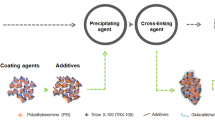
Abstract
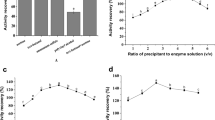
Cross-linked enzyme aggregates (CLEAs) of lipase were prepared after fractional precipitation with 40–50% ammonium sulfate and then cross-linking with glutaraldehyde. The process variables for the preparation of lipase-CLEAs such as glutaraldehyde concentration, cross-linking period, and initial pH of medium were optimized. The optimized conditions for the preparation of lipase-CLEAs were 25 mM/80 min/pH 7.0, and 31.62 mM/90 min/pH 6.0 with one factor at a time approach and numerical optimization with central composite design, respectively. Lipase-CLEAs were characterized by particle size analysis, SEM, and FTIR. Cross-linking not only shifted the optimal pH and temperature from 7.0 to 7.5 and 40–45 to 45–50 °C, but also altered the secondary structure. Lipase-CLEAs showed an increase in Km by 7.70%, and a decrease in Vmax by 16.63%. Lipase-CLEAs presented better thermostability than free lipase as evident from thermal inactivation constants (t1/2, D and Ed value), and thermodynamic parameters (Ed, ΔH°, ΔG°, and ΔS°) in the range of 50–70 °C. Lipase-CLEAs retained more than 65% activity up to four cycles and showed good storage stability for 12 days when stored at 4 ± 2 °C. They were successfully utilized for the epoxidation of lemongrass oil which was confirmed by changes in iodine value, epoxide value, and FTIR spectra.
Graphic abstract









Similar content being viewed by others
References
Saddiq AA, Khayyat SA (2010) Chemical and antimicrobial studies of monoterpene: Citral. Pestic Biochem Phys 98(1):89–93. https://doi.org/10.1016/j.pestbp.2010.05.004
Veloza LA, Orozco LM, Sepúlveda-Arias JC (2011) Use of dimethyldioxirane in the epoxidation of the main constituents of the essential oils obtained from Tagetes lucida, Cymbopogon citratus, Lippia alba and Eucalyptus citriodora. Nat Prod Commun 6(7):925–930. https://doi.org/10.1177/1934578X1100600701
Boukhatem MN, Ferhat MA, Kameli A, Saidi F, Kebir HT (2014) Lemon grass (Cymbopogon citratus) essential oil as a potent anti-inflammatory and antifungal drugs. Libyan J Med 9(1):1–29. https://doi.org/10.3402/ljm.v9.25431
Piccolo D, Vianello C, Lorenzetti A, Maschio G (2019) Epoxidation of soybean oil enhanced by microwave radiation. Chem Eng J 377:120113. https://doi.org/10.1016/j.cej.2018.10.050
Liu W, Chen J, Liu R, Bi Y (2016) Revisiting the enzymatic epoxidation of vegetable oils by perfatty acid: perbutyric acid effect on the oil with low acid value. J Am Oil Chem Soc 93(11):1479–1486. https://doi.org/10.1007/s11746-016-2897-3
Portilla-Zúñiga O, Mosquera-Ramírez MF, Martín-Franco J, Hoyos-Saavedra OL, Cuervo-Ochoa G (2016) Epoxidation of Neral/Geranial using a Jacobsen-Katsuki Mn catalyst by chemical and electrochemical methods. J Mex Chem Soc 60(1):3–12
Zhang T, Ma Y, Tan CP, Hollmann F, Wang J, Yang B, Wang Y (2019) An efficient strategy for the production of epoxidized oils: natural deep eutectic solvent-based enzymatic epoxidation. J Am Oil Chem Soc 96(6):671–679. https://doi.org/10.1002/aocs.12220
Ariffin MF, Idris A, Ngadiman NH (2019) Optimization of lipase immobilization on maghemite and its physico-chemical properties. Braz J Chem Eng 36(1):171–179. https://doi.org/10.1590/0104-6632.20190361s20180168
Cui J, Zhao Y, Liu R, Zhong C, Jia S (2016) Surfactant-activated lipase hybrid nanoflowers with enhanced enzymatic performance. Sci Rep 6:27928. https://doi.org/10.1038/srep27928
