Abstract
The demand for lithium has increased significantly during the last decade as it has become key for the development of industrial products, especially batteries for electronic devices and electric vehicles. This article reviews sources, extraction and production, uses, and recovery and recycling, all of which are important aspects when evaluating lithium as a key resource. First, it describes the estimated reserves and lithium production from brine and pegmatites, including the material and energy requirements. Then, it continues with a description about the current uses of lithium focusing on its application in batteries and concludes with a description of the opportunities for recovery and recycling and the future demand forecast. The article concludes that the demand of lithium for electronic vehicles will increase from 30% to almost 60% by 2020. Thus, in the next years, the recovery and recycling of lithium from batteries is decisive to ensure the long-term viability of the metal.
Similar content being viewed by others
Introduction
New technologies often mean new ways of producing and consuming material and energy sources. In general, technologies are becoming more sophisticated, and products require the use of materials that are often nonrenewable and scarce. Among those materials, metals have potentially important applications in technologies such as rechargeable batteries for hybrid and electric cars, permanent magnets for maglev trains, wind turbines and motors, and solar panels.1 Even though such metals are used in low concentrations, demand has risen significantly, and consequently, their availability and potential recovery needs to be considered.2,3 Some of these metals are geologically scarce or sometimes not found in conveniently recoverable concentrations.4 Their recovery is also difficult and not economically feasible because they are used in alloys with other metals such as iron or in low concentration. For example neodymium (Nd), a rare-earth metal used for neodymium-iron-boron (Nd-Fe-B) magnets in hard disk drives for personal computers, forms extremely stable compounds with elements like oxygen, which makes its reuse and recycling very difficult. As a result, almost the entire amount of neodymium is dissipated and ends as a waste.5 We are especially concerned with the increase in the demand for certain metals due to the rapid development of new technologies, particularly because their availability can limit the lifetime of such technologies.
Although lithium has a low supply risk and there are possible substitutes depending on its applications, it is considered a critical metal due to its high economic importance.6,7 Most of its economic importance is as a material for the production of batteries for portable information technologies devices, as laptop computers and mobile phones, and as a key component for electric vehicles.8 Lithium is the lightest and the most highly reducing of metals, which confers to batteries the highest gravimetric and volumetric energy densities (typically over 160 Wh/kg and 400 Wh/L), 50% greater than conventional batteries.9 Even though the initial uses of lithium were as a hardener in lead alloy-bearing material, as an additive in frits and glass formulations, and as an industrial catalyst, currently, among those applications its employment in secondary batteries is the most rapidly expanding market.10 Between 2000 and 2007, the production of lithium secondary batteries grew by 25%.11 For instance, lithium ion secondary batteries are replacing nickel metal hybrid (NiMH) batteries used in the first commercialized electric vehicles because they have higher energy densities, which improve operation. Electric vehicle mass production started in 2011–2012 and is expected to increase progressively between 3% and 10% from 2020 to 2025.12 As result, accounting for the material and energy flows related to the life cycle of lithium, particularly in batteries has turned a necessity in order to assess the feasibility of future technologies containing lithium derived materials.
The aim of this article is to describe the sources, production, and uses of lithium from a strictly resource point of view to shed some light on the availability of lithium-containing technologies. First, the article explains the sources of lithium, analyzes its current production processes, and describes its uses on a global scale. Then, it describes the current recovery and recycling, and it estimates how increasing demand for lithium batteries can affect its production. The article finishes with a forecast on the future demand of lithium for batteries of electric vehicles.
Sources
The major sources of lithium are contained in brine lake deposits (also referred as salarsFootnote 1) and pegmatites. Brines with high lithium (about 0.3%) concentration are located in Salars of Chile, Bolivia, and Argentina. Salars with lower lithium concentration are located in the United States and the Tibetan Plateau.13 Pegmatites are coarse-grained igneous rocks formed by the crystallization of magma at depth in the crust. Lithium is currently extracted from 13 pegmatite deposits; the largest production mine is Greenbushes in Australia.14 Other potential sources of supply of lithium are clays and seawater.13,15 Lithium from clays can be recovered by limestone-gypsum roasting and selective chlorination, and by limestone-gypsum roast-water leach process at recovery rates of 20% and 80%, respectively.10 Lithium concentration in seawater is rather small (0.17 ppm) compared with concentration in salars (1000–3000 ppm) and the magnesium lithium ratio is high.16 About 20% of the lithium in seawater can be recovered by ion-exchange resins, solvent extraction, co-precipitation, membrane processes, and adsorption. Among the listed methods, adsorption using manganese dioxides (λ-MnO2) to recover lithium as a chloride salt seems to be the most promising because of its high sorption capacity in alkaline medium.17 Although the energy requirement has been reduced significantly from 1386 GJ to 288 GJ per kilogram of lithium, it is still too high to develop the process at industrial scale.17
Lithium reservesFootnote 2 estimates vary from 4 million tonnes to 30 million tonnes.13,18 In 2011, a detailed study examining data from 103 deposits containing lithium estimated lithium reserves in 39 million tonnes.14 Such differences in reserves are due to the availability of information and the assumptions for quantifying the feasibility of recovering lithium. The economic feasibility depends on the size of the deposit, the content of lithium, the content of other elements (such calcium and magnesium, which might interfere during extraction and processing), and the processes used to remove the lithium-bearing material and extract lithium from it. Kunasz19 argued that some of those estimates are overvalued as calculations followed hard rock deposit guidelines. Brines are fluids, as various elements occur as ions in a dynamic fluid, rather than being chemically bonded in a solid. A preliminary resource estimate should include the flow potential and hydraulic parameters, as there are fine-grained sediments that will not release brine upon pumping and thus must not be included for the resource estimates.19 In addition, the classification of a deposit as resource or reserve can change as extraction and production technology develops further and more resources will become reserves in time.
