sheep. This research contributes to the genetic variability of SNPs for thermotolerance adaptability in sheep.
Similar content being viewed by others
Introduction
Climate change is a complex phenomenon with disastrous consequences in almost all aspects of human societies, including in the livestock sector. Human activities would have contributed to a 1.0 °C increase in global temperature above pre-industrial levels. If current trends continue, global warming will likely approach 1.5 °C between 2030 and 20521. Furthermore, according to reports from several international organizations involved in climate change research, practically all sectors of the economy and all regimes around the world will suffer from the negative effects of climate change, although to varying degrees, such as the irreversible loss of some natural ecosystems2,3,4,5. Particular attention should be paid to temperature variations as the most significant stressor on the efficiency of livestock production systems, affecting animal development, growth, production outputs and reproduction efficiency.
Compared to other ruminants, sheep tend to perform better in harsh environments, nevertheless, heat stress was shown to negatively impact physiology6,7,8, production parameters (milk production quality and quantity9,10, growth rates11), reproduction (male12 and female fertility13), sheep health14, and sheep welfare15, lowering farms economic returns and efficiency. Van Wettere et al.16 reported that when temperatures drop below 12 °C or increase over 31 °C, thermoregulatory mechanisms are severely disrupted and sheep's ability to maintain homeothermy is disturbed, with the impacts on performance and welfare being severe.
Heat stress is cytotoxic as it changes biological molecules, disrupts cell activities, affects metabolic responses, causes oxidative cell damage, and activates apoptosis and necrosis pathways17,18,19. In ewes, heat stress reduces estradiol concentration and aromatase activity, negatively influencing estrous occurrence and duration20,21,22. For each additional day of high-temperature exposure (> 32 °C) in a week prior to estrous onset, 2.7% of the fertilization rate and 3.5% of the lambing rate reduction were observed16. Heat stress also leads to higher circulating progesterone levels during the luteal phase, implying increased progesterone synthesis and decreased clearance, which impacts the timing and development of pre-ovulatory follicle growth13. Research with Malpura sheep housed in a climatic chamber for 6 h at 42 °C and 54% relative humidity found a significant drop in hemoglobin and packed cell volume levels as more water is transported through the thermoregulation circulatory system. Furthermore, higher plasma cortisol levels and increased cholesterol catabolism were involved in improving gluconeogenesis and providing more energy to heat-stressed rams. Because of the lower Gonadotropin releasing hormone (GnRH) release from the hypothalamus, plasma triiodothyronine (T3), thyroxine (T4) and testosterone levels were lowered, as were the sexual behaviour and semen volume, with less progressive sperm motility and sperm concentration23,24.
A critical component of adaptation to climate change is represented by the animals' genetic capacity to survive under stressful climatic conditions. Since it results in a permanent and cumulative transformation, the selection of thermoresistant animals could represent an efficient method of increasing livestock productivity during periods of high environmental temperature. Identifying and utilizing thermo-tolerant genotypes in sheep is critical due to the changing climate scenario, having the potential to significantly influence sheep productivity.
Thermotolerant genes in sheep have been studied by employing various genomic tools, for example, in heat stress protein genes; HSP90 and HSP70 genes polymorphism were investigated in Indian sheep using PCR–RFLP method25, FGF2, GNAI13 and PLCB1 melanogenesis candidate genes were studied in Egyptian Barki sheep breed, generated by 50K SNPs Beadchips26, and HSP5 and HSP40 in Brazilian sheep using the 50K SNP Chip27. Given that genetics has a complex role in affecting an individual’s capacity to respond to a stressful situation, more research into mechanisms and the development of more appropriate tools is required.
This study aimed to investigate the polymorphism of 51 SNPs in 29 genes involved in thermotolerance throughout the use of Kompetitive Allele Specific PCR (KASP-PCR) technique in 17 sheep breeds originating from different climatic conditions. The main goal was to identify and describe polymorphisms related to climatic adaptation that could be used to develop future thermal resilience in sheep through genomic selection.
Results
Allele and genotype frequency
A total of 601 animals (83.47%) from the total 720 animals were successfully genotyped and 32 SNPs (62.74%) among the initial set of 51 SNPs were successfully genotyped in this study; 17 of them were found to be polymorphic (33.33%) (see Table S1 of the Supplementary data), which were HSPA12A, HSP90AA1, IL33, DIO2, BTNL2, CSN2, ABCG1, CSN1S1, GHR, HSPA8, STAT3, and HCRT. Allelic and genotypic frequencies presented in Tables S2 and S3 of the Supplementary data, were different from one population to another. Four SNPs were successfully genotyped in all breeds: rs161504783-HSP12A, rs588145625-HSPA8, rs588498137-STAT3 and rs602521720-HCRT.