Muley AB, Mulchandani KH, Singhal RS (2020) Immobilization of enzymes on iron oxide magnetic nanoparticles: synthesis, characterization, kinetics and thermodynamics. Methods Enzymol 630:39–79. https://doi.org/10.1016/bs.mie.2019.10.016
Ladole MR, Nair RR, Bhutada YD, Amritkar VD, Pandit AB (2018) Synergistic effect of ultrasonication and co-immobilized enzymes on tomato peels for lycopene extraction. Ultrason Sonochem 48:453–462. https://doi.org/10.1016/j.ultsonch.2018.06.013
Ren S, Li C, Jiao X, Jia S, Jiang Y, Bilal M, Cui J (2019) Recent progress in multienzymes co-immobilization and multienzyme system applications. Chem Eng J 373:1254–1278. https://doi.org/10.1016/j.cej.2019.05.141
Cui J, Cui L, Jia S, Su Z, Zhang S (2016) Hybrid cross-linked lipase aggregates with magnetic nanoparticles: a robust and recyclable biocatalysis for epoxidation of oleic acid. J Agric Food Chem 64(38):7179–7187. https://doi.org/10.1021/acs.jafc.6b01939
Verri F, Diaz U, Macario A, Corma A, Giordano G (2016) Optimized hybrid nanospheres immobilizing Rhizomucor miehei lipase for chiral biotransformation. Proc Biochem 51(2):240–248. https://doi.org/10.1016/j.procbio.2015.11.020
Mohd Hussin FNN, Abdul Wahab RA (2017) Optimization parameters for lipase immobilization: effects of time, temperature and cross-linker concentration. Proc Chem 2(1):70–75
Shao Y, Jing T, Tian J, Zheng Y (2015) Graphene oxide-based Fe3O4 nanoparticles as a novel scaffold for the immobilization of porcine pancreatic lipase. RSC Adv 5(126):103943–103955. https://doi.org/10.1039/C5RA19276E
Cruz J, Barbosa O, Rodrigues RC, Fernandez-Lafuente R, Torres R, Ortiz C (2012) Optimized preparation of CALB-CLEAs by response surface methodology: the necessity to employ a feeder to have an effective crosslinking. J Mol Catal B Enzym 80:7–14. https://doi.org/10.1016/j.molcatb.2012.04.013
Zhong L, Feng Y, Wang G, Wang Z, Lv H, Jia S, Cui J (2020) Production and use of immobilized lipases in/on nanomaterials: a review from the waste to biodiesel production. Int J Biol Macromol 152:207–222. https://doi.org/10.1016/j.ijbiomac.2020.02.258
Kulkarni NH, Muley AB, Bedade DK, Singhal RS (2020) Cross-linked enzyme aggregates of arylamidase from Cupriavidus oxalaticus ICTDB921: process optimization, characterization, and application for mitigation of acrylamide in industrial wastewater. Bioproc Biosyst Eng 43(3):457–471. https://doi.org/10.1007/s00449-019-02240-4
Khanahmadi S, Yusof F, Amid A, Mahmod SS, Mahat MK (2015) Optimized preparation and characterization of CLEA-lipase fromcocoa pod husk. J Biotechnol 202:153–161. https://doi.org/10.1016/j.jbiotec.2014.11.015
Cui J, Lin T, Feng Y, Tan Z, Jia S (2017) Preparation of spherical cross-linked lipase aggregates with improved activity, stability and reusability characteristic in water-in-ionic liquid microemulsion. J Chem Tech Biotech 92:1785–1793. https://doi.org/10.1002/jctb.5179
Cui JD, Jia SR (2015) Optimization protocols and improved strategies of cross-linked enzyme aggregates technology: current development and future challenges. Crit Rev Biotechnol 35(1):15–28. https://doi.org/10.3109/07388551.2013.795516
Sheldon RA (2019) CLEAs, Combi-CLEAs and ‘Smart’Magnetic CLEAs: Biocatalysis in a bio-based economy. Catalysts 9(3):261. https://doi.org/10.3390/catal9030261
Bilal M, Cui J, Iqbal HMN (2019) Tailoring enzyme microenvironment: State-of-the-art strategy to fulfill the quest for efficient bio-catalysis. Int J Biol Macromol 130:186–196. https://doi.org/10.1016/j.ijbiomac.2019.02.141
Cui JD, Zhao YM, Li HB, Feng YX, Lin T, Zhong CH, Tan ZHL, Jia SR (2017) Encapsulation of spherical cross-linked phenylalanine ammonia lyase aggregates in mesoporous biosilica. J Agric Food Chem 65:618–625. https://doi.org/10.1021/acs.jafc.6b05003