Despite the market downturn from 2009, new companies are exploring for lithium reserves. In June 2010, vast lithium deposits were discovered in northern Afghanistan.20 Lithium is available in three main types of deposits: pegmatite and spodumene, mineralized springs, and salar sediments, which are estimated in 1.27 million tonnes of lithium oxide (Li2O) with grades from 1% to 2.31%.21 As consequence, Afghanistan could eventually be transformed into one of the most important mining centers in the world and change the future of lithium market.
Production and Extraction of Lithium
The production capacities and amounts of metals reported in statistics show that the metallurgical industry is a rapidly moving sector, especially with the increasing application of metals by new technologies. Lithium is one of the metals whose demand has almost doubled in the past 5 years. In 2011, the world lithium production was 34800 tonnes, an increase of almost 30% from that of 2010, and 77% more than that of 2009.22,23 Almost 60% of the world’s lithium is still obtained from brines. The amount of lithium from pegmatites almost doubled its production from 2010, despite its high energy and transport costs of pegmatites as spodumene occurs in relatively small deposits. In recent years, the production of lithium from spodumene has gained importance (I) as its price and application in batteries has increased and (II) as an additional source of tantalum, a scarce metal with high economic value used for capacitors in most of electrical and electronic circuits.15
Figure 1 shows the sources of the world production of lithium in 2011. From brine, 108100 tonnes were recovered, which supplied 20690 tonnes of lithium. More than 60% of the production of lithium from brines originated from Chile. China and Argentina supplied 20% and 14%, respectively. The rest of lithium production (14110 tonnes) was supplied by the extraction of pegmatites. Almost 85% was produced from spodumene in Greenbushes (Australia), and the rest was obtained from a mixture of pegmatites in Zimbabwe and concentrates from Brazil and China, which used spodumene imported from Australia.18,22 Pegmatites are generally further processed to lithium carbonate and lithium chloride, although there is not enough information to quantify their production from each source in Fig. 1.
Production sources of lithium in 2011 (in tonnes)22
Figure 1 can also serve to estimate the waste generated from lithium production in 2011. In overall, only 6% of the total amount of lithium compounds extracted is actually lithium, the remaining 94% of the resources are other substances that will end up as waste. As illustrated, each tonne of lithium requires 5.22 tonnes and 35.56 tonnes of brine and pegmatite, respectively. Thus, in terms of mass, the production of lithium from brine is more efficient than the production from pegmatites. Capacity use among the current lithium producers is more than 80%, reflecting a relatively tight market between lithium production and consumption.22 As result, worldwide lithium resource exploration has increased significantly since 2010, and most lithium producers plan to increase their capacities in the next years. For instance, the company Sociedad Química y Minera de Chile, which supplies 31% of the world lithium market, increased lithium carbonate and lithium hydroxide production capacities to 48000 tonnes and 6000 tonnes, respectively, in 2011.22
From Brine Lakes
Lithium from brine is obtained as lithium carbonate (Li2CO3) by the lime soda evaporation process, which consists on evaporating salty water for 12–18 months in ponds using solar energy. Because evaporation is done using solar energy, the production of lithium from dry lakes is the most affordable and competitive of all processes.10 Brine contains a mixture of salts such as chlorides and sulfates of sodium, potassium, calcium, magnesium, boron, and lithium that are recovered by evaporation in ponds. The most interfering substance is magnesium, which is removed by two-step precipitation using sodium carbonate (Na2CO3) and lime (CaO).16
The best evaporation rates are achieved in strong solar radiation, low humidity, moderately intense winds, and low rainfall conditions. During evaporation processes, other important factors to take into account are lithium concentration and the magnesium lithium ratio. High magnesium lithium ratios slow down evaporation rates and reduce the yield.10 For example, lithium recovery is not possible in Salar Uyumi, the world largest lithium resource due to its elevated location and high magnesium lithium ratio. The world’s greatest lithium salt deposits are Salar de Atacama in Chile and Salar del Hombre Muerto located in Argentina. Salar de Atacama’s brine has a lithium content of 0.15% and a high magnesium lithium ratio (6.4), but the climate is ideal for achieving high rates of evaporation. The lithium concentration of Salar del Hombre Muerto is half that of Atacama but the magnesium lithium ratio is lower; thus, solar evaporation is feasible.15
The extraction of lithium carbonate (Li2CO3) from Salars generates sodium chloride (NaCl) as a by-product. Each tonne of lithium carbonate (Li2CO3) requires 1.8 tonnes of sodium carbonate (Na2CO3) and approximately between 12.53 and 18.80 GJ/m2 of solar radiation.15
From Pegmatites
Lithium is found in more than 145 different minerals, but it is extracted only from spodumene (Li2O·Al2O3·4SiO2), lepidolite (KLi2Al(Al,Si)3O10(F,OH)2), petalite (LiAlSi4O10), amblygonite ((Li,Na)AlPO4(F,OH)), and eucriptite (LiAlSiO4). Among those, spodumene is the most abundant lithium ore. The naturally occurring form contains 8% pure lithium oxide (Li2O), but commercial ores usually contain only 1–3%. Spodumene concentrate is used to produce lithium carbonate (Li2CO3) and then lithium metal. Lithium carbonate (Li2CO3) is economically more competitive because of its higher lithium content, but for certain applications such as pharmaceutical and plastics, lithium metal is still preferred.
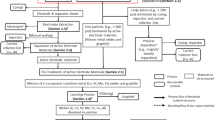
Table I gives the material and energy inputs required for the production of 1 tonne of lithium carbonate (Li2CO3). The production of lithium from spodumene starts with a heating process in a rotary kiln at 1100°C to change α-spodumene to β-spodumene, a more amenable form to chemical attack.
