Abstract
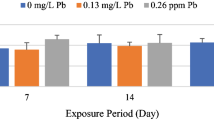
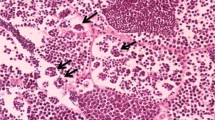
Sera 17β-estradiol (E2), 11-ketotestesteron (11-KT) and 17,20-β-dihydroxy-4-pregnen-3-one (17,20βP) levels together with histological changes in liver and gonad tissues were determined after exposing sexually mature female C.carpio to 0.13 and 0.26 mg L− 1 lead for 7, 14 and 21 days. Effects of lead on hepatosomatic - gonadosomatic indices of female fish were also determined after these exposure periods. No statistically significant differences were found between the E2, 11-KT and 17,20βP levels of control and lead exposed fish at the exposure periods tested. GSI values increased after exposure to high levels of Pb for 14 days and HSI values decreased after exposure to high levels of Pb after 7 and 21 days. Histopathological inspection showed dilatation in bile ducts and sinusoids, central vein congestions and pigment accumulation. Moreover melanomacrophage centres were determined in the centre of liver parenchyma on day 21. No distinct pathology was observable in ovary tissues compared to controls. No endocrine disrupted characteristic, widespread pathologic disruption and effect on ovary tissues were detected in female C.carpio exposed to the mentioned concentrations of Pb at the periods studied.






Similar content being viewed by others
References
Abdel-Moneim AM, Al-Kahtani MA, Elmenshawy OM (2012) Histopathological biomarkers in gills and liver of Oreochromis niloticus from polluted wetland environments. Saudi Arab Chemosphere 88:1028–1035
Alam M, Maughan O (1992) The effect of malathion, diazinon, and various concentrations of zinc, copper, nickel, lead, iron, and mercury on fish . Biol Trace Elem Res 34:225–236
Arellano JM, Storch V, Sarasquete C (1999) Histological changes and copper accumulation in liver and gills of the Senegales sole Solea senegalensis. Ecotoxicol Environ Saf 44:62–72
Barannikova I, Bayunova L, Semenkova T (2004) Serum levels of testosterone, 11-ketotestosterone and oestradiol‐17β in three species of sturgeon during gonadal development and final maturation induced by hormonal treatment. J Fish Biol 64:1330–1338
Biswas S, Ghosh AR (2016) Lead induced histological alterations in ovarian tissue of freshwater teleost Mastacembelus pancalus (Hamilton. Int J Adv Sci Res 2:45–51
Camargo MM, Martinez CB (2007) Histopathology of gills, kidney and liver of a Neotropical fish caged in an urban stream. Neotropical Ichthyol 5:327–336
Celander MC (2011) Cocktail effects on biomarker responses in fish. Aquat Toxicol 105:72–77
Choe S-Y, Kim S-J, Kim H-G, Lee JH, Choi Y, Lee H, Kim Y (2003) Evaluation of estrogenicity of major heavy metals. Sci Total Environ 312:15–21
Choi SM, Yoo SD, Lee BM (2004) Toxicological characteristics of endocrine-disrupting chemicals: developmental toxicity, carcinogenicity, and mutagenicity. J Toxicol Environ Health Part B 7:1–23
Costa PM et al (2009) Histological biomarkers in liver and gills of juvenile Solea senegalensis exposed to contaminated estuarine sediments: a weighted indices approach. Aquat Toxicol 92:202–212
Dawson A (2000) Mechanisms of endocrine disruption with particular reference to occurrence in avian wildlife: a review. Ecotoxicology 9:59–69
do Carmo Silva JP, da Costa MR, Araújo FG (2019) Energy acquisition and allocation to the gonadal development of Cynoscion leiachus (Perciformes, Sciaenidae) in a tropical Brazilian bay. Mar Biol Res 15:170–180
Drevnick PE, Sandheinrich MB (2003) Effects of dietary methylmercury on reproductive endocrinology of fathead minnows. Environ Sci Technol 37:4390–4396
Driessnack MK, Jamwal A, Niyogi S (2017) Effects of chronic waterborne cadmium and zinc interactions on tissue-specific metal accumulation and reproduction in fathead minnow (Pimephales promelas). Ecotoxicol Environ Saf 140:65–75
Ebrahimi M, Taherianfard M (2010) Concentration of four heavy metals (cadmium, lead, mercury, and arsenic) in organs of two cyprinid fish (Cyprinus carpio and Capoeta sp.) from the Kor River. (Iran) Environ Monit Assess 168:575–585
Ebrahimi M, Taherianfard M (2011) The effects of heavy metals exposure on reproductive systems of cyprinid fish from Kor River Iranian. J Fish Sci 10:13–26