Sheldon RA, van Pelt S (2013) Enzyme immobilisation in biocatalysis: why, what and how. Chem Soc Rev 42(15):6223–6235. https://doi.org/10.1039/C3CS60075K
Yildirim D, Tükel SS, Alagöz D (2014) Crosslinked enzyme aggregates of hydroxynitrile lyase partially purified from Prunus dulcis seeds and its application for the synthesis of enantiopure cyanohydrins. Biotechnol Prog 30(4):818–827. https://doi.org/10.1002/btpr.1925
Asgher M, Bashir F, Iqbal HM (2018) Protease-based cross-linked enzyme aggregates with improved catalytic stability, silver removal, and dehairing potentials. Int J Biol Macromol 118:1247–1256. https://doi.org/10.1016/j.ijbiomac.2018.06.107
Samoylova Y, Sorokina K, Piligaev A, Parmon V (2018) Preparation of stable cross-linked enzyme aggregates (CLEAs) of a Ureibacillus thermosphaericus esterase for application in malathion removal from wastewater. Catalysts 8(4):154. https://doi.org/10.3390/catal8040154
Shuddhodana GMN, Bisaria VS (2018) Effectiveness of cross-linked enzyme aggregates of cellulolytic enzymes in hydrolyzing wheat straw. J Biosci Bioeng 126(4):445–450. https://doi.org/10.1016/j.jbiosc.2018.04.007
Perzon A, Dicko C, Çobanoğlu O, Yükselen O, Eryilmaz J, Dey ES (2017) Cellulase cross-linked enzyme aggregates (CLEA) activities can be modulated and enhanced by precipitant selection. J Chem Technol Biotechnol 92(7):1645–1649. https://doi.org/10.1002/jctb.5160
Diaz-Ramos MD, Miranda LP, Fernandez-Lafuente R, Kopp W, Tardioli PW (2019) Improving the yields and reaction rate in the ethanolysis of soybean oil by using mixtures of lipase CLEAs. Molecules 24:4392. https://doi.org/10.3390/molecules24234392
Badoei-Dalfard A, Karami Z, Malekabadi S (2019) Construction of CLEAs-lipase on magnetic graphene oxide nanocomposite: an efficient nanobiocatalyst for biodiesel production. Biores Technol 278:473–476. https://doi.org/10.1016/j.biortech.2019.01.050
Xing X, Jia JQ, Zhang JF, Zhou ZW, Li J, Wang N, Yu XQ (2019) CALB immobilized onto magnetic nanoparticles for efficient kinetic resolution of racemic secondary alcohols: long-term stability and reusability. Molecules 24:490. https://doi.org/10.3390/molecules24030490
Diaz-Vidal T, Armenta-Perez VP, Rosales-Rivera LC, Mateos-Díaz JC, Rodríguezz JA (2019) Cross-linked enzyme aggregates of recombinant Candida antarctica lipase B for the efficient synthesis of olvanil, a nonpungent capsaicin analogue. Biotechnol Prog 35:2807. https://doi.org/10.1002/btpr.2807
Zhang W, Yang H, Liu W, Wang N, Yu X (2017) Improved performance of magnetic cross-linked lipase aggregates by interfacial activation: a robust and magnetically recyclable biocatalyst for transesterification of jatropha oil. Molecules 22:2157. https://doi.org/10.3390/molecules22122157
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
He Z, Wang Y, Zhao T, Ye Z, Huang H (2014) Ultrasonication-assisted rapid determination of epoxide values in polymer mixtures containing epoxy resin. Anal Methods 6(12):4257–4261. https://doi.org/10.1039/C4AY00439F
Kotoski SP, Srigley CT (2018) Determination of iodine value in hydrogenated oils: comparison of titration and gas chromatography with flame-ionization detection methodologies. Lipids 53(7):755–763. https://doi.org/10.1002/lipd.12079
Muley AB, Thorat AS, Singhal RS, Babu KH (2018) A tri-enzyme co-immobilized magnetic complex: process details, kinetics, thermodynamics and applications. Int J Biol Macromol 118:1781–1795. https://doi.org/10.1016/j.ijbiomac.2018.07.022