The heterozygote TC for rs161504783-HSPA12A was dominant, except for Hungarian Racka, Transylvanian Merino, Hungarian Merino, Botosani Karakul and Sardi. The T and C allele frequencies were almost equally frequent in most breeds, except in Sardi (T allele = 0.107 and C alleles = 0.828) and Botosani Karakul (T allele = 0.735 and C alleles = 0.265).
The heterozygote GA for SNP rs588145625-HSPA8 was absent in the heat tolerance breeds, except the Transylvanian Merino, and in breeds with high cold tolerance (Hungarian Racka, Babolna Tetra, Hungarian Tsigai, Romanian Racka, Pramenka and Turcana). The homozygote GG was present in all heat-tolerant breeds and some cold-tolerant breeds (Suffolk, Ile de France and Hungarian Merino). The G allele was dominant in all breeds, ranging from 0.760 to 1, and the A allele has only appeared in cold-tolerant breeds and Transylvanian Merino with frequency ranging from 0 to 0.308.
No patterns have been found for allelic and genotypic frequencies for rs588498137-STAT3 and rs602521720-HCRT SNPs.
The GG genotype and G allele were dominant in all breeds for SNP rs588498137-STAT3, with G allelic frequency varying from 0.750 to 1. Similarly, the CC genotype and C allele for SNP rs602521720-HCRT was dominant in all breeds, with C allelic frequency varying from 0.546 to 1.
The Hardy–Weinberg equilibrium (HWE) was investigated with Chi-square (x2) test using allelic frequencies, observed and expected genotypes, and P value of the polymorphic genes are summarized in Table S2. Botosani Karakul breed from Romania was found to be the breed with most SNPs deviated from HWE (P ≤ 0.05); rs416259751-IL33, rs411181557-DIO2, rs414917134-BTNL2 and rs420611298-ABCG1.
Genetic diversity and interrelationship between SNPs
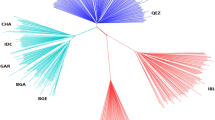
SNPs data were used to perform Principal Component Analysis (PCA) to highlight differences between either the breeds (Fig. 1a) or the climatic characteristics (Fig. 1b).
Score biplot of Principal Component Analysis of the 17 SNPs and 601 animals. (a) Individuals of different breeds are differently colored; (AWAS Hungarian Awassi, BG Béni Guil, DM D’Man, HUMER Hungarian Merino, HURAC Hungarian Racka, HUTSI Hungarian Tsigai, IDF Ile de France, KAR Botosani Karakul, PRA Pramenka, RORAC Romanian Racka, ROTSI Romanian Tsigai, SAR Sardi, SUF Suffolk, TET Babolna Tetra, TIM Timahdite, TRANSMER Transylvanian Merino and TUR Turcana). (b) Breeds grouped by climatic characteristics; COLD: cold-tolerant breeds (Suffolk, Babolna Tetra, Ile de France, Hungarian Tsigai, Hungarian Racka, Hungarian Merino, Pramenka, Romanian Racka and Turcana), HOT: heat tolerant breeds originated from Morocco (Béni Guil, D’Man, Timahdite and Sardi), HOT EU: Heat tolerant breeds reared in Europe (Hungarian Awassi, Botosani Karakul, Transylvanian Merino and Romanian Tsigai).
As displayed in Fig. 1, the PC1 and PC2 account for 11.21% and 9.98% of the total variation in the 17 breeds, respectively. Both PC1 and PC2 were unable to clearly separate neither the breeds (Fig. 1a), nor the climatic regions (Fig. 1b) even after we separated the heat tolerant breeds reared in the EU and in Morocco. All breeds were mostly concentrated in − 2.50 < PC1 < 1.12; cold tolerant breeds were outspread in PC1 score of − 5.593 to − 5.013 and PC2 score of − 4.149 to 4.020, heat-tolerant breeds were outspread in PC1 score of − 0.452 to 0.460 and PC2 score of − 0.157 to 1.183, while heat tolerant breeds reared in Europe had PC1 and PC2 ranging from − 0.881 to 0.686 and − 3.987 to 2.931, respectively. The highest contributions to the principal component were by rs397514117-HSP90AA1 (c) and rs397514272-HSP90AA1 (e), whereas rs410259751-IL33 (g) also had a positive contribution (Fig. 2). The loading value and score value of PCA are available on Tables S4 and S5 of the Supplementary data.
