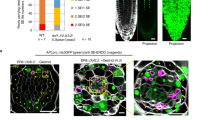
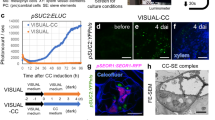
Abstract
During phloem unloading, multiple cell-to-cell transport events move organic substances to the root meristem. Although the primary unloading event from the sieve elements to the phloem pole pericycle has been characterized to some extent, little is known about post-sieve element unloading. Here, we report a novel gene, PHLOEM UNLOADING MODULATOR (PLM), in the absence of which plasmodesmata-mediated symplastic transport through the phloem pole pericycle–endodermis interface is specifically enhanced. Increased unloading is attributable to a defect in the formation of the endoplasmic reticulum–plasma membrane tethers during plasmodesmal morphogenesis, resulting in the majority of pores lacking a visible cytoplasmic sleeve. PLM encodes a putative enzyme required for the biosynthesis of sphingolipids with very-long-chain fatty acid. Taken together, our results indicate that post-sieve element unloading involves sphingolipid metabolism, which affects plasmodesmal ultrastructure. They also raise the question of how and why plasmodesmata with no cytoplasmic sleeve facilitate molecular trafficking.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data underlying the findings are available from the corresponding authors on request.
Change history
16 August 2019
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Lucas, W. J. Plasmodesmata: intercellular channels for macromolecular transport in plants. Curr. Opin. Cell Biol. 7, 673–680 (1995).
Kim, I. & Zambryski, P. C. Cell-to-cell communication via plasmodesmata during Arabidopsis embryogenesis. Curr. Opin. Plant Biol. 8, 593–599 (2005).
Otero, S., Helariutta, Y. & Benitez-Alfonso, Y. Symplastic communication in organ formation and tissue patterning. Curr. Opin. Plant Biol. 29, 21–28 (2016).
Lim, G. H. et al. Plasmodesmata localizing proteins regulate transport and signaling during systemic acquired immunity in plants. Cell Host Microbe 19, 541–549 (2016).
Yadav, S. R., Yan, D., Sevilem, I. & Helariutta, Y. Plasmodesmata-mediated intercellular signaling during plant growth and development. Front. Plant Sci. 5, 44 (2014).
Benitez-Alfonso, Y. et al. Symplastic intercellular connectivity regulates lateral root patterning. Dev. Cell 26, 136–147 (2013).
Sivaguru, M. et al. Aluminum-induced 1→3-β-d-glucan inhibits cell-to-cell trafficking of molecules through plasmodesmata. A new mechanism of aluminum toxicity in plants. Plant Physiol. 124, 991–1006 (2000).
Tylewicz, S. et al. Photoperiodic control of seasonal growth is mediated by ABA acting on cell–cell communication. Science 360, 212–214 (2018).
Oparka, K. J. et al. Simple, but not branched, plasmodesmata allow the nonspecific trafficking of proteins in developing tobacco leaves. Cell 97, 743–754 (1999).
Tilsner, J., Amari, K. & Torrance, L. Plasmodesmata viewed as specialised membrane adhesion sites. Protoplasma 248, 39–60 (2011).
Ding, B., Turgeon, R. & Parthasarathy, M. V. Substructure of freeze-substituted plasmodesmata. Protoplasma 169, 28–41 (1992).
Tilsner, J., Nicolas, W., Rosado, A. & Bayer, E. M. Staying tight: plasmodesmal membrane contact sites and the control of cell-to-cell connectivity in plants. Annu. Rev. Plant Biol. 67, 337–364 (2016).
Nicolas, W. J. et al. Architecture and permeability of post-cytokinesis plasmodesmata lacking cytoplasmic sleeves. Nat. Plants 3, 17082 (2017).
Roberts, A. G. & Oparka, K. J. Plasmodesmata and the control of symplastic transport. Plant Cell Environ. 26, 103–124 (2003).
Burch-Smith, T. M., Stonebloom, S., Xu, M. & Zambryski, P. C. Plasmodesmata during development: re-examination of the importance of primary, secondary, and branched plasmodesmata structure versus function. Protoplasma 248, 61–74 (2011).
Schulz, A. Plasmodesmal widening accompanies the short-term increase in symplasmic phloem unloading in pea root-tips under osmotic-stress. Protoplasma 188, 22–37 (1995).