Chaudhari SA, Singhal RS (2017) A strategic approach for direct recovery and stabilization of Fusarium sp. ICT SAC1 cutinase from solid state fermented broth by carrier free cross-linked enzyme aggregates. Int J Biol Macromol 98:610–621. https://doi.org/10.1016/j.ijbiomac.2017.02.033
Muley AB, Chaudhari SA, Bankar SB, Singhal RS (2019) Stabilization of cutinase by covalent attachment on magnetic nanoparticles and improvement of its catalytic activity by ultrasonication. Ultrason Sonochem 75:174–185. https://doi.org/10.1016/j.ultsonch.2019.02.019
Garcia-Galan C, Berenguer-Murcia Á, Fernandez-Lafuente R, Rodrigues RC (2011) Potential of different enzyme immobilization strategies to improve enzyme performance. Adv Synth Catal 353:2885–2904. https://doi.org/10.1002/adsc.201100534
Bedade DK, Muley AB, Singhal RS (2019) Magnetic cross-linked enzyme aggregates of acrylamidase from Cupriavidus oxalaticus ICTDB921 for biodegradation of acrylamide from industrial waste water. Bioresour Technol 272:137–145. https://doi.org/10.1016/j.biortech.2018.10.015
Gupta K, Jana AK, Kumar S, Jana MM (2015) Solid state fermentation with recovery of amyloglucosidase from extract by direct immobilization in cross linked enzyme aggregate for starch hydrolysis. Biocatal Agricult Biotechnol 4:486–492. https://doi.org/10.1016/j.bcab.2015.07.007
Verma R, Kumar A, Kumar S (2019) Synthesis and characterization of cross-linked enzyme aggregates (CLEAs) of thermostable xylanase from Geobacillus thermodenitrificans X1. Proc Biochem 80:72–79. https://doi.org/10.1016/j.procbio.2019.01.019
Miao C, Li H, Zhuang X, Wang Z, Yang L, Lv P, Luo W (2019) Synthesis and properties of porous CLEAs lipase by the calcium carbonate template method and its application in biodiesel production. RSC Adv 9(51):29665–29675. https://doi.org/10.1039/c9ra04365a
Mafra ACO, Koppa W, Beltrameb MB, Giordanoa RLC, Ribeirob MPA, Tardiolia PW (2016) Diffusion effects of bovine serum albumin on cross-linked aggregates of catalase. J Mol Catal B Enzym 133:107–116. https://doi.org/10.1016/j.molcatb.2016.08.002
Yavuz S, Kocabay S, Cetinkaya S, Akkaya B, Akkaya R, Yenidunya AF, Bakici MZ (2017) Production, purification, and characterization of metalloprotease from Candida kefyr 41 PSB. Int J Biol Macromol 94:106–113. https://doi.org/10.1016/j.ijbiomac.2016.10.006
Ladole MR, Muley AB, Patil ID, Talib M, Parate VR (2014) Immobilization of tropizyme-P on amino-functionalized magnetic nanoparticles for fruit juice clarification. J Biochem Technol 5(4):838–845
Talekar S, Joshi A, Kambale S, Jadhav S, Nadar S, Ladole M (2017) A tri-enzyme magnetic nanobiocatalyst with one pot starch hydrolytic activity. Chem Eng J 325:80–90. https://doi.org/10.1016/j.cej.2017.05.054
Muley AB, Chaudhari SA, Singhal RS (2017) Non-covalent conjugation of cutinase from Fusarium sp. ICT SAC1 with pectin for enhanced stability: process minutiae, kinetics, thermodynamics and structural study. Int J Biol Macromol 102:729–740. https://doi.org/10.1016/j.ijbiomac.2017.04.072
Muley AB, Chaudhari SA, Mulchandani KH, Singhal RS (2018) Extraction and characterization of chitosan from prawn shell waste and its conjugation with cutinase for enhanced thermo-stability. Int J Biol Macromol 111:1047–1058. https://doi.org/10.1016/j.ijbiomac.2018.01.115
Ladole MR, Pokale PB, Patil SS, Belokar PG, Pandit AB (2020) Laccase immobilized peroxidase mimicking magnetic metal organic frameworks for industrial dye degradation. Biores Technol 317:124035. https://doi.org/10.1016/j.biortech.2020.124035