Discussion
The genetic background of thermotolerance adaptation in sheep represents a great interest for current research, especially when faced with the imminent increase of the earth's temperature because of climate change. Different breeds from different regions were used in this study, with the aim to see the different genetic backgrounds of thermotolerance adaptation according to geographical origins.
The application of KASP-PCR assay in this study did not show a high assay success rate as in previous studies carried out on goats28 and dairy cattle29. As much as 62.74% of SNPs were successfully genotyped in this study. These relatively high (37.26%) unsuccessful genotypes could be the consequence of sample damage during transportation from the laboratory to the subcontracting laboratory. Furthermore, quality control results in lower data for the analysis.
Only four SNPs were successfully genotyped in all investigated populations, and the focus was given to SNPs HSPA12A and HSPA8 as they are the only ones that showed allelic and genotypic frequency patterns with the climatic characteristics. HSPs are a wide family of chaperone proteins categorized according to their molecular size and amino acid sequence similarity. The HSPA12A and HSPA8 are members of the HSP70 family with a molecular weight of 70 kDa30. It is the biggest, most numerous and most conserved protein family throughout evolution, as well as the most extensively studied protein family across a wide range of species31,32,33. It is found on chromosome 15 and is made up of nine exons in sheep. This gene is widely studied for thermal adaptability. It controls cellular survival to heat stress with a highly dynamic nature, and these proteins are responsible for maintaining the organism's equilibrium and acclimating to heat stress34,35.
HSPA12A affects aspects like cellular senescence and how cells respond to heat stress36. It was found to be more active in ruminants during the summer, which helps them adapt to a hostile environment. This gene was found to be highly associated with heat tolerance in indicine cattle (Bos taurus indicus) and also has an important role in the water holding capacity in beef breeds, which might also act as a mechanism in surviving heat stress through the increased adrenaline and pH change in muscles37,38. According to the genotype and allele frequency results, the heterozygote TC for rs161504783-HSPA12A was dominant in most of the breeds, except Hungarian Racka, Hungarian Merino, Transylvanian Merino, Botosani Karakul and Sardi. Béni Guil, D’Man and Timahdite, breeds from a hot region, were not in HWE for HSPA12A.
In HSP70, exon 1 is non-coding, while the following eight exons combine to form the HSPA8 protein, with 650 amino acids of 71 kDa. HSPA8 aids in the day-to-day cell functions of protein folding and unfolding, polypeptide aggregation prevention, disassembly of large protein complexes and protein translocation across cellular compartments35,39. Given that increasing HSPA8 levels have been discovered to be positively correlated with heat tolerance, this gene has been employed as a candidate for heat resistance in many livestock species40,41,42.
In this study, the homozygote GG was carried by all heat-tolerant breeds and some cold-tolerant breeds (Suffolk, Ile de France and Romanian Tsigai), while the heterozygote GA for SNP rs588145625-HSPA8 was only found in cold-tolerant breeds (Hungarian Racka, Babolna Tetra, Hungarian Tsigai, Romanian Racka, Transylvanian Merino, Pramenka and Turcana). The A allele was absent in heat tolerant breeds, which is opposed to the observation made by25 in a study of gene expression in Indian sheep, which discovered that animals with A allele have better hot climate adaptability, compared to animals with the G allele. The AA genotype is more adaptable to a hot environment and has lower HSPA8 gene expression, than animals with the AG genotype. Similarly, a study by41 showed that the GG genotype has the least ability to survive heat stress in Awassi and Arabi sheep.
In this study, Botosani Karakul has shown to be the breed with the largest number of SNPs deviating from HWE. Lower heterozygosity was observed in 10 SNPs, and 4 of them deviated from HWE. One possible reason for this deviation of HWE was because Botosani Karakul is one of the Romanian crossbreeds that has undergone extensive genetic mixing from its German and Austria lines, and other Romanian breeds since its introduction from Russia at the beginning of the nineteenth century43,44.
PCA was unable to differentiate each breed in a clear and distinct manner; however, a relative clustering was observed based on the three different categories of climatic regions (Fig. 1b). This could be attributed to the fact that heat-tolerant breeds that are kept in the EU have become acclimated to the subtropical environment, in addition to the fact that genetic ad-mixture has occurred as a result of the widespread use of reproductive technologies, which has led to a less distinct genetic divergence between breeds, with previous studies reporting high levels of ad-mixture between different sheep breeds reared in Hungary and Romania, with the aim to increase the productivity45,46,47,48,49. We acknowledge that the cold breeds used in this study were not from year-around cold regions (e.g. Iceland, Finland, Norway), which was one of our limitations due to our inability to obtain samples from these regions. However, we strongly believe that the cold tolerance breeds from temperate climates utilized in this study are sufficiently contrasting with the Moroccan heat tolerant breeds.