Then, β-spodumene is cooled at 65°C, grounded (< 149 μm), mixed, and roasted with concentrated sulfuric acid (H2SO4) at 250°C. Through this process, the hydrogen of the sulfuric acid is replaced by lithium ions to generate lithium sulfate (Li2SO4) and an insoluble ore residue. The excess of sulfuric acid is neutralized with limestone (CaCO3). The resulting slurry is after that filtered to separate ore residues resulting in a concentrated calcium sulfate (Ca2SO4) solution free of iron and aluminum. Magnesium content is precipitated using lime (CaO) and then calcium using soda ash (Na2CO3) generated as by-products during precipitation of sodium sulfate (Na2SO4). After filtration, the solution is pH adjusted with sulfuric acid (H2SO4) and concentrated by multiple-effect evaporation, then the lithium carbonate (Li2CO3) is precipitated at 90°C to 100°C with a soda ash (Na2CO3) solution, centrifuged, washed, and dried.10,15,24
Production of Lithium Manganese Oxide (LMO) for Batteries
Lithium carbonate is the raw material to produce many lithium-derived compounds, including the cathode and electrolyte material for lithium ion batteries (LIBs). Dunn et al.25 estimated that the energy use to produce 1 kg of LMO in Chile and the United States is 30 and 36 MJ, respectively. Despite the energy use to transport soda ash for Li2CO3 production from the United States to Chile, LMO from the United States still has the greatest energy demand due to more dilute lithium in brine, higher lime consumption, and combustion of residual oil. For a battery used in an electric vehicle (EV), the total energy use is 75 MJ per kg of battery. The cathode material contributes between 10% and 14% of the cradle-to-gate energy use whereas battery assembly adds 6%.
Uses of Lithium
Lithium is extracted from brine and spodumene as lithium carbonate (Li2CO3), which is directly used or further processed.22,26 Spodumene and lithium carbonate (Li2CO3) are used to lower the boiling points and increase resistance to thermal expansion in ceramic and glass applications. Lithium carbonate (Li2CO3) is further processed to lithium hydroxide (LiOH) and lithium chloride (LiCl). Lithium hydroxide (LiOH) is used for producing special inorganic compounds as absorbers of carbon dioxide or further processed to lithium phosphate (Li3PO4), lithium hypochlorite (LiOCl), lithium oxide (Li2O), peroxide (Li2O2), and others to be used as catalysts, in sanitation, neutron absorber, and photographic developer solutions. Lithium chloride (LiCl) is used as electrolyte in batteries or further processed to produce lithium metal for lead and magnesium alloys, lithium hydride (LiH) for high-purity silane, and lithium nitride (Li3N) used as catalyst.10 Lithium has also some dissipative uses as lubricating greases, medical and pharmaceutical use, air treatment, and sanitation.
Figure 2 shows the main applications of lithium-containing chemicals and the quantities used in each application accounted for in tonnes of lithium.18 As observed in the figure, more than 40% of lithium is used in the form of lithium carbonate (Li2CO3) for primary aluminum production, continuous casting, and ceramics and glass, as well as in batteries. The rest of lithium is used for producing intermediates as lithium hydroxide (LiOH), lithium chloride (LiCl), and metal lithium. Unfortunately, the amounts of intermediates are not available, and current published data do not permit to develop a more precise substance flow analysis of lithium. For instance, lithium used in batteries, which is estimated to be 6940 tonnes, can be in the form of lithium carbonate, lithium hydroxide, and lithium metal. The amount of each of these substances is not disclosed in current statistics.
As illustrated in Fig. 2, almost 75% of lithium is added to the stock of end products as aluminum, casting, glass and ceramics, and batteries. Depending on the lifetime of these products, this lithium could in theory be recovered at some point in the future. Batteries, for example, which are responsible for the consumption of 6940 tonnes of Li in 2011, can have a lifetime between 2 and 10 years at the end of which they could either be recycled, kept in stock “forever,” or be discarded as waste. The remaining 25% of lithium used in end-use products such as lubricants, greases, rubber, and pharmaceuticals is regarded as dissipative uses and assumed to end up as waste. In some uses such as catalysts or absorbers, lithium is most likely recycled within the process but eventually will become waste because this is not a recoverable fraction. In 2011, about 3% of lithium was recycled and reused within the battery manufacturing industries, as can be seen in Fig. 2. The increase in demand for lithium and the recycling targets set by some economies, as the European Commission, is expected to drive more interest to its recycling.
Lithium in Batteries
Lithium’s use in secondary batteries has experienced the largest market growth among all the other sectors. For instance, between 2000 and 2009, the number of secondary batteries increased from 500 million cells to 3.1 million cells, and it is still due to increase.27 Lithium in batteries can be used in many combinations of active materials: for the anode, cathode, and electrolyte. Each combination affects voltage, energy density, and charging/discharging cycles. Lithium batteries can be divided in primary (one use) and secondary batteries (rechargeable). Primary batteries use metallic lithium as an anode and a salt of lithium dissolved in an organic solvent as an electrolyte. The most commercialized lithium primary batteries use manganese dioxide (MnO2), thionyl chloride (SOCl2), iron sulfide (FeS2), and sulfur dioxide (SO2) as a cathode.28 Primary batteries are button and cylindrical shaped and are used in calculators, cameras, computers, electronic games, watches, and other devices. Secondary batteries use graphite as an anode, lithium metal oxide (LiMeO2) as a cathode, and a lithium salt in an organic solvent as an electrolyte. The most commercialized lithium secondary batteries are lithium ion (Li-ion) and polymer (Li-poly). In 2008, the lithium cathode most used in lithium ion batteries was 75% lithium cobalt oxide (LiCoO2), 8% lithium manganese oxide (LiMn2O4), and 2% lithium ferrophosphate (LiFePO4).27 The electrolytes used are lithium hexafluorophosphate (LiPF6), lithium perchlorate (LiClO4), and lithium tetrafluoroborate (LiBF4).29 Lithium polymer batteries use as electrolyte a polymer as polyethylene oxide (PEG) and polyacrylonitrile (PAN) instead of a lithium salt. Secondary lithium batteries are used in cordless tools, portable computers and telephones, video cameras, tablets, and electric vehicles.
