Abstract
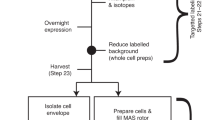
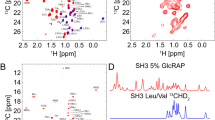
Solid-state NMR (ssNMR) is a technique that allows the study of protein structure and dynamics at atomic detail. In contrast to X-ray crystallography and cryo-electron microscopy, proteins can be studied under physiological conditions—for example, in a lipid bilayer and at room temperature (0–35 °C). However, ssNMR requires considerable amounts (milligram quantities) of isotopically labeled samples. In recent years, 1H-detection of perdeuterated protein samples has been proposed as a method of alleviating the sensitivity issue. Such methods are, however, substantially more demanding to the spectroscopist, as compared with traditional 13C-detected approaches. As a guide, this protocol describes a procedure for the chemical shift assignment of the backbone atoms of proteins in the solid state by 1H-detected ssNMR. It requires a perdeuterated, uniformly 13C- and 15N-labeled protein sample with subsequent proton back-exchange to the labile sites. The sample needs to be spun at a minimum of 40 kHz in the NMR spectrometer. With a minimal set of five 3D NMR spectra, the protein backbone and some of the side-chain atoms can be completely assigned. These spectra correlate resonances within one amino acid residue and between neighboring residues; taken together, these correlations allow for complete chemical shift assignment via a 'backbone walk'. This results in a backbone chemical shift table, which is the basis for further analysis of the protein structure and/or dynamics by ssNMR. Depending on the spectral quality and complexity of the protein, data acquisition and analysis are possible within 2 months.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
















Similar content being viewed by others
Change history
27 March 2017
In the supplementary information originally posted online, the file: Supplementary Data 1–10 was not attached. The error has been corrected in the HTML and PDF versions as of 27 March 2017.
References
Wallin, E. & von Heijne, G. Genome-wide analysis of integral membrane proteins from eubacterial, archaean, and eukaryotic organisms. Protein Sci. 7, 1029–1038 (1998).
Moraes, I., Evans, G., Sanchez-Weatherby, J., Newstead, S. & Stewart, P.D. Membrane protein structure determination - the next generation. Biochim. Biophys. Acta 1838, 78–87 (2014).
Ostermeier, C. & Michel, H. Crystallization of membrane proteins. Curr. Opin. Struct. Biol. 7, 697–701 (1997).
Hwang, P.M. & Kay, L.E. Solution structure and dynamics of integral membrane proteins by NMR: a case study involving the enzyme PagP. Methods Enzymol. 394, 335–350 (2005).
Sanders, C.R. & Sonnichsen, F. Solution NMR of membrane proteins: practice and challenges. Magn. Reson. Chem. 44, S24–S40 (2006).
Tamm, L.K. & Liang, B.Y. NMR of membrane proteins in solution. Prog. Nucl. Magn. Reson. Spectrosc. 48, 201–210 (2006).
Zhao, X. Protein structure determination by solid-state NMR. Top. Curr. Chem. 326, 187–213 (2012).
Naito, A. & Kawamura, I. Solid-state NMR as a method to reveal structure and membrane-interaction of amyloidogenic proteins and peptides. Biochim. Biophys. Acta 1768, 1900–1912 (2007).
Zech, S.G., Wand, A.J. & McDermott, A.E. Protein structure determination by high-resolution solid-state NMR spectroscopy: application to microcrystalline ubiquitin. J. Am. Chem. Soc. 127, 8618–8626 (2005).
Lange, A. et al. A concept for rapid protein-structure determination by solid-state NMR spectroscopy. Angew. Chem. Int. Ed. Engl. 44, 2089–2092 (2005).
Patching, S.G. Solid-state NMR structures of integral membrane proteins. Mol. Membr. Biol. 32, 156–178 (2015).
Baker, L.A. et al. Magic-angle-spinning solid-state NMR of membrane proteins. Methods Enzymol. 557, 307–328 (2015).