From the loading biplot, HSP90AA1 and IL33 SNPs significantly contributed to PC1 and PC2. HSP90AA1 has been confirmed to be associated with thermal stress susceptibility of sheep in previous studies50,51, while IL33 was found to affect the sheep immunity and resistance to gastrointestinal intestinal nematode infection52,53, which is also associated because heat stress promotes to immune suppression and increases animal vulnerability to illnesses54. Our PCA findings validate these two SNPs as potential candidates for heat adaptability across the sheep breeds investigated in the current study, although more research is needed in order to clarify the relationship between the investigated SNPs and heat resistance.
Conclusion
Based on our 17 SNP polymorphism analyses performed on 601 animals, we found that the KASP-PCR method represents a feasible method for investigating polymorphisms in different sheep breeds. Furthermore, based on allele and genotype frequency, we validated that HSPA12A and HSPA8 SNPs are potential candidate markers for thermotolerance adaptation in sheep, whereas principal component analysis confirmed that HSP90AA1 and IL33 SNPs were the primary potential candidates. The results contribute to an increase in knowledge regarding the genetic variability of SNPs for thermotolerance adaptation in sheep. However, more studies are needed in order to clarify the relationship between the studied SNPs and heat resistance in sheep.
Method
Genomic DNA extraction
Samples were collected from 720 sheep belonging to17 breeds adapted to different climatic conditions, originating from 4 countries (Table 1). The majority of the breeds studied are indigenous to the country of origin, with some exotic breeds being included to determine if their acclimatization has contributed to adaptation. The breed characteristic (hot or cold tolerant) was determined based on the origin and development history of each breed in that particular country, as well as the environmental conditions under which the sample was collected (Table 2). All research activities were conducted in compliance with the European Union's Animal Experimentation Directive (Directive 2010/63/EU). FAO/IAEA55 recommended method from hair follicles and56 method from the blood was used for DNA genomic isolation. The DNA was kept at − 20 °C until analysis. The concentration of DNA was determined using a NanoDrop Spectrophotometer (Thermo Scientific, Waltham, MA, USA). All samples were diluted to a uniform concentration, and genotyping was performed using the equivalent of 50 ng of DNA per sample.
Selection of SNPs
Based on the findings of previous genome-wide association studies (GWAS) and marker-assisted selection studies across the sheep genome25,41,57,58,59, a SNP panel of 51 SNPs from 29 genes related to heat stress was selected, which included loci distributed on 18 chromosomes (Table 3). The Ovis SNP data were obtained from the Single Nucleotide Polymorphism Database (dbSNP) from the National Center for Biotechnology Information (NCBI) or Ensembl a few years ago, some of the RS reference IDs might have changed up to the present time. Due to the frequently changing genotyping database and varying reference sequences from those available at the European Variation Archive (EVA), only 10 SNPs out of 17 SNPs are deposited on EVA.
Genotyping and quality control
The bi-allelic discrimination of the selected 51 SNPs was performed using Kompetitive Allele Specific PCR (KASPTM, LGC Genomics, Teddington, Middlesex, UK). SNP Viewer software version 1.99 (Hoddesdon, UK) was used to visualize the results. All genotype data were exported for statistical analysis. Only SNPs that appeared in at least 50% of the breeds were considered. Data quality control of genotyped data included discarding animals with a call rate of less than 50% and the SNPs with call rates < 50%. This led to discrepancies in either the number of animals per breed or the number of SNPs per animal.
Data analysis
The raw allele calls obtained from LGC Genomics were analyzed using LGC Genomics' KlusterCaller program. Gene diversity, allele and genotype frequencies, and their accordance with or deviation from the Hardy–Weinberg equilibrium were determined by POPGENE software version 1.3160
The Principal Component Analysis was done using FactoMineR61 and ggplot262 packages from the R Program63 to visualize the genetic divergences between sheep breeds that were divided according to their climatic characteristics; cold-tolerant breeds (Babolna Tetra, Hungarian Merino, Hungarian Racka, Hungarian Tsigai, Ile de France, Pramenka, Romanian Racka, Suffolk and Turcana), heat tolerance breeds originated from Morocco (Béni Guil, D’Man, Timahdite and Sardi), and heat tolerant breeds reared in Europe (Hungarian Awassi, Botosani Karakul, Transylvanian Merino, and Romanian Tsigai).