Abdel-Naby MA, Fouad AA, El-Refai HA (2015) Catalytic and thermodynamic properties of glycosylated Bacillus cereus cyclodextrin glycosyltransferase. Int J Biol Macromol 76:132–137. https://doi.org/10.1016/j.ijbiomac.2015.02.017
Maisuria VB, Nerurkar AS (2012) Biochemical properties and thermal behavior of pectate lyase produced by Pectobacterium carotovorum subsp. carotovorum BR1 with industrial potentials. Biochem Eng J 63:22–30. https://doi.org/10.1016/j.bej.2012.01.007
Mohapatra BR (2017) Kinetic and thermodynamic properties of alginate lyase and cellulase co-produced by Exiguobacterium species Alg-S5. Int J Biol Macromol 98:103–110. https://doi.org/10.1016/j.ijbiomac.2017.01.091
Shirke AN, Basore D, Butterfoss GL, Bonneau R, Bystroff C, Gross RA (2016) Toward rational thermostabilization of Aspergillus oryzae cutinase: insights into catalytic and structural stability. Proteins Struct Funct Bioinf 84(1):60–72. https://doi.org/10.1002/prot.24955
de Castro RJS, Ohara A, Nishide TG, Albernaz JRM, Soares MH, Sato HH (2015) A new approach for proteases production by Aspergillus niger based on the kinetic and thermodynamic parameters of the enzymes obtained. Biocatal Agric Biotechnol 4(2):199–207. https://doi.org/10.1016/j.bcab.2014.12.001
Tayefi-Nasrabadi H, Asadpour A (2008) Effect of heat treatment on buffalo (Bubalus bubalis) lactoperoxidase activity in raw milk. J Biol Sci 8(8):1310–1315. https://doi.org/10.3923/jbs.2008.1310.1315
Panwar D, Kaira GS, Kapoor M (2017) Cross-linked enzyme aggregates (CLEAs) and magnetic nanocomposite grafted CLEAs of GH26 endo-β-1, 4-mannanase: Improved activity, stability and reusability. Int J Biol Macromol 105:1289–1299. https://doi.org/10.1016/j.ijbiomac.2017.07.154
Bhalerao MS, Kulkarni VM, Patwardhan AW (2017) Ultrasound-assisted chemoenzymatic epoxidation of soybean oil by using lipase as biocatalyst. Ultrason Sonochem 40:912–920. https://doi.org/10.1016/j.ultsonch.2017.08.042
Melchiors MS, Vieira TY, Pereira LPS, Carciofi BAM, de Araujo PHH, de Oliveira D, Sayer C (2019) Epoxidation of (R)-(+)-limonene to 1,2-limonene oxide mediated by low-cost immobilized Candida antarctica lipase Fraction B. Ind Eng Chem Res 58:13918–13925. https://doi.org/10.1021/acs.iecr.9b02168
Sun S, Ke X, Cui L, Yang G, Bi Y, Song F, Xu X (2011) Enzymatic epoxidation of Sapindus mukorossi seed oil by perstearic acid optimized using response surface methodology. Ind Crops Prod 33:676–682. https://doi.org/10.1016/j.indcrop.2011.01.002
Acknowledgements
The authors are grateful to University Grants Commission, Government of India, for providing financial supports by awarding fellowship under BSR scheme (Award number: F.25-1/2014-15(BSR)/No.F.5-62/2007(BSR) dated 16 Feb 2015) to carry out this research work.
Author information
Authors and Affiliations
Contributions
Design of experiments: ABM, SA, and RSS; performance of experiment: ABM and NLJ; analysis and interpretation of data: ABM, PPB, and NLJ; manuscript draft and compilation: ABM, SA, and PPB; manuscript correction and revision: ABM, SA, PPB, NLJ, and RSS.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not include any studies with human participants or animals performed by any authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Muley, A.B., Awasthi, S., Bhalerao, P.P. et al. Preparation of cross-linked enzyme aggregates of lipase from Aspergillus niger: process optimization, characterization, stability, and application for epoxidation of lemongrass oil. Bioprocess Biosyst Eng 44, 1383–1404 (2021). https://doi.org/10.1007/s00449-021-02509-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-021-02509-7