Brunkard, J. O., Runkel, A. M. & Zambryski, P. C. The cytosol must flow: intercellular transport through plasmodesmata. Curr. Opin. Cell Biol. 35, 13–20 (2015).
Zavaliev, R., Ueki, S., Epel, B. L. & Citovsky, V. Biology of callose (β-1,3-glucan) turnover at plasmodesmata. Protoplasma 248, 117–130 (2011).
De Storme, N. & Geelen, D. Callose homeostasis at plasmodesmata: molecular regulators and developmental relevance. Front. Plant Sci. 5, 138 (2014).
Cui, W. & Lee, J. Y. Arabidopsis callose synthases CalS1/8 regulate plasmodesmal permeability during stress. Nat. Plants 2, 16034 (2016).
Fernandez-Calvino, L. et al. Arabidopsis plasmodesmal proteome. PLoS ONE 6, e18880 (2011).
Kraner, M. E., Muller, C. & Sonnewald, U. Comparative proteomic profiling of the choline transporter-like1 (CHER1) mutant provides insights into plasmodesmata composition of fully developed Arabidopsis thaliana leaves. Plant J. 92, 696–709 (2017).
Diao, M. et al. Arabidopsis formin 2 regulates cell-to-cell trafficking by capping and stabilizing actin filaments at plasmodesmata. eLife 7, e36316 (2018).
Grison, M. S. et al. Specific membrane lipid composition is important for plasmodesmata function in Arabidopsis. Plant Cell 27, 1228–1250 (2015).
Zhang, Z. et al. Suppressing a putative sterol carrier gene reduces plasmodesmal permeability and activates sucrose transporter genes during cotton fiber elongation. Plant Cell 29, 2027–2046 (2017).
Ross-Elliott, T. J. et al. Phloem unloading in Arabidopsis roots is convective and regulated by the phloem-pole pericycle. eLife 6, e24125 (2017).
Vaten, A. et al. Callose biosynthesis regulates symplastic trafficking during root development. Dev. Cell 21, 1144–1155 (2011).
Ullman, M. D. & Radin, N. S. The enzymatic formation of sphingomyelin from ceramide and lecithin in mouse liver. J. Biol. Chem. 249, 1506–1512 (1974).
Elmendorf, H. G. & Haldar, K. Plasmodium falciparum exports the Golgi marker sphingomyelin synthase into a tubovesicular network in the cytoplasm of mature erythrocytes. J. Cell Biol. 124, 449–462 (1994).
Tafesse, F. G., Ternes, P. & Holthuis, J. C. M. The multigenic sphingomyelin synthase family. J. Biol. Chem. 281, 29421–29425 (2006).
Voelker, D. R. & Kennedy, E. P. Cellular and enzymic synthesis of sphingomyelin. Biochemistry 21, 2753–2759 (1982).
Huitema, K., van den Dikkenberg, J., Brouwers, J. F. H. M. & Holthuis, J. C. M. Identification of a family of animal sphingomyelin synthases. EMBO J. 23, 33–44 (2004).
Yachi, R. et al. Subcellular localization of sphingomyelin revealed by two toxin-based probes in mammalian cells. Genes Cells 17, 720–727 (2012).
Wang, W. et al. An inositolphosphorylceramide synthase is involved in regulation of plant programmed cell death associated with defense in Arabidopsis. Plant Cell 20, 3163–3179 (2008).
Knox, K. et al. Putting the squeeze on plasmodesmata: a role for reticulons in primary plasmodesmata formation. Plant Physiol. 168, 1563–1572 (2015).
Tolley, N. et al. Transmembrane domain length is responsible for the ability of a plant reticulon to shape endoplasmic reticulum tubules in vivo. Plant J. 64, 411–418 (2010).
Markham, J. E. & Jaworski, J. G. Rapid measurement of sphingolipids from Arabidopsis thaliana by reversed-phase high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 21, 1304–1314 (2007).
Markham, J. E., Li, J., Cahoon, E. B. & Jaworski, J. G. Separation and identification of major plant sphingolipid classes from leaves. J. Biol. Chem. 281, 22684–22694 (2006).
Gronnier, J., Germain, V., Gouguet, P., Cacas, J. L. & Mongrand, S. GIPC: glycosyl inositol phospho ceramides, the major sphingolipids on earth. Plant Signal. Behav. 11, e1152438 (2016).