Ethical approval
The authors confirm that the experiment complied with the European Union's Directive on Animal Experimentation (Directive 2010/63/EU) and ARRIVE guidelines. All animals in the experiment underwent standard procedures without experiencing any harm or discomfort, and all procedures were carried out in compliance with applicable guidelines and regulations. The study was approved by the Scientific and Ethics Committee of Centre for Agricultural Genomics and Biotechnology, University of Debrecen (Ethics statement No. 07).
Data availability
The 10 SNPs genotype datasets generated and analyzed in this study are only partially available at the EVA under accession number ERZ6760182. The remaining 7 SNPs genotype data is available in the supplementary material file (Table S6) (https://www.ebi.ac.uk/ena/browser/view/ERZ7485042?show=analyses).
References
IPCC. Summary for Policymakers. In (Masson-Delmotte, V., P. Zhai, H.-O. Pörtner, D. Roberts, J. Skea, P.R. Shukla, A. Pirani, W. Moufouma-Okia, C. Péan, R. Pidcock, S. Connors, J.B.R. Matthews, Y. Chen, X. Zhou, M.I. Gomis, E. Lonnoy, T. Maycock, M. Tignor, and T. Waterfield, eds) Global Warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. In Press (2018).
Malhi, Y. et al. Climate change and ecosystems: Threats, opportunities and solutions. Philos. Trans. R. Soc. B Biol. Sci. 375(1794), 20190104. https://doi.org/10.1098/rstb.2019.0104 (2020).
McElwee, P. Climate change and biodiversity loss. Curr. Hist. 120(829), 295–300. https://doi.org/10.1525/curh.2021.120.829.295 (2021).
Dickinson, M. G., Orme, C. D. L., Suttle, K. B. & Mace, G. M. Separating sensitivity from exposure in assessing extinction risk from climate change. Sci. Rep. 4(1), 6898. https://doi.org/10.1038/srep06898 (2015).
UNFCCC (United Nations Framework Convention on Climate Change). Global Warming Potentials http://unfccc.int/ghg_data/items/3825.php (2014).
BelhadjSlimen, I., Chniter, M., Najar, T. & Ghram, A. Meta-analysis of some physiologic, metabolic and oxidative responses of sheep exposed to environmental heat stress. Livestock Sci. 229, 179–187. https://doi.org/10.1016/j.livsci.2019.09.026 (2019).
Wojtas, K., Cwynar, P. & Kołacz, R. Effect of thermal stress on physiological and blood parameters in merino sheep. Bull. Vet. Inst. Pulawy 58(2), 283–288. https://doi.org/10.2478/bvip-2014-0043 (2014).
Gavojdian, D., Cziszter, L. T., Budai, C. & Kusza, S. Effects of behavioral reactivity on production and reproduction traits in Dorper sheep breed. J. Vet. Behav. 10(4), 365–368. https://doi.org/10.1016/j.jveb.2015.03.012 (2015).
Mehaba, N., Coloma-Garcia, W., Such, X., Caja, G. & Salama, A. A. K. Heat stress affects some physiological and productive variables and alters metabolism in dairy ewes. J. Dairy Sci. 104(1), 1099–1110. https://doi.org/10.3168/jds.2020-18943 (2021).
Ramón, M., Díaz, C., Pérez-Guzman, M. D. & Carabaño, M. J. Effect of exposure to adverse climatic conditions on production in Manchega dairy sheep. J. Dairy Sci. 99(7), 5764–6577. https://doi.org/10.3168/jds.2016-10909 (2016).
Mahjoubi, E. et al. The effect of cyclical and severe heat stress on growth performance and metabolism in Afshari lambs1. J. Anim. Sci. 93(4), 1632–1640. https://doi.org/10.2527/jas.2014-8641 (2015).
dos Hamilton, T. R. S. et al. Evaluation of lasting effects of heat stress on sperm profile and oxidative status of ram semen and epididymal sperm. Oxid. Med. Cell. Longev. 1–12, 2016. https://doi.org/10.1155/2016/1687657 (2016).
Romo-Barron, C. B. et al. Impact of heat stress on the reproductive performance and physiology of ewes: A systematic review and meta-analyses. Int. J. Biometeorol. 63(7), 949–962. https://doi.org/10.1007/s00484-019-01707-z (2019).
Caroprese, M. et al. Glucocorticoid effects on sheep peripheral blood mononuclear cell proliferation and cytokine production under in vitro hyperthermia. J. Dairy Sci. 101(9), 8544–8551. https://doi.org/10.3168/jds.2018-14471 (2018).
