Abstract
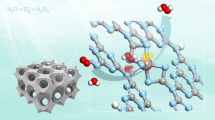
Overall water splitting, evolving hydrogen and oxygen in a 2:1 stoichiometric ratio, using particulate photocatalysts is a potential means of achieving scalable and economically viable solar hydrogen production. To obtain high solar energy conversion efficiency, the quantum efficiency of the photocatalytic reaction must be increased over a wide range of wavelengths and semiconductors with narrow bandgaps need to be designed. However, the quantum efficiency associated with overall water splitting using existing photocatalysts is typically lower than ten per cent1,2. Thus, whether a particulate photocatalyst can enable a quantum efficiency of 100 per cent for the greatly endergonic water-splitting reaction remains an open question. Here we demonstrate overall water splitting at an external quantum efficiency of up to 96 per cent at wavelengths between 350 and 360 nanometres, which is equivalent to an internal quantum efficiency of almost unity, using a modified aluminium-doped strontium titanate (SrTiO3:Al) photocatalyst3,4. By selectively photodepositing the cocatalysts Rh/Cr2O3 (ref. 5) and CoOOH (refs. 3,6) for the hydrogen and oxygen evolution reactions, respectively, on different crystal facets of the semiconductor particles using anisotropic charge transport, the hydrogen and oxygen evolution reactions could be promoted separately. This enabled multiple consecutive forward charge transfers without backward charge transfer, reaching the upper limit of quantum efficiency for overall water splitting. Our work demonstrates the feasibility of overall water splitting free from charge recombination losses and introduces an ideal cocatalyst/photocatalyst structure for efficient water splitting.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Supporting data are available at the Shinshu University Institutional Repository at http://hdl.handle.net/10091/00021822.
References
Kudo, A. & Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 38, 253–278 (2009).
Chen, S., Takata, T. & Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2, 17050 (2017).
Goto, Y. et al. A particulate photocatalyst water-splitting panel for large-scale solar hydrogen production. Joule 2, 509–520 (2018).
Ham, Y. et al. Flux-mediated doping of SrTiO3 photocatalysts for efficient overall water splitting. J. Mater. Chem. A 4, 3027–3033 (2016).
Maeda, K. & Domen, K. Photocatalytic overall water splitting: recent progress and future challenges. J. Phys. Chem. Lett. 1, 2655–2661 (2010).
Lyu, H. et al. An Al-doped SrTiO3 photocatalyst maintaining sunlight-driven overall water splitting activity over 1,000 h of constant illumination. Chem. Sci. 10, 3196–3201 (2019).
Sakata, Y., Hayashi, T., Yasunaga, R., Yanaga, N. & Imamura, H. Remarkably high apparent quantum yield of the overall photocatalytic H2O splitting achieved by utilizing Zn ion added Ga2O3 prepared using dilute CaCl2. Chem. Commun. 51, 12935–12938 (2015).
Kato, H., Asakura, K. & Kudo, A. Highly efficient water splitting into H2 and O2 over lanthanum-doped NaTaO3 photocatalysts with high crystallinity and surface nanostructure. J. Am. Chem. Soc. 125, 3082–3089 (2003).
Chiang, T. H. et al. Efficient photocatalytic water splitting using Al-doped SrTiO3 coloaded with molybdenum oxide and rhodium-chromium oxide. ACS Catal. 8, 2782–2788 (2018).
Li, Y. et al. Photocatalytic water splitting by N-TiO2 on MgO(111) with exceptional quantum efficiency at elevated temperatures. Nat. Commun. 10, 4421 (2019).
Wagner, F. T. & Somorojai, G. A. Photocatalytic and photoelectrochemical hydrogen production on strontium titanate single crystals. J. Am. Chem. Soc. 102, 5494–5502 (1980).
Domen, K. et al. Photocatalytic decomposition of water vapour on an NiO-SrTiO3 catalyst. J. Chem. Soc. Chem. Commun. 543–544 (1980).
Lehn, J. M., Sauvage, J. P., Zlessel, R. & Hilaire, L. Water photolysis by UV irradiation of rhodium loaded strontium titanate catalysts. Relation between catalytic activity and nature of the deposit from combined photolysis and ESCA studies. Isr. J. Chem. 22, 168–172 (1982).