The lithium content in batteries varies from 0.60 g to 4.00 g in primary batteries and from 0.35 g to 26.00 g in secondary batteries. Table II shows how the lithium content of different types of primary and secondary lithium batteries varies also with the chemistry of the anode and cathode.
The worldwide rechargeable battery market is dominated by lithium ion batteries (51%) followed by NiMH (22%), NiCd (17%), and lithium polymer (10%).27 Lithium batteries reduce the weight by half and volume by 20% to 50% compared to the same capacity NiCd and NiMH. Lithium ion batteries also provide three times the voltage of NiCd and NiMH; thus, it helps reduce the dimension of electronic devices and allows partial charging.
In 2011, the major applications of lithium batteries are in portable personal computers (41%) and mobile phones (24%), and the remaining 35% are others like tablets (6%), power tools (5%), e-bikes (5%), automobiles (5%), digital cameras and camcorders (5%), toys and video games (2%), household devices (2%), MP3 players (1%), and other electronic devices (4%).30 Only in 2009, the units of lithium secondary cells increased from 500 million to 3100 million, which contains 4140 tonnes of lithium.27 The demand for lithium batteries is still expected to increase from the portable electronics and automotive industries. As China is recognized as a major base of production for lithium batteries, major automobile and established battery manufacturers have taken different actions to secure low-cost supply of lithium. Such actions include purchasing a part of lithium-producing companies, diversifying lithium sources, establishing partnerships to build battery plants for hybrid and electric-drive vehicles, and beginning mass production of Li ion batteries. On the other hand, spent batteries are becoming an attractive source for lithium supply.25
Recovery and Recycling
Lithium recovery and recycling can happen during mining and processing (preconsumer recycling) and at the disposal of lithium-containing products (postconsumer recycling). Estimating the recycling rates of pre-consumer recycling is easier because the sources of waste generation are well known and also waste is generated continuously and scaled in relation to product production. Postconsumer recycling is harder to estimate as some lithium applications, such as lubricating greases, medical and pharmaceutical use, and sanitation, are dissipative. Nondissipative uses of lithium, such as in aluminum production and casting, metal alloys, and batteries, are also hard to estimate due to its low content and the time to reach the waste management sector. Among nondissipative uses, batteries are attracting the most attention as they represent a high market share of lithium uses (27%), and battery production is due to increase as result of the implementation of electric vehicles.
Primary batteries use metallic lithium as an anode and a salt of lithium dissolved in an organic solvent as an electrolyte. Lithium anodes can be used to produce secondary lithium batteries, and lithium electrolyte can be separated and converted to lithium carbonate (Li2CO3) for resale.31 Secondary batteries use a lithium metal oxide as a cathode (LiCoO2, LiNiO2, and LiMn2O4) and an organic liquid dissolved with substances like LiClO4, LiBF4, and LiPF6 as an electrolyte. In secondary batteries, lithium can be recovered from cathodes.
The processes used for recycling rechargeable batteries are as follows: hydrometallurgical, intermediate physical, direct physical, and pyrometallurgical.32 The recovered lithium from hydrometallurgical, intermediate, and direct physical processes must undergo further processing to regenerate it into a useable material.33 Hydrometallurgy is the main method to recycle lithium cobalt oxide (LiCoO2) from spent LIBs. The leaching of LiCoO2 is usually carried out by using inorganic acids such as sulfuric acid (H2SO4), hydrochloric acid (HCl), and nitric acid (HNO3) as leaching agents, and hydrogen peroxide (H2O2) is usually added to convert cobalt to the +2 state for subsequent recovery by electrochemical, precipitation, or solvent extraction techniques.34 Hydrometallurgy can also be used to recover lithium from lithium manganese oxide (LiMn2O4). By this process, the cathode-containing lithium compounds are treated by a bath of N-methylpyrrolidone to separate aluminum. Next it is calcined, ground, and the metals are leached with hydrogen peroxide (H2O2) and organic acid. The lithium can then precipitate as Li2CO3, and next it is fired with manganese oxide (Mn2O3) to produce LiMn2O4.25 By intermediate physical processes, spent batteries are shredded and then separated in components (metals, paper, plastic, and a black mass) by a series of physical steps. The black mass is further chemically processed with sodium carbonate (Na2CO3) to produce lithium carbonate (Li2CO3). Lithium is recovered as lithium carbonate (Li2CO3), which can be combined with virgin Mn2O3 to yield LiMn2O4.25 By direct physical processing, LIBs are discharged and disassembled to the cell level. Then, the electrolyte is separated from the cell by supercritical carbon dioxide (CO2). The cell undergoes pulverization or other size-reduction steps, and the components are separated by electronic conductivity, density, or other techniques to separate out the metals. By this process, lithium is recovered as lithium cobalt oxide (LiCoO2). Dunn et al.25 reviewed all these three technologies to recover lithium from automotive LIBs using LiMn2O4 as a cathode. The energy to recover 1 kg of LiMn2O4 from batteries varies from 4 MJ to 7 MJ, and it increases to 29 MJ when the processes to produce LiMn2O4 are included, which is still lower than the 30–37 MJ to obtain 1 kg of virgin LiMn2O4. Among the three technologies overviewed, direct physical processing reports the greatest energy savings, between 23 and 30 MJ depending on the origin of lithium.