Fent K, Stegeman JJ (1991) Effects of tributyltin chloride in vitro on the hepatic microsomal monooxygenase system in the fish Stenotomus chrysops. Aquat Toxicol 20:159–168
Friedmann A, Watzin M, Leiter J, Brinck-Johnsen T (1996) Effects of environmental mercury on gonadal function in Lake Champlain northern pike (Esox lucius). Bull Environ Contam Toxicol 56:486–492
Gautam GJ, Chaube R (2018) Differential effects of heavy metals (Cadmium, Cobalt, Lead and Mercury) on oocyte maturation and ovulation of the catfish Heteropneustes fossilis: an in vitro study Turkish. J Fish Aquat Sci 18:1205–1214
Georgescu B, Georgescu C, Dărăban S, Bouaru A, Paşcalău S (2011) Heavy metals acting as endocrine disrupters. Sci Pap Anim Sci Biotechnol 44:89–93
Ghasemi A, Zahediasl S (2012) Normality tests for statistical analysis: a guide for non-statisticians. Int J Endocrinol Metab 10:486
Goksøyr A (2006) Endocrine disruptors in the marine environment: mechanisms of toxicity and their influence on reproductive processes in fish. J Toxicol Environ Health Part A 69:175–184
Guével RL, Petit F, Goff PL, Métivier R, Valotaire Y, Pakdel F (2000) Inhibition of rainbow trout (Oncorhynchus mykiss) estrogen receptor activity by cadmium. Biol Reprod 63:259–266
Heath AG (1995) Water pollution and fish physiology. CRC Press, Boca Raton
Hong Y, Yu B, Sherman M, Yuan Y-C, Zhou D, Chen S (2007) Molecular basis for the aromatization reaction and exemestane-mediated irreversible inhibition of human aromatase. Mol Endocrinol 21:401–414
Iavicoli I, Fontana L, Bergamaschi A (2009) The effects of metals as endocrine disruptors. J Toxicol Environ Health Part B 12:206–223
Jansen MS, Nagel SC, Miranda PJ, Lobenhofer EK, Afshari CA, McDonnell DP (2004) Short-chain fatty acids enhance nuclear receptor activity through mitogen-activated protein kinase activation and histone deacetylase inhibition. Proc Natl Acad Sci 101:7199–7204
Jobling S, Tyler CR (2003) Endocrine disruption in wild freshwater fish. Pure Appl Chem 75:2219–2234
Katti SR, Sathyanesan A (1987) Lead nitrate-induced nuclear inclusions in the oocytes of the catfish Clarias batrachus (L). Environ Res (United States) 44
Khan I, Thomas P (2000) Lead and Aroclor 1254 disrupt reproductive neuroendocrine function in Atlantic croaker. Mar Environ Res 50:119–123
Kime DE (1995) The effects of pollution on reproduction in fish. Rev Fish Biol Fish 5:52–95
Kime DE (2012) Endocrine disruption in fish. Springer Science & Business Media, New York
Kloas W, Lutz I, Einspanier R (1999) Amphibians as a model to study endocrine disruptors: II. Estrogenic activity of environmental chemicals in vitro and in vivo. Sci Total Environ 225:59–68
Kumar S, Pant S (1984) Comparative effects of the sublethal poisoning of zinc, copper and lead on the gonads of the teleost Puntius conchonius. Ham Toxicol Lett 23:189–194
Kunz PY, Fent K (2006) Estrogenic activity of UV filter mixtures. Toxicol Appl Pharmcol 217:86–99
Levesque HM, Dorval J, Hontela A, Van Der Kraak GJ, Campbell PG (2003) Hormonal, morphological, and physiological responses of yellow perch (Perca flavescens) to chronic environmental metal exposures. J Toxicol Environ Health Part A 66:657–676
Luo Y et al (2015) Subchronic effects of cadmium on the gonads, expressions of steroid hormones and sex-related genes in tilapia Oreochromis niloticus. Ecotoxicology 24:2213–2223
Mantovani A, Stazi A, Macri C, Maranghi F, Ricciardi C (1999) Problems in testing and risk assessment of endocrine disrupting chemicals with regard to developmental toxicology. Chemosphere 39:1293–1300
Maqbool F, Mostafalou S, Bahadar H, Abdollahi M (2016) Review of endocrine disorders associated with environmental toxicants and possible involved mechanisms. Life Sci 145:265–273
Mela M, Randi M, Ventura D, Carvalho C, Pelletier E, Ribeiro CO (2007) Effects of dietary methylmercury on liver and kidney histology in the neotropical fish Hoplias malabaricus. Ecotoxicol Environ Saf 68:426–435
Mishra AK, Mohanty B (2008) Acute toxicity impacts of hexavalent chromium on behavior and histopathology of gill, kidney and liver of the freshwater fish, Channa punctatus (Bloch. Environ Toxicol Pharmacol 26:136–141
Muramoto S (1983) Elimination of copper from Cu-contaminated fish by long‐term exposure to EDTA and fresh water. J Environ Sci Health Part A 18:455–461