Takata, T. & Domen, K. Defect engineering of photocatalyst by doping of aliovalent metal cations for efficient water splitting. J. Phys. Chem. C 113, 19386–19388 (2009).
Kanan, M. W. & Nocera, D. G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 321, 1072–1075 (2008).
Ohno, T., Sarukawa, K. & Matsumura, M. Crystal faces of rutile and anatase TiO2 particles and their roles in photocatalytic reactions. New J. Chem. 26, 1167–1170 (2002).
Liu, G., Yu, J. C., Lu, G. Q. & Cheng, H. M. Crystal facet engineering of semiconductor photocatalysts: motivations, advances and unique properties. Chem. Commun. 47, 6763–6783 (2011).
Xie, Y. P., Liu, G., Yina, L. & Cheng, H. M. Crystal facet-dependent photocatalytic oxidation and reduction reactivity of monoclinic WO3 for solar energy conversion. J. Mater. Chem. 22, 6746–6751 (2012).
Zhen, C., Yu, J. C., Liu, G. & Cheng, H. M. Selective deposition of redox co-catalyst(s) to improve the photocatalytic activity of single-domain ferroelectric PbTiO3 nanoplates. Chem. Commun. 50, 10416–10419 (2014).
Li, R. et al. Spatial separation of photogenerated electrons and holes among {010} and {110} crystal facets of BiVO4. Nat. Commun. 4, 1432 (2013).
Mu, L. et al. Enhancing charge separation on high symmetry SrTiO3 exposed with anisotropic facets for photocatalytic water splitting. Energy Environ. Sci. 9, 2463–2469 (2016).
Luo, Y. et al. Construction of spatial charge separation facets on BaTaO2N crystals by flux growth approach for visible-light-driven H2 production. ACS Appl. Mater. Interfaces 11, 22264–22271 (2019).
Wang, Z. et al. Overall water splitting by Ta3N5 nanorod single crystals grown on the edges of KTaO3 particles. Nat. Catal. 1, 756–763 (2018).
Wang, Q. et al. Oxysulfide photocatalyst for visible-light-driven overall water splitting. Nat. Mater. 18, 827–832 (2019).
Frederikse, H., Thurber, W. & Hosler, W. Electronic transport in SrTiO3. Phys. Rev. 134, A442–A445 (1964).
Weaver, H. Dielectric properties of single crystals of SrTiO3 at room temperatures. J. Phys. Chem. Solids 11, 275–277 (1959).
Zhao, Z. et al. Electronic structure basis for enhanced overall water splitting photocatalysis with aluminum doped SrTiO3 in natural sunlight. Energy Environ. Sci. 12, 1385–1395 (2019).
Konta, R., Ishii, T., Kato, H. & Kudo, A. Photocatalytic activities of noble metal ion doped SrTiO3 under visible light irradiation. J. Phys. Chem. B 108, 8992–8995 (2004).
Cohen, M. I. & Blunt, R. F. Optical properties of SrTiO3 in the region of absorption edge. Phys. Rev. 168, 929–933 (1968).
Zollner, S. et al. Optical properties of bulk and thin film SrTiO3 on Si and Pt. J. Vac. Sci. Technol. B 18, 2242–2254 (2000).
Sze, S. M. Physics of Semiconductor Devices (John Wiley & Sons, 1981).
Acknowledgements
This work was primarily supported by the Artificial Photosynthesis Project of the New Energy and Industrial Technology Development Organization (NEDO) and partly supported by JSPS KAKENHI grant number JP19K05669, JP16K06862. A part of this work was conducted at the Advanced Characterization Nanotechnology Platform of the University of Tokyo, supported by “Nanotechnology Platform” of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan (grant number JPMXP09A-19-UT-0023). We are grateful to M. Yamaguchi and Y. Kuromiya at the University of Tokyo for the preparation and evaluation of photocatalysts.