Pyrometallurgical process use thermal treatments to recover cobalt and/or nickel, which have a higher economic value, but the process cannot recover the lithium itself.35 LIBs are introduced in a smelter where nickel and cobalt are separated and sent for refining, whereas lithium is gone in the slag together with aluminum, silicon, and calcium. Using recycled cobalt and nickel in new batteries reduces fossil fuel use by 45.3% and nuclear energy demand by 57.2%. It also saves 51% of natural resources.8
A less common recycling process to recover lithium from batteries and preconsumer scrap is cryogenization.36 The cryogenic process consists of freezing still charged batteries with liquid nitrogen (at −163°C) before being shredded to reduce the reactivity of cells to zero. Lithium is then extracted by flooding the battery chambers in a caustic bath that dissolves lithium salts, which are filtered out and used to produce lithium carbonate (Li2CO3). The remaining sludge is processed to recover cobalt for battery electrodes.37 Current research on recycling batteries is focused on developing biometallurgical processes that use microorganisms (such as chemolithotrophic, acidophilic bacteria, and Acidithiobacillus ferrooxidans) to produce metabolites like sulfuric acid and ferric ion in the leaching medium to obtain cobalt and lithium.32,38 These processes, which are still under development, are due to replace conventional metallurgical processes as they are more efficient and have lower cost.
Currently, recycling of lithium batteries is done by a few companies in Asia, Europe, and North America. Although there is an increasing number of companies recycling lithium, statistical data state that preconsumer and postconsumer lithium recycling is insignificant due to the low lithium concentration in final products.18,39,40 In many cases, spent secondary lithium batteries are recovered as an important source of cobalt and nickel, which have a higher market value and are scarce. Dewulf et al.8 recently demonstrated that the recycling of cobalt and nickel in secondary batteries results in a 51% natural resource savings besides decreasing the dependency on raw material supply. Table III summarizes the companies and their location, the type of batteries treated, the recycling processes used and the final metals obtained.
With the aim of increasing the recycling of batteries, the EU has set as target to collect at least 25% of spent batteries and recycle 50% of that into materials for batteries or other uses by 2012.41 In 2007, France, Germany, Austria, Belgium, and the Netherlands reached the 25% collection target, nine EU countries transposedFootnote 3 the 2006 directive, and three EU countries have partially transposed it.42 Overall, the collection average rate reached 13.6%.43 The amount of spent batteries collected for recycling tripled to 27200 tonnes from 2000 to 2007 in EU-27.44 Of the collected batteries, only 3% were lithium based being 40% primary and 60% lithium ion.43
The collection and recycling of lithium batteries are due to increase in the near future as spent lithium batteries start reaching the waste management sector. Batteries from electronics are deposed between 1 years and 3 years, but those from automobiles can take up to 15 years from the date of purchase to be disposed of. It is difficult estimating batteries and lithium recycling rates.45 There are also other factors as the proliferation of secondary markets for electric and electronic devices that may affect considerably the potential recycling and recovery of lithium. In secondary markets, used electric and electronic devices generally from developed economies are bought and sold to developing countries. This is partially because those retired devices tend to be in good condition as they are currently replaced before the end of their technical life.46 For instance, in 2006 Taiwan imported 2256 tonnes of used lithium batteries from more than 20 countries. Most of the LIBs were imported from China (880 tonnes), Japan (826 tonnes), Korea (324 tonnes), and Indonesia (136 tonnes), with only 23 tonnes of batteries from Europe.31 From those imported batteries, 53% were refurbished and used for the fabrication of new batteries, 47% were commercialized directly in the domestic market, and 7% reached the waste management stage where batteries were incinerated without recovering any metal. The creation of secondary markets for batteries in Taiwan helped increase the useful life of a battery by a second use phase; however, as the waste management infrastructure and legislation are less stringent, proper recycling and recovery of metals is not assured.
Demand Forecast
The demand for lithium is due to increase drastically in the battery sector mainly because of the growth of electric vehicles and electronic devices (mainly mobile phones, portable computers, and tablets). The EU has published two directives to promote electric vehicles: Directive 2009/33/EC of the European Parliament and of the Council of 23 April 2009 on the promotion of clean and energy-efficient road transport vehicles and the Directive 2006/32/EC of the European Parliament and of the Council of 5 April 2006 on energy end-use efficiency and energy services.41 The EU has also included the Green Car Initiative in the European Economic Recovery Plan.47 Additionally, the Transport and Energy General direction (DG TREN) of the European Commission is supporting a large European “electromobility” project on electric vehicles and related infrastructure with a total budget of around 50 million Euros as part of the Green Car Initiative.48 A number of European countries has also launched national programs and promotion strategies for electric cars ranging from support for research and development to purchase incentives such as the reduction of value-added tax and other taxes, insurance facilities, parking and charging facilities (including free recharging on street or in the parking areas), free road tax, toll free travel on highways, and exemption from congestion charging, among other initiatives.49 France is investing in building a countrywide network of charging stations, as well as a plant to produce electric car batteries. Britain is projected to have Europe’s biggest electric car plant at the Nissan Sunderland factory. London has confirmed up to 20 million Euros (£17 million) for electric vehicle infrastructure, revealing ambitious plans to make London the electric vehicle capital of Europe.50 In Denmark, the biggest power company together with the Californian Company Better Place will build a nationwide grid to support electric cars, composed of thousands of charging stations. Electric vehicles are only taxed at 25% compared to 180% + 25% charged to petrol. Free parking is also offered to electric vehicles in Copenhagen and other cities, and there is free recharging at some parking spaces. Portugal is gearing up to be one of the first markets for Renault-Nissan’s electric cars in 2011. Spain aims to have 1 million electric or hybrid cars on the road by 2014. The U.S. Department of Energy also invested in the deployment of electric vehicles by funding 1800 million Euros ($2.4 billion) in grants to accelerate the development of batteries and electric-drive components in 2009 (the largest investment ever made in battery technology for electric vehicles). Approximately 40% of the funding has been granted to lithium battery material suppliers, manufacturers, and recyclers.51 Despite the economic downturn, in the coming years it is expected to see a great progress on the lithium industry, particularly in supplying batteries to the automotive sector.