Axelrod, D., Koppel, D. E., Schlessinger, J., Elson, E. & Webb, W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J. 16, 1055–1069 (1976).
Merrill, A. H., Wang, E., Gilchrist, D. G. & Riley, R. T. Fumonisins and other inhibitors of de-novo sphingolipid biosynthesis. Adv. Lipid Res. 26, 215–234 (1993).
Markham, J. E. et al. Sphingolipids containing very-long-chain fatty acids define a secretory pathway for specific polar plasma membrane protein targeting in Arabidopsis. Plant Cell 23, 2362–2378 (2011).
Benitez-Alfonso, Y. et al. Control of Arabidopsis meristem development by thioredoxin-dependent regulation of intercellular transport. Proc. Natl Acad. Sci. USA 106, 3615–3620 (2009).
Li, W. M. et al. Depletion of ceramides with very long chain fatty acids causes defective skin permeability barrier function, and neonatal lethality in ELOVL4 deficient mice. Int. J. Biol. Sci. 3, 120–128 (2007).
Schneiter, R. et al. A yeast acetyl coenzyme a carboxylase mutant links very-long-chain fatty acid synthesis to the structure and function of the nuclear membrane-pore complex. Mol. Cell. Biol. 16, 7161–7172 (1996).
Eisenkolb, M., Zenzmaier, C., Leitner, E. & Schneiter, R. A specific structural requirement for ergosterol in long-chain fatty acid synthesis mutants important for maintaining raft domains in yeast. Mol. Biol. Cell 13, 4414–4428 (2002).
Cacas, J. L. et al. Revisiting plant plasma membrane lipids in tobacco: a focus on sphingolipids. Plant Physiol. 170, 367–384 (2016).
Pinto, S. N., Silva, L. C., Futerman, A. H. & Prieto, M. Effect of ceramide structure on membrane biophysical properties: the role of acyl chain length and unsaturation. Biochim. Biophys. Acta 1808, 2753–2760 (2011).
Obayashi, T. et al. ATTED-II in 2018: a plant coexpression database based on investigation of statistical property of the mutual rank index. Plant Cell Physiol. 59, e3 (2018).
Shang, B. S. et al. Very-long-chain fatty acids restrict regeneration capacity by confining pericycle competence for callus formation in Arabidopsis. Proc. Natl Acad. Sci. USA 113, 5101–5106 (2016).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Wilke, S. A. et al. Deconstructing complexity: serial block-face electron microscopic analysis of the hippocampal mossy fiber synapse. J. Neurosci. 33, 507–522 (2013).
Furuta, K. M. et al. Arabidopsis NAC45/86 direct sieve element morphogenesis culminating in enucleation. Science 345, 933–937 (2014).
Belevich, I., Joensuu, M., Kumar, D., Vihinen, H. & Jokitalo, E. Microscopy image browser: a platform for segmentation and analysis of multidimensional datasets. PLoS Biol. 14, e1002340 (2016).
Nicolas, W. J., Bayer, E. & Brocard, L. Electron tomography to study the three-dimensional structure of plasmodesmata in plant tissues—from high pressure freezing preparation to ultrathin section collection. Bio-protocol 8, e2681 (2018).
Kremer, J. R., Mastronarde, D. N. & McIntosh, J. R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 116, 71–76 (1996).
Mortimer, J. C. et al. Abnormal glycosphingolipid mannosylation triggers salicylic acid-mediated responses in Arabidopsis. Plant Cell 25, 1881–1894 (2013).
Bure, C. et al. Fast screening of highly glycosylated plant sphingolipids by tandem mass spectrometry. Rapid Commun. Mass Spectrom. 25, 3131–3145 (2011).
Fang, L. et al. Loss of inositol phosphorylceramide sphingolipid mannosylation induces plant immune responses and reduces cellulose content in Arabidopsis. Plant Cell 28, 2991–3004 (2016).
Welti, R. et al. Profiling membrane lipids in plant stress responses. Role of phospholipase Dα in freezing-induced lipid changes in Arabidopsis. J. Biol. Chem. 277, 31994–32002 (2002).
Xiao, S. et al. Overexpression of Arabidopsis acyl-CoA binding protein ACBP3 promotes starvation-induced and age-dependent leaf senescence. Plant Cell 22, 1463–1482 (2010).