Brown, L.S. & Ladizhansky, V. Membrane proteins in their native habitat as seen by solid-state NMR spectroscopy. Protein Sci. 24, 1333–1346 (2015).
Wang, S. & Ladizhansky, V. Recent advances in magic angle spinning solid state NMR of membrane proteins. Prog. Nucl. Magn. Reson. Spectrosc. 82, 1–26 (2014).
Loquet, A., Habenstein, B. & Lange, A. Structural investigations of molecular machines by solid-state NMR. Acc. Chem. Res. 46, 2070–2079 (2013).
Sengupta, I., Nadaud, P.S. & Jaroniec, C.P. Protein structure determination with paramagnetic solid-state NMR spectroscopy. Acc. Chem. Res. 46, 2117–2126 (2013).
Lewandowski, J.R., Halse, M.E., Blackledge, M. & Emsley, L. Protein dynamics. Direct observation of hierarchical protein dynamics. Science 348, 578–581 (2015).
Lamley, J.M., Oster, C., Stevens, R.A. & Lewandowski, J.R. Intermolecular interactions and protein dynamics by solid-state NMR spectroscopy. Angew. Chem. Int. Ed. Engl. 54, 15374–15378 (2015).
Lamley, J.M. et al. Solid-state NMR of a protein in a precipitated complex with a full-length antibody. J. Am. Chem. Soc. 136, 16800–16806 (2014).
Lewandowski, J.R. Advances in solid-state relaxation methodology for probing site-specific protein dynamics. Acc. Chem. Res. 46, 2018–2027 (2013).
Schanda, P. & Ernst, M. Studying dynamics by magic-angle spinning solid-state NMR spectroscopy: principles and applications to biomolecules. Prog. Nucl. Magn. Reson. Spectrosc. 96, 1–46 (2016).
Ma, P.X. et al. Probing transient conformational states of proteins by solid-state R(1ρ) relaxation-dispersion NMR spectroscopy. Angew. Chem. Int. Ed. Engl. 53, 4312–4317 (2014).
Tollinger, M., Sivertsen, A.C., Meier, B.H., Ernst, M. & Schanda, P. Site-resolved measurement of microsecond-to-millisecond conformational-exchange processes in proteins by solid-state NMR spectroscopy. J. Am. Chem. Soc. 134, 14800–14807 (2012).
Tycko, R. Solid-state NMR studies of amyloid fibril structure. Annu. Rev. Phys. Chem. 62, 279–299 (2011).
Hong, M. Resonance assignment of 13C/15N labeled solid proteins by two- and three-dimensional magic-angle-spinning NMR. J. Biomol. NMR 15, 1–14 (1999).
Vijayan, V. et al. Low-power solid-state NMR experiments for resonance assignment under fast magic-angle spinning. Chemphyschem 10, 2205–2208 (2009).
Fung, B.M., Khitrin, A.K. & Ermolaev, K. An improved broadband decoupling sequence for liquid crystals and solids. J. Magn. Reson. 142, 97–101 (2000).
Loquet, A. et al. Atomic model of the type III secretion system needle. Nature 486, 276–279 (2012).
Fricke, P., Chevelkov, V., Shi, C. & Lange, A. Strategies for solid-state NMR investigations of supramolecular assemblies with large subunit sizes. J. Magn. Reson. 253, 2–9 (2015).
Ward, M.E. et al. Proton detection for signal enhancement in solid-state NMR experiments on mobile species in membrane proteins. J. Biomol. NMR 63, 375–388 (2015).
Ishii, Y. & Tycko, R. Sensitivity enhancement in solid state 15N NMR by indirect detection with high-speed magic angle spinning. J. Magn. Reson. 142, 199–204 (2000).
Chevelkov, V., Rehbein, K., Diehl, A. & Reif, B. Ultrahigh resolution in proton solid-state NMR spectroscopy at high levels of deuteration. Angew Chem Int Ed. Engl. 45, 3878–3881 (2006).
McDermott, A.E., Creuzet, F.J., Kolbert, A.C. & Griffin, R.G. High-resolution magic-angle-spinning NMR spectra of protons in deuterated solids. J. Magn. Reson. 98, 408–413 (1992).