There are three main types of electric vehicles: EVs, hybrid electric vehicles (HEVs), and plug-in hybrid electric vehicles (PHEVs). EVs are 100% powered by an electric battery charged by plugging the vehicle into the electric power grid. HEVs and PHEVs are powered by an electric battery and an internal combustion engine or a hydrogen fuel cell. The battery of HEV is charged by the gasoline engine and regenerative braking. PHEV can be additionally charged by a power grid.52 About 90% of current battery research is focused in lithium ion batteries as they are the most promising technology for electric vehicles since NiMH are nearing its fundamental technical limits and further technical progress is not foreseen.53 LIBs will become the dominant technology in future electric vehicles.54 Table IV shows that the amount of lithium for LIB varies depending on the battery chemistry and type of electric vehicle.
As shown in Table IV, batteries using LMO as a cathode and graphite as an anode require the lowest amount of lithium, which varies from 0.17 kg for HEVs to 3.38 kg for EVs. LMO batteries using lithium titanium oxide require the greatest amount of lithium—almost 13 kg for EV. Based on this information, we can establish that an electric car requires a minimum of 0.17 kg and 12.68 kg as maximum. Hsiao and Richter estimated that the automobile battery cathode chemistry most used will be NCA-G, and therefore, an LIB will contain a minimum amount of 0.37 kg and a maximum amount 7.39 kg of lithium for EV.55 Other authors suggest slightly higher amount—8.4–9 kg of lithium for a battery of 30 kWh.15,56 LFP and LMO are lower cost alternatives, resulting from the substitution of cobalt. However, these two cathode materials are seen as a less attractive option because they have lower density and capacity.55 For instance, the energy capacity and density of LMO batteries are roughly a third less than lithium cobalt oxide, a significant factor when considering use in vehicles.
The total worldwide hybrid car registration was 735000 units in 2009, and reached almost 1.5 million units in 2012.30,57 The leading hybrid market is dominated by Japan (54%), United States (29%), Europe (10%), and the remaining 7% from other countries. Toyota (Toyota City, Japan) remains the leading HEV manufacturer with almost 80% of the market share. Honda has about 12% of the market, and the remaining 8% is from other HEV manufacturers as Hyundai (Seoul, South Korea), Ford (Dearborn, MI), General Motors (Detroit, MI), BMW (Munich, Germany), and others.58 In 2012, LIBs were used for PHEV and in less amount for HEVs. The PHEV bestselling models were Chevrolet Volt (General Motors), Toyota Prius Plug in, and Nissan Leaf (Nissan, Yokohama, Japan) in the United States.58 The Volt and Leaf use an LMO-G battery, whereas the Prius Plug in uses LFP. PHEVs required 76 tonnes of lithium for their batteries. The sales of HEVs were led by Toyota Prius, Toyota Camry Hybrid, Hyundai Sonata, Lexus CT200h (Toyota), Chevrolet Malibu Hybrid, and Ford Fusion hybrid, which represented more than 75% of the market. All these HEVs use NiMH batteries, except for the Hyundai Sonata, which uses a lithium polymer battery pack. In 2012, Chevrolet and Ford announced that they will replace the NiMH of their HEVs by NCM LIB batteries in 2013.59
There are several estimates about the global EV market and the demand for lithium. Gaines and Nelson60 did a detailed study to estimate the light vehicle sales to 2050 extending the U.S. Energy Information Administration transportation projections for 2030 for the United States. They expect that the maximum total annual sales of vehicles with electric drive occur in 2050, when they reach 21 million units, of which plug-in light trucks represent over 8 million units, PHEVs begin to stabilize, and sales of EVs account for about 2.4 million new vehicles.60 In the United States, the cumulative total sales of all types of electric vehicle is estimated to be 465 million vehicles until 2050. Assuming that all EVs use the current NCA-G chemistry, the demand for lithium is expected to be over 50000 tonnes annually by 2050. Gaines and Nelson60 estimated that the demand of mined lithium for batteries would peak to 25000 tonnes after 2030 and then decline progressively as spent LIB become available for recycling.
Navingan30 estimated that HEV will grow annually at 6% and PHEVs (combined plug-in hybrid and battery electric) at annual growth rate of 39% between 2012 and 2020. As result, the annual worldwide sales of all EVs will reach 3.8 million by 2020.61 Pillot30 estimated that the global HEV sales will reach 2.2 million units by 2015, and they will rise to almost 4.5 million units by 2020. In 2020, the greatest demand for LIB would be almost 75% for electronic devices. For automobiles, the demand for LIB would be mostly from EVs (22%), followed by PHEVs (3%) and HEVs (2%).30 Considering that NCA-G chemistry would be the most widely used, as Hsiao and Richter55 assumed, the global demand for lithium for EV would be 11800–23000 tonnes in 2020, in line with estimate given by Gaines and Nelson.60 As result, the amount of lithium used for batteries (6990 tonnes) would need to increase between 30% and 60%.
Even though lithium estimated reserves can provide such demand, there is a need to increase production in a short term, as lithium producers are working at 80% of their capacity and the overall demand is due to almost double during the next years. A possible way to increase its production is by its recovery from batteries, which is still low and has still to be improved. Optimizing the cycle of lithium by improving its recovery and recycling will help lithium to remain a viable source over the long term.
Conclusion
Lithium has been considered as critical metal due to its high economic and technological importance. Reserves of lithium have been recently estimated to be 39 million tonnes. Lithium is mainly produced from brine, which has a low energy demand for the process (it uses principally solar energy) and generates eight times less solid waste than its production from spodumene. One of the major uses of lithium is in batteries. In 2011, the battery sector consumed 6990 tonnes of lithium, and it is due to increase as lithium batteries are fully implemented in electric vehicles. In 2020, the expected demand of lithium is estimated to be 11800–23000 tonnes. Recycling of lithium is still incipient; in 2011, less than 3% of the total annual production was recycled. However, as the collection and recycling targets set by the EU are reached, it will become an important source of lithium and other metals as cobalt and nickel.