Marcone, G., Kaart, T., Piirsalu, P. & Arney, D. R. Panting scores as a measure of heat stress evaluation in sheep with access and with no access to shade. Appl. Anim. Behav. Sci. 240, 105350. https://doi.org/10.1016/j.applanim.2021.105350 (2021).
Van Wettere, W. H. E. J. et al. Review of the impact of heat stress on reproductive performance of sheep. J. Anim. Sci. Biotechnol. 12(1), 26. https://doi.org/10.1186/s40104-020-00537-z (2021).
Belhadj Slimen, I., Najar, T., Ghram, A. & Abdrrabba, M. Heat stress effects on livestock: Molecular, cellular and metabolic aspects, a review. J. Anim. Physiol. Anim. Nutr. 100(3), 401–412. https://doi.org/10.1111/jpn.12379 (2016).
Guo, Z., Gao, S., Ouyang, J., Ma, L. & Bu, D. Impacts of heat stress-induced oxidative stress on the milk protein biosynthesis of dairy cows. Animals 11(3), 726. https://doi.org/10.3390/ani11030726 (2021).
Liu, Z. et al. Heat stress in dairy cattle alters lipid composition of milk. Sci. Rep. 7(1), 961. https://doi.org/10.1038/s41598-017-01120-9 (2017).
Krishnan, G. et al. Mitigation of the heat stress impact in Livestock reproduction. In Theriogenology (InTech, 2017).
Robertson, S. & Friend, M. Strategies to ameliorate heat stress effects on sheep reproduction. In Climate Change and Livestock Production: Recent Advances and Future Perspectives 175–183 (Springer, 2021). https://doi.org/10.1007/978-981-16-9836-1_15.
Sawyer, G. & Narayan, E. J. A review on the influence of climate change on sheep reproduction. In Comparative Endocrinology of Animals (Intech Open, 2019). https://doi.org/10.5772/intechopen.86799.
Maurya, V. P., Sejian, V., Kumar, D. & Naqvi, S. M. K. Biological ability of Malpura rams to counter heat stress challenges and its consequences on production performance in a semi-arid tropical environment. Biol. Rhythm. Res. 49(3), 479–493. https://doi.org/10.1080/09291016.2017.1381451 (2018).
Shahat, A. M., Rizzoto, G. & Kastelic, J. P. Amelioration of heat stress-induced damage to testes and sperm quality. Theriogenology 158, 84–96. https://doi.org/10.1016/j.theriogenology.2020.08.034 (2020).
Singh, K. M. et al. Association of heat stress protein 90 and 70 gene polymorphism with adaptability traits in Indian sheep (Ovis aries). Cell Stress Chaperones 22(5), 675–684. https://doi.org/10.1007/s12192-017-0770-4 (2017).
Kim, E.-S. et al. Multiple genomic signatures of selection in goats and sheep indigenous to a hot arid environment. Heredity 116(3), 255–264. https://doi.org/10.1038/hdy.2015.94 (2016).
do Paim, T. P., Alves dos Santos, C., de Faria, D. A., Paiva, S. R. & McManus, C. Genomic selection signatures in Brazilian sheep breeds reared in a tropical environment. Livestock Sci. 258, 104865. https://doi.org/10.1016/j.livsci.2022.104865 (2022).
Kusza, S. et al. Kompetitive Allele Specific PCR (KASPTM) genotyping of 48 polymorphisms at different caprine loci in French Alpine and Saanen goat breeds and their association with milk composition. PeerJ 6, e4416. https://doi.org/10.7717/peerj.4416 (2018).
Zhang, Y. et al. Technical note: Development and application of KASP assays for rapid screening of 8 genetic defects in Holstein cattle. J. Dairy Sci. 103(1), 619–624. https://doi.org/10.3168/jds.2019-16345 (2020).
Chaari, A. Molecular chaperones biochemistry and role in neurodegenerative diseases. Int. J. Biol. Macromol. 131, 396–411. https://doi.org/10.1016/j.ijbiomac.2019.02.148 (2019).
Tripathy, K., Sodhi, M., Kataria, R. S., Chopra, M. & Mukesh, M. In silico analysis of HSP70 gene family in bovine genome. Biochem. Genet. 59(1), 134–158. https://doi.org/10.1007/s10528-020-09994-7 (2021).
Rehman, S. et al. Genomic identification, evolution and sequence analysis of the heat-shock protein gene family in buffalo. Genes 11(11), 1388. https://doi.org/10.3390/genes11111388 (2020).