Brunner, E., Freude, D., Gerstein, B.C. & Pfeifer, H. Residual linewidths of NMR spectra of spin-1/2 systems under magic-angle spinning. J. Magn. Reson. 90, 90–99 (1990).
Waugh, J.S., Huber, L.M. & Haeberle, U. Approach to high-resolution NMR in solids. Phys. Rev. Lett. 20, 180–182 (1968).
Chevelkov, V. et al. 1H detection in MAS solid-state NMR spectroscopy of biomacromolecules employing pulsed field gradients for residual solvent suppression. J. Am. Chem. Soc. 125, 7788–7789 (2003).
Paulson, E.K. et al. Sensitive high resolution inverse detection NMR spectroscopy of proteins in the solid state. J. Am. Chem. Soc. 125, 15831–15836 (2003).
Ward, M.E. et al. Proton-detected solid-state NMR reveals intramembrane polar networks in a seven-helical transmembrane protein proteorhodopsin. J. Am. Chem. Soc. 133, 17434–17443 (2011).
Agarwal, V. et al. De novo 3D structure determination from sub-milligram protein samples by solid-state 100 kHz MAS NMR spectroscopy. Angew. Chem. Int. Ed. Engl. 53, 12253–12256 (2014).
Zhou, D.H. et al. Proton-detected solid-state NMR spectroscopy of fully protonated proteins at 40 kHz magic-angle spinning. J. Am. Chem. Soc. 129, 11791–11801 (2007).
Marchetti, A. et al. Backbone assignment of fully protonated solid proteins by 1H detection and ultrafast magic-angle-spinning NMR spectroscopy. Angew. Chem. Int. Ed. Engl. 51, 10756–10759 (2012).
Xiang, S.Q., Biernat, J., Mandelkow, E., Becker, S. & Linser, R. Backbone assignment for minimal protein amounts of low structural homogeneity in the absence of deuteration. Chem. Commun. 52, 4002–4005 (2016).
Weingarth, M. et al. Quantitative analysis of the water occupancy around the selectivity filter of a K+ channel in different gating modes. J. Am. Chem. Soc. 136, 2000–2007 (2014).
Knight, M.J. et al. Fast resonance assignment and fold determination of human superoxide dismutase by high-resolution proton-detected solid-state MAS NMR spectroscopy. Angew. Chem. Int. Ed. Engl. 50, 11697–11701 (2011).
Zhou, D.H. et al. Solid-state NMR analysis of membrane proteins and protein aggregates by proton detected spectroscopy. J. Biomol. NMR 54, 291–305 (2012).
Chevelkov, V. et al. Proton-detected MAS NMR experiments based on dipolar transfers for backbone assignment of highly deuterated proteins. J. Magn. Reson. 242, 180–188 (2014).
Verel, R., Ernst, M. & Meier, B.H. Adiabatic dipolar recoupling in solid-state NMR: the DREAM scheme. J. Magn. Reson. 150, 81–99 (2001).
Nielsen, N.C., Bildsoe, H., Jakobsen, H.J. & Levitt, M.H. Double-quantum homonuclear rotary resonance - efficient dipolar recovery in magic-angle-spinning nuclear-magnetic-resonance. J. Chem. Phys. 101, 1805–1812 (1994).
Shi, C. et al. Atomic-resolution structure of cytoskeletal bactofilin by solid-state NMR. Sci. Adv. 1, e1501087 (2015).
Barbet-Massin, E. et al. Rapid proton-detected NMR assignment for proteins with fast magic angle spinning. J. Am. Chem. Soc. 136, 12489–12497 (2014).
Linser, R., Fink, U. & Reif, B. Proton-detected scalar coupling based assignment strategies in MAS solid-state NMR spectroscopy applied to perdeuterated proteins. J. Magn. Reson. 193, 89–93 (2008).
Xiang, S., Chevelkov, V., Becker, S. & Lange, A. Towards automatic protein backbone assignment using proton-detected 4D solid-state NMR data. J. Biomol. NMR 60, 85–90 (2014).