Notes
A salar, also referred as a dry lake, is a superficial lake consisting in fine-grained sediments with high concentration of alkali salts (chlorines, sulfates, nitrates, borates, etc.).
Reserves are the part of the resource that can be currently economically extracted or produced.
EU directives become laws once each member state transposes them into national law within the set deadline.
References
D. Cohen, New Scientist. 2605, 34 (2007).
M. Weil, S. Ziemann, and L. Schebek (Paper presented at the World Congress Resource Management and Technology for Material and Energy Efficiency, Nagoya, Japan, 2009).
D.E. Sullivan, Recycled Cell Phones—A Treasure Trove of Valuable Metals (Reston, VA: U.S. Geological Survey, 2006), p. 4.
L. Talens Peiró, G. Villalba Méndez, and R.U. Ayres, Environ. Sci. Technol. 47, 2939 (2013).
O. Takeda, T.H. Okabe, and Y. Umetsu, J. Alloys Compd. 408–412, 387 (2006).
U.S. National Research Council and Committee on Critical Mineral Impacts of the U.S. Economy, Minerals, Critical Minerals and the US Economy (Washington DC: National Academy Press, 2008).
M. Buchert, D. Schueler, and D. Bleher, Critical Metals for Future Sustainable Technologies and Their Recycling Potential, in Sustainable Innovation and Technology Transfer Industrial Sector Studies (Paris, France: United Nations Environment Program, 2009). http://www.unep.fr/shared/publications/pdf/DTIx1202xPA-Critical%20Metals%20and%20their%20Recycling%20Potential.pdf.
J. Dewulf, G. Van der Vorst, K. Denturck, H. Van Langenhove, W. Ghyoot, J. Tytgat, and K. Vandeputte, Resour. Conserv. Recycl. 54, 229 (2010).
S. Martinet, F. Le Cras, H. Rouault, and J.Y. Poinso, Clefs CEA (50–51), 130 (2004–2005).
C. Kamienski, D. McDonald, M. Stark, and J. Papcun, Kirk-Othmer Encyclopedia of Chemical Technology (New York: Wiley, 2004).
Roskill Information Services Ltd., The Economics of Lithium 2009 (London: Roskill Information Services, Ltd., 2009).
European Automobile Manufacturers Association, Electric Vehicles: Turning Buzz into Reality (Brussels, Belgium: European Automobile Manufacturers Association, 2010).
A. Yaksic Beckdorf and J. Tilton, Resour. Policy 34, 185 (2009).
P.W. Gruber, P.A. Medina, G.A. Keoleian, S.E. Kesler, M.P. Everson, and T.J. Wallington, J. Ind. Ecol. 15, 760 (2011).
W. Tahil, The Trouble with Lithium, Implications of Future PHEV Production for Lithium Demand, 2007, http://www.meridian-int-res.com/Projects/Lithium_Problem_2.pdf.
W. Tahil, The Trouble with Lithium, 2006, http://www.meridian-int-res.com/Projects/Lithium_Problem_2.pdf.
K. Yoshizuka, A. Kitajou, and M. Holba, Ars. Sep. Acta 4, 78 (2006).
B.W. Jaskula, Minerals Commodity Summaries: Lithium, ed. U.S. Department of the Interior (Washington, DC: United States Geological Survey, 2013), p. 94–95.
I.A. Kunasz, Brines Resources and Reserves. Analysis of and Practical Recommendations for CIM’s Publication, Best Practices for Resource and Reserve Estimation for Lithium Brines (Tucson, AZ: TRU Group, 2013), pp. 1–7.
J. Risen, U.S. Identifies Vast Mineral Riches in Afghanistan, The New York Times, 13 June 2010.
Afghanistan Geological Survey, Rare-Metal Deposits, in Minerals in Afghanistan, Kabul, 6 (2010).
B.W. Jaskula, 2011 Minerals Yearbook: Lithium, U.S. Geological Survey (Reston, VA: US Department of the Interior and US Geological Survey, 2012), pp. 44.1–44.13. http://minerals.usgs.gov/minerals/pubs/commodity/lithium/myb1-2011-lithi.pdf.
B.W. Jaskula, 2010 Minerals Yearbook: Lithium, U.S. Geological Survey (Reston, VA: US Department of the Interior and US Geological Survey, 2011), pp. 44.1–44.11. http://minerals.usgs.gov/minerals/pubs/commodity/lithium/myb1-2010-lithi.pdf.
W.L. Faith, D.B. Keyes, and R.C. Clark, Industrial Chemicals, 1st ed. (New York: Wiley-Interscience, 1950).
J.B. Dunn, L. Gaines, J. Sullivan, and M.Q. Wang, Environ. Sci. Technol. 46, 12704 (2012).
A. Ebensperger, P. Maxwell, and C. Moscoso, Resour. Pol. 30, 218 (2005).
C. Pillot (Paper presented at Batteries 2009, The International Power Supply Conference and Exhibition, Cannes-Mandelieu, France, 2009).
D.R. Wilburn, Material Use in the United States-Selected Case Studies for Cadmium, Cobalt, Lithium and Nickel in Rechargeable Batteries (Reston, VA: United States Geological Survey, 2009), pp. 1–18.
Y. Wang, P. He, and H. Zhou, Energ. Environ. Sci. 4, 805 (2011).
C. Pillot (Paper presented at the European Electric Vehicle Congress EEVC, Brussels, Belgium, 2012).
T.C. Chang, S.J. You, B.S. Yu, and K.F. Yao, J. Hazard. Mater. 163, 910 (2009).