Huo, C. et al. Chronic heat stress negatively affects the immune functions of both spleens and intestinal mucosal system in pigs through the inhibition of apoptosis. Microbial Pathog. 136, 103672. https://doi.org/10.1016/j.micpath.2019.103672 (2019).
Morange, M. HSFs in development. In Molecular Chaperones in Health and Disease 153–169 (Springer, 2006). https://doi.org/10.1007/3-540-29717-0_7.
Hoter, A., El-Sabban, M. & Naim, H. The HSP90 family: Structure, regulation, function, and implications in health and disease. Int. J. Mol. Sci. 19(9), 2560. https://doi.org/10.3390/ijms19092560 (2018).
Vanselow, J., Vernunft, A., Koczan, D., Spitschak, M. & Kuhla, B. Exposure of lactating dairy cows to acute pre-ovulatory heat stress affects granulosa cell-specific gene expression profiles in dominant follicles. PLoS One 11(8), e0160600. https://doi.org/10.1371/journal.pone.0160600 (2016).
Joy, A. et al. Resilience of small ruminants to climate change and increased environmental temperature: A review. Animals 10(5), 86. https://doi.org/10.3390/ani10050867 (2020).
Saravanan, K. A. et al. Genomic scans for selection signatures revealed candidate genes for adaptation and production traits in a variety of cattle breeds. Genomics 113(3), 955–963. https://doi.org/10.1016/j.ygeno.2021.02.009 (2021).
Singh, A. K., Upadhyay, R. C., Malakar, D., Kumar, S. & Singh, S. V. Effect of thermal stress on HSP70 expression in dermal fibroblast of zebu (Tharparkar) and crossbred (Karan-Fries) cattle. J. Therm. Biol 43, 46–53. https://doi.org/10.1016/j.jtherbio.2014.04.006 (2014).
Verma, N., Gupta, I. D., Verma, A., Kumar, R. & Das, R. Novel SNPs in HSPB8 gene and their association with heat tolerance traits in Sahiwal indigenous cattle. Trop. Anim. Health Prod. 48(1), 175–180. https://doi.org/10.1007/s11250-015-0938-9 (2016).
Al-Thuwaini, T. M., Al-Shuhaib, M. B. S. & Hussein, Z. M. A novel T177P missense variant in the HSPA8 gene associated with the low tolerance of Awassi sheep to heat stress. Trop. Anim. Health Prod. 52(5), 2405–2416. https://doi.org/10.1007/s11250-020-02267-w (2020).
Onasanya, G. O. et al. Heterozygous single-nucleotide polymorphism genotypes at heat shock protein 70 gene potentially influence thermo-tolerance among four Zebu breeds of Nigeria. Front. Genet. https://doi.org/10.3389/fgene.2021.642213 (2021).
Pascal, C. Researches regarding quality of sheep skins obtained from Karakul from Botosani sheep. Biotechnol. Anim. Husband. 27(3), 1123–1130. https://doi.org/10.2298/BAH1103123P (2011).
Kevorkian, S. E. M., Zǎuleţ, M., Manea, M. A., Georgescu, S. E. & Costache, M. Analysis of the ORF region of the prion protein gene in the Botosani Karakul sheep breed from Romania. Turk. J. Vet. Anim. Sci. 35(2), 105–109. https://doi.org/10.3906/vet-0909-124 (2011).
Kusza, S. et al. Mitochondrial DNA variability in Gyimesi Racka and Turcana sheep breeds. Acta Biochim. Pol. 62(2), 273–280. https://doi.org/10.18388/abp.2015_978 (2015).
Gavojdian, D. et al. Effects of using indigenous heritage sheep breeds in organic and low-input production systems on production efficiency and animal welfare in Romania. Landbauforschung Volkenrode 66(4), 290–297. https://doi.org/10.3220/LBF1483607712000 (2016).
Gavojdian, D. et al. Reproduction efficiency and health traits in Dorper, White Dorper, and Tsigai sheep breeds under temperate European conditions. Asian Australas. J. Anim. Sci. 28(4), 599–603. https://doi.org/10.5713/ajas.14.0659 (2015).
Kusza, S. et al. The genetic variability of Hungarian Tsigai sheep. Archiv Tierzuch 53(3), 309–317 (2010).
Kusza, S. et al. Study of genetic differences among Slovak Tsigai populations using microsatellite markers. Czeh J. Anim. Sci. 54(10), 468–474. https://doi.org/10.17221/1670-CJAS (2009).
Marcos-Carcavilla, A. et al. Polymorphisms in the HSP90AA1 5′ flanking region are associated with scrapie incubation period in sheep. Cell Stress Chaperones 15(4), 343–349. https://doi.org/10.1007/s12192-009-0149-2 (2010).