Zuo, J., Niu, Q. W. & Chua, N. H. Technical advance: an estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 24, 265–273 (2000).
Melnyk, C. W. Grafting with Arabidopsis thaliana. Methods Mol. Biol. 1497, 9–18 (2017).
Acknowledgements
This work was supported by the Finnish Centre of Excellence in Molecular Biology of Primary Producers (Academy of Finland CoE program 2014–2019, decision no. 271832), the Gatsby Foundation (GAT3395/PR3), the National Science Foundation Biotechnology and Biological Sciences Research Council grant (BB/N013158/1), the University of Helsinki (award 799992091), the European Research Council Advanced Investigator Grant SYMDEV (no. 323052) and the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant no. 772103-BRIDGING to E.M.B.). J.D.P. is funded by a PhD fellowship from the Formation à la Recherche dans l’Industrie et l’Agriculture (grant no. 1.E.096.18), Belgium. This work was also funded as part of the US Department of Energy Joint BioEnergy Institute (http://www.jbei.org) supported by the US Department of Energy, Office of Science, Office of Biological and Environmental Research, through contract DE-AC02-05CH11231 between the Lawrence Berkeley National Laboratory and the US Department of Energy. We thank R. Wightman for assistance with confocal imaging, M. Bourdon for help in conducting immunolocalization and K. Abley for help with the seed germination assay. The SB-EM imaging was supported by the Biocenter Finland (I.B. and E.J.), and we thank M. Lindman, A. Salminen and M. Veikkolainen for sample preparation for SB-EM. We thank M. Roth and R. Welti for polar lipid analysis, A. Shevchenko and K. Schuchmann for sphingomyelin analysis and P. Dupree for data interpretation. Electron tomography was performed at the plant pole of the Bordeaux Imaging Centre (http://www.bic.u-bordeaux.fr/). The Region Aquitaine also supported the acquisition of the electron microscope (grant no. 2011 13 04 007 PFM), and FranceBioImaging Infrastructure supported the acquisition of the AFS2 and ultra-microtome. The sterol analyses were performed at the Functional Genomic Center of Bordeaux, Metabolome/Lipidome platform (https://metabolome.cgfb.u-bordeaux.fr/en), funded by Grant MetaboHUB-ANR-11-INBS-0010.
Author information
Authors and Affiliations
Contributions
D.Y., A.P., S.R.Y., E.M.B. and Y.H. designed the experiments. D.Y., A.P., S.R.Y., E.M.B. and Y.H. wrote the manuscript with input from other authors. D.Y., S.R.Y., A.V. and J.-Y.L. generated the Arabidopsis lines. S.R.Y., A.V., L.K. and S.e.-S. carried out the suppressor screen and gene mapping. D.Y., S.R.Y. and A.P. performed the growth phenotype analysis. D.Y. and J.E.M. carried out the sphingolipid profiles, and M.S.G. carried out the sterol analyses. A.P., W.J.N., J.D.P. and L.B. carried out the electron tomography analysis with support from E.M.B. M.K. provided support for the FRAP experiment. A.P., E.J. and I.B. carried out the SB-EM analysis. G.M.M. and J.M. performed the GIPC glycosylation assay. J.D.P. carried out the transient co-expression assay. The rest of experiments were performed by D.Y.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–17 and Supplementary Tables 1–3.
Rights and permissions
About this article
Cite this article
Yan, D., Yadav, S.R., Paterlini, A. et al. Sphingolipid biosynthesis modulates plasmodesmal ultrastructure and phloem unloading. Nat. Plants 5, 604–615 (2019). https://doi.org/10.1038/s41477-019-0429-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-019-0429-5
This article is cited by
-
Plasmodesmata mediate cell-to-cell transport of brassinosteroid hormones
Nature Chemical Biology (2023)
-
Sphingolipids in plant immunity
Phytopathology Research (2022)
-
The walnut shell network: 3D visualisation of symplastic and apoplastic transport routes in sclerenchyma tissue
Planta (2022)
-
HPC enables efficient 3D membrane segmentation in electron tomography
The Journal of Supercomputing (2022)
-
Intercellular trafficking via plasmodesmata: molecular layers of complexity
Cellular and Molecular Life Sciences (2021)