J. Xu, H.R. Thomas, R.W. Francis, K.R. Lum, J. Wang, and B. Liang, J. Power Sources 177, 512 (2008).
J.B. Dunn, L. Gaines, M. Barnes, J. Sullivan, and M. Wang, Material and Energy Flows in the Materials Production, Assembly and End-of-Life Stages of the Automotive Li-Ion Battery Life Cycle, ed. U.S. Department of Energy (Argonne, IL: Argonne National Laboratory, 2012), pp. 1–73.
S.-G. Zhu, W.-Z. He, G.-M. Li, X. Zhou, X.-J. Zhang, and J.-W. Huang, Trans. Nonferrous Met. Soc. China 22, 2274 (2012).
K. Fisher, M. Collins, P. Laenen, E. Wallen, P. Garrett, and S. Aumonier, Battery Waste Management. Life Cycle Assessment (London, U.K.: Department for Environment, Food and Rural Affairs, 2006), pp. 1–230.
Toxco Inc., Inside Toxco’s Battery Recycling Facilities, 2003, http://www.toxco.com/facilities.html.
T. Hamilton, Lithium battery recycling gets a boost, MIT Technology Review, 12 August 2009.
J.R. Cui and L.F. Zhang, J. Hazard. Mater. 158, 228 (2008).
M. Buchert, A. Manhart, D. Bleher, and D. Pingel, Recycling Critical Raw Materials from Waste Electronic Equipment, in Sustainable Innovation and Technology Transfer Industrial Sector Studies (Freiburg, Germany: Oeko-Institut e.V., 2012).
European Commission, Critical Raw Materials for the European Union (Brussels, Belgium: European Commission, 2010).
Commission to the European Parliament and Council, Economic Growth and the Environment: Some Implications for Economic Policy Making (Brussels, Belgium: Commission to the European Parliament and Council, 1994).
G. Van der Have, Recycl. Int. 60 (2008).
European Battery Recycling Association, Only 27,200 Tons of Portable Batteries Recycled in 2007 in the 27-EU Member States 2008 (Brussels, Belgium: European Recycling Association, 2008), pp. 1–4.
B. Schutz and E. Beaurepaire, 10 Years of Battery Recycling in Europe (Brussels, Belgium: European Recycling Association, 2008).
Association, E.p.b. Recycling Around Europe, 2010, http://www.epbaeurope.net/recycling.html#collectionrate.
R. Geyer and V.D. Blass, Int. J. Adv. Manuf. Technol. 47, 515 (2010).
European Commission, European Green Cars Initiative, 2008, http://www.green-cars-initiative.eu/.
European Commission, Clean Urban Transport. Electric Vehicles, 2008, http://ec.europa.eu/transport/urban/vehicles/road/electric_en.htm.
European Association for Battery Hybrid and Fuel Cell Electric Vehicles, EU State Subsidies (Brussels, Belgium: European Association for Battery Hybrid and Fuel Cell Electric Vehicles [AVERE], 2006).
R. Massey, Nissan’s Sunderland factory to become Europe’s biggest green car plant, Daily Mail, 18 March 2010.
United States Geological Survey, Minerals Yearbook, Vol. I: Metals and Minerals (Washington, DC: United States Geological Survey, 2010).
World Electric Vehicle Association, Types of Electric Drive, 2013, http://wevaonline.net/.
M.A. Kromer and J.B. Heywood, Electric Powertrains: Opportunities and Challenges in the U.S Light-Duty Vehicle Fleet (Cambridge, MA: MIT Laboratory for Energy and the Environment, 2007), p. 153.
F. Hacker, R. Harthan, F. Matthes, and W. Zimmer, Environmental Impacts and Impact on the Electricity Market of a Large Scale Introduction of Electric Cars in Europe, European Topic Centre on Air and Climate Change, 2009, p. 169.
E. Hsiao and C. Richter, Electric Vehicles Special Report-Lithium Nirvana-Powering the Car of Tomorrow (Beijing, China: CLSA Asia-Pacific Markets, 2008), p. 44.
R. Lache, R. Galves, and P. Nolan, Electric Cars: Plugged In. Batteries Must Be Included (New York: Deutsche Bank Global Market Research, 2008), pp. 1–55.
C. Pillot (Paper presented at the 27th International Battery Seminar and Exhibition, Fort Lauderdale, FL, 2010).
J. Cobb, December 2012 Dashboard, 2013, http://www.hybridcars.com/december-2012-dashboard.
Hybridcars.com, Ohio-Made Lithium-Ion Battery Cathodes, 2012, http://www.hybridcars.com/ohio-made-lithium-ion-battery-cathodes-61054/.
L.L. Gaines and P. Nelson, Lithium-Ion Batteries—Possible Materials Issues, U.S. Department of Transportation (Chicago, IL: Argonne National Laboratory, 2009), pp. 1–16. http://www.transportation.anl.gov/pdfs/B/583.PDF.
Navigant Research, 2013 Electric Vehicle Market Forecasts (Boulder, CO: Navigant Research, 2013).
J. Sutter, Life Cycle Inventories of Highly Pure Chemicals (Duebendorf and St. Gallen: Swiss Centre for Life Cycle Inventory, ETHZ, 2007).
Acknowledgements
Part of this research has been developed under the framework of the project “Development and Application of a Standardized Methodology for the PROspective SUstaInability assessment of Technologies (PROSUITE)” funded by the European Union (Grant 227078) and Marie Curie fellowship (FP7-PLEOPLE-2010-IEF 272206).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Talens Peiró, L., Villalba Méndez, G. & Ayres, R.U. Lithium: Sources, Production, Uses, and Recovery Outlook. JOM 65, 986–996 (2013). https://doi.org/10.1007/s11837-013-0666-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-013-0666-4