Author information
Authors and Affiliations
Contributions
T.T. conceived the photocatalyst design and performed the photocatalytic reactions. T.H. synthesized Al-doped SrTiO3. J.J. and Y.S. performed quantum efficiency measurements. M.N. and N.S. performed electron microscopy measurements. V.N. and K.S. performed electrical simulations. K.D. supervised the project. T.T., T.H. and K.D. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks David Tilley, Martijn Zwijnenburg and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Fig. 1 Dependence of water-splitting activities on the amounts and components of cocatalysts.
a, b, Dependence of water-splitting activity on the amount of Cr2O3 added to Rh (0.1 wt%)-loaded SrTiO3:Al (a) and CoOOH added to Rh (0.1 wt%)/Cr2O3(0.05 wt%)-loaded SrTiO3:Al (b). c, Time course of H2 and O2 evolution over SrTiO3:Al unloaded (i) and loaded with Rh (0.1 wt%) (ii), Rh (0.1 wt%)/CoOOH (0.05 wt%) (iii), CoOOH (0.05 wt%) (iv) and CoOOH (0.05 wt%)/Cr2O3 (0.05 wt%) (v).
Extended Data Fig. 2 Reproducibility and stability of water-splitting activity of SrTiO3:Al loaded with Rh (0.1 wt%)/Cr2O3 (0.05 wt%)/CoOOH (0.05 wt%).
a, Variation of water-splitting activity of SrTiO3:Al loaded with Rh (0.1 wt%)/Cr2O3 (0.05 wt%)/CoOOH (0.05 wt%) (25 samples) from different batches (B) and lots (L). Batches and lots represent SrTiO3:Al samples and the cocatalysts synthesized and loaded at different timings, respectively. The averages (μ) of the H2 and O2 evolution rates were 3.54 mmol h−1 and 1.78 mmol h−1 and their standard deviations (σ) were 0.26 mmol h−1 and 0.12 mmol h−1, respectively. All the gas evolution rates were within μ ± 2σ. b, Stability test of SrTiO3:Al loaded with Rh (0.1 wt%)/Cr2O3 (0.05 wt%)/CoOOH (0.05 wt%) during photocatalytic water splitting. The produced H2 and O2 accumulated in the reaction system were evacuated every 2.5 h. The inner wall of the reactor window was cleaned before every evacuation.
Extended Data Fig. 3
Time course of H2 and O2 evolution over SrTiO3:Al photodeposited with Rh (0.1 wt%)/Cr2O3 (0.05 wt%)/CoOOH (0.05 wt%) under simulated sunlight irradiation.
Extended Data Fig. 4
Effect of initial background pressure on the photocatalytic water-splitting activity of SrTiO3:Al photodeposited with Rh (0.1 wt%)/Cr2O3 (0.05 wt%)/CoOOH (0.05 wt%).
Extended Data Fig. 5 Simulations of graded band energy values and resulting anisotropic charge separation based on various differences in work function between the {110} and {100} facets.
a, Maps of conduction band energy and logarithms of the electron and hole densities (n and p, respectively). b, c, Energy band diagrams (b) and electron and hole densities (c) as functions of position. See Methods for further discussion.
Extended Data Fig. 6
Power spectrum of the Xe lamp used in the study.
Extended Data Fig. 7
XRD pattern of the synthesized SrTiO3:Al.
Extended Data Fig. 8 EQE measurement.
a, Photographs of the devices used in the measurement. Left, side view of the measurement system. Middle left, top view of the measurement system. Middle right, arrangement of the lamp and reactor. Right, arrangement of the Si photodiode. b, c, Illustrations of water-splitting reactor and photon-counting system (b) and illumination unit (c). d, Example of light intensity distribution (365-nm bandpass filter and 0.1-optical-density neutral density filter; left) and model used to calculate the number of incident photons (right).
Rights and permissions
About this article
Cite this article
Takata, T., Jiang, J., Sakata, Y. et al. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 581, 411–414 (2020). https://doi.org/10.1038/s41586-020-2278-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2278-9
This article is cited by
-
Electrolyte-assisted polarization leading to enhanced charge separation and solar-to-hydrogen conversion efficiency of seawater splitting
Nature Catalysis (2024)
-
Bubble-water/catalyst triphase interface microenvironment accelerates photocatalytic OER via optimizing semi-hydrophobic OH radical
Nature Communications (2024)
-
The reformation of catalyst: From a trial-and-error synthesis to rational design
Nano Research (2024)
-
Multiple exciton generation effect in photocatalytic overall water splitting
Science China Chemistry (2024)
-
Small-sized Ni-Co/Mo2C/Co6Mo6C2@C for efficient alkaline and acidic hydrogen evolution reaction by an anchoring calcination strategy
Frontiers of Chemical Science and Engineering (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.