Akbey, U. et al. Optimum levels of exchangeable protons in perdeuterated proteins for proton detection in MAS solid-state NMR spectroscopy. J. Biomol. NMR 46, 67–73 (2010).
Nieuwkoop, A.J. et al. Sensitivity and resolution of proton detected spectra of a deuterated protein at 40 and 60 kHz magic-angle-spinning. J. Biomol. NMR 61, 161–171 (2015).
Mance, D. et al. An efficient labelling approach to harness backbone and side-chain protons in H-1-detected solid-state NMR spectroscopy. Angew. Chem. Int. Ed. Engl. 54, 15799–15803 (2015).
Wishart, D.S. Interpreting protein chemical shift data. Prog. Nucl. Magn. Reson. Spectrosc. 58, 62–87 (2011).
Shaka, A.J., Keeler, J., Frenkiel, T. & Freeman, R. An improved sequence for broad-band decoupling - WALTZ-16. J. Magn. Reson. 52, 335–338 (1983).
Shaka, A.J., Keeler, J. & Freeman, R. Evaluation of a new broad-band decoupling sequence - WALTZ-16. J. Magn. Reson. 53, 313–340 (1983).
Zhou, D.H. & Rienstra, C.M. High-performance solvent suppression for proton detected solid-state NMR. J. Magn. Reson. 192, 167–172 (2008).
Baldus, M., Petkova, A.T., Herzfeld, J. & Griffin, R.G. Cross polarization in the tilted frame: assignment and spectral simplification in heteronuclear spin systems. Mol. Phys. 95, 1197–1207 (1998).
Emsley, L. & Bodenhausen, G. Optimization of shaped selective pulses for NMR using a quaternion description of their overall propagators. J. Magn. Reson. 97, 135–148 (1992).
Hu, K.N., Qiang, W. & Tycko, R. A general Monte Carlo/simulated annealing algorithm for resonance assignment in NMR of uniformly labeled biopolymers. J. Biomol. NMR 50, 267–276 (2011).
Pines, A., Waugh, J.S. & Gibby, M.G. Proton-enhanced nuclear induction spectroscopy - method for high-resolution NMR of dilute spins in solids. J. Chem. Phys. 56, 1776–1777 (1972).
Morris, G.A. & Freeman, R. Enhancement of nuclear magnetic-resonance signals by polarization transfer. J. Am. Chem. Soc. 101, 760–762 (1979).
Biological Magnetic Resonance Data Bank (BMRB). Full chemical shift statistics. Accessed 2 September 2016, http://www.bmrb.wisc.edu/ref_info/statsel.htm.
Böckmann, A. et al. Characterization of different water pools in solid-state NMR protein samples. J. Biomol. NMR 45, 319–327 (2009).
Morcombe, C.R. & Zilm, K.W. Chemical shift referencing in MAS solid state NMR. J. Magn. Reson. 162, 479–486 (2003).
Acknowledgements
We thank S. Lange and E. Ousby for valuable discussions. This work was supported by the Leibniz-Institut für Molekulare Pharmakologie, the Max Planck Society, the European Research Council (ERC Starting Grant to A.L.), the German Research Foundation (Deutsche Forschungsgemeinschaft; Emmy Noether Fellowship to A.L.) and the Fonds der Chemischen Industrie (Kekulé Scholarship to P.F.).
Author information
Authors and Affiliations
Contributions
P.F., V.C. and M.Z. implemented the protocol; K.G. and S.B. produced the protein sample; V.C. and A.L. designed the research; P.F. and A.L. wrote the manuscript; and all authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Data 1–10 (ZIP 14 kb)
Supplementary Information
Supplementary Data 1–10 (PDF 627 kb)
Rights and permissions
About this article
Cite this article
Fricke, P., Chevelkov, V., Zinke, M. et al. Backbone assignment of perdeuterated proteins by solid-state NMR using proton detection and ultrafast magic-angle spinning. Nat Protoc 12, 764–782 (2017). https://doi.org