Oishi S (2002) Effects of propyl paraben on the male reproductive system. Food Chem Toxicol 40:1807–1813
Olojo E, Olurin K, Mbaka G, Oluwemimo A (2005) Histopathology of the gill and liver tissues of the African catfish Clarias gariepinus exposed to lead African. J Biotechnol 4:117–122
Paulos P, Runnalls TJ, Nallani G, La Point T, Scott AP, Sumpter JP, Huggett DB (2010) Reproductive responses in fathead minnow and Japanese medaka following exposure to a synthetic progestin Norethindrone. Aquat Toxicol 99:256–262
Poleksic V, Lenhardt M, Jaric I, Djordjevic D, Gacic Z, Cvijanovic G, Raskovic B (2010) Liver, gills, and skin histopathology and heavy metal content of the Danube sterlet (Acipenser ruthenus Linnaeus, 1758) . Environ Toxicol Chem 29:515–521
Rabitto I et al (2005) Effects of dietary Pb (II) and tributyltin on neotropical fish, Hoplias malabaricus: histopathological and biochemical findings. Ecotoxicol Environ Saf 60:147–156
Raldúa D, Díez S, Bayona JM, Barceló D (2007) Mercury levels and liver pathology in feral fish living in the vicinity of a mercury cell chlor-alkali factory. Chemosphere 66:1217–1225
Rotchell J, Ostrander G (2003) Molecular markers of endocrine disruption in aquatic organisms. J Toxicol Environ Health Part B 6:453–496
Roy D, Palangat M, Chen C-W, Thomas RD, Colerangle J, Atkinson A, Yan Z-J (1997) Biochemical and molecular changes at the cellular level in response to exposure to environmental estrogen-like chemicals. J Toxicol Environ Health Part A 50:1–30
Ruby S, Hull R, Anderson P (2000) Sublethal lead affects pituitary function of rainbow trout during exogenous vitellogenesis. Arch Environ Contam Toxicol 38:46–51
Short S, Meyers TR (2001) Histology for finfish NWFHS laboratory procedures manual Version 1
Singh H (1989) Interaction of xenobiotics with reproductive endocrine functions in a protogynous teleost Monopterus albus. Mar Environ Res 28:285–289
Stoica A, Katzenellenbogen BS, Martin MB (2000) Activation of estrogen receptor-α by the heavy metal cadmium. Mol Endocrinol 14:545–553
Thomas P (1988) Reproductive endocrine function in female Atlantic croaker exposed to pollutants. Mar Environ Res 24:179–183
Thomas P (1990) Teleost model for studying the effects of chemicals on female reproductive endocrine function. J Exp Zool 256:126–128
Tseng C-H, Chong C-K, Tseng C-P, Hsueh Y-M, Chiou H-Y, Tseng C-C, Chen C-J (2003) Long-term arsenic exposure and ischemic heart disease in arseniasis-hyperendemic villages in Taiwan. Toxicol Lett 137:15–21
Tulasi S, Reddy P, Rao JR (1989) Effects of lead on the spawning potential of the fresh water fish Anabas testudineus. Bull Environ Contam Toxicol 43:858–863
Van Dyk J, Pieterse G, Van Vuren J (2007) Histological changes in the liver of Oreochromis mossambicus (Cichlidae) after exposure to cadmium and zinc . Ecotoxicol Environ Safety 66:432–440
Velma V, Tchounwou PB (2010) Chromium-induced biochemical, genotoxic and histopathologic effects in liver and kidney of goldfish Carassius auratus. Mutat Res/Genetic Toxicology Environmental Mutagenesis 698:43–51
Vinodhini R, Narayanan M (2009) Heavy Metal Induced Histopathological Alterations in Selected Organs of the Cyprinus carpio L.(Common Carp). Int J Environ Res 3
Weber D (1993) Exposure to sublethal levels of waterborne lead alters reproductive behavior patterns in fathead minnows (Pimephales promelas. Neurotoxicology 14:347–358
Yamaguchi S et al (2007) Effects of lead, molybdenum, rubidium, arsenic and organochlorines on spermatogenesis in fish: Monitoring at Mekong Delta area and in vitro experiment. Aquat Toxicol 83:43–51
Funding
This study was funded by Research Project Coordination Unit of Mersin University (2016-2-TP3-1835).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors Cengiz Korkmaz, Özcan Ay, Ahmet Erdem Dönmez and Cahit Erdem declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Korkmaz, C., Ay, Ö., Dönmez, A.E. et al. Influence of Lead on Reproductive Physiology and Gonad and Liver Histology of Female Cyprinus carpio. Thalassas 36, 597–606 (2020). https://doi.org/10.1007/s41208-020-00232-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41208-020-00232-w