Salces-Ortiz, J. et al. Looking for adaptive footprints in the HSP90AA1 ovine gene. BMC Evol. Biol. 15(1), 7. https://doi.org/10.1186/s12862-015-0280-x (2015).
Toscano, J. H. B. et al. Innate immune responses associated with resistance against Haemonchus contortus in Morada Nova Sheep. J. Immunol. Res. 2019, 1–10. https://doi.org/10.1155/2019/3562672 (2019).
Estrada-Reyes, Z. M. et al. Signatures of selection for resistance to Haemonchus contortus in sheep and goats. BMC Genom. 20(1), 735. https://doi.org/10.1186/s12864-019-6150-y (2019).
Caroprese, M., Bradford, B. J. & Rhoads, R. P. Editorial: Impact of climate change on immune responses in agricultural animals. Front. Vet. Sci. https://doi.org/10.3389/fvets.2021.732203 (2021).
FAO/IAEA. Agriculture biotechnology laboratory—handbook of laboratory exercises. Seibersdorf: IAEA Laboratories, 18 (2004).
Zsolnai, A. & Orbán, L. Accelerated separation of random complex DNA patterns in gels: Comparing the performance of discontinuous and continuous buffers. Electrophoresis 20(7), 1462–1468. https://doi.org/10.1002/(SICI)1522-2683(19990601)20:7%3c1462::AID-ELPS1462%3e3.0.CO;2-0 (1999).
Cavalcanti, L. C. G. et al. Genetic characterization of coat color genes in Brazilian Crioula sheep from a conservation nucleus. Pesq. Agrop. Brasil. 52(8), 615–622. https://doi.org/10.1590/s0100-204x2017000800007 (2017).
Li, Y. et al. Heat stress-responsive transcriptome analysis in the liver tissue of Hu sheep. Genes 10(5), 395. https://doi.org/10.3390/genes10050395 (2019).
Younis, F. Expression pattern of heat shock protein genes in sheep. Mansoura Vet. Med. J. 21(1), 1–5. https://doi.org/10.35943/mvmj.2020.21.001 (2020).
Yeh F. C., Boyle R., Yang R. C., Ye Z., Mao J. X. & Yeh D. POPGENE version 1.32. Computer program and documentation distributed by the author. http://www.ualberta.ca/∼fyeh/popgene.html (1999).
Lê, S., Josse, J. & Husson, F. FactoMineR: A package for multivariate analysis. J. Stat. Softw. 25(1), 1–18. https://doi.org/10.18637/jss.v025.i01 (2008).
Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer. https://ggplot2.tidyverse.org (2016) (ISBN 978-3-319-24277-4).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ (2020).
Acknowledgements
The work was supported by the EFOP-3.6.2-16-2017-00001 project, entitled: "Complex rural economic development and sustainability research, development of the service network in the Carpathian Basin" and Hungarian Eötvös State Scholarship, and a grant of the Romanian National Authority for Scientific Research and Innovation, CNCS-UEFISCDI, project number PN-II-RU-TE-2014-4-0023. This study was also supported by the bilateral S&T cooperation programme, within the project “Genetic characterization of native sheep in Carpathian basin and Morocco as a potential factor for climate change adaptation” from the National Development, Research and Innovation Fund (2021-1.2.4-TÉT-2021-00014). This publication is based upon work from COST Action G-BiKE, CA 18134, supported by COST (European Cooperation in Science and Technology), www.cost.eu. P.K.A. and G.W were supported by Tempus Public Foundation within the Stipendium Hungaricum Programme. We would also like to thank Ismaïl Boujenane and Mohammed Piro for helping in sample collection and Universitas Gadjah Mada for supporting P.K.A.’s study.
Author information
Authors and Affiliations
Contributions
Conceptualization, S.K.; methodology, S.K.; data analysis, P.K.A; resources, S.K., D.E.I., D.G., B.B., H.O., E.P.J., A.J.; writing, P.K.A; writing-review and editing, G.W., D.E.I., D.G., B.B., H.O., E.P.J., Z.B., A.J., S.K.; visualization, G.W., P.K.A.; supervision, S.K.; funding, S.K., D.G., A.J.; acquisition, S.K. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Astuti, P.K., Ilie, D.E., Gavojdian, D. et al. Validation of SNP markers for thermotolerance adaptation in Ovis aries adapted to different climatic regions using KASP-PCR technique. Sci Rep 12, 22348 (2022). https://doi.org/10.1038/s41598-022-26909-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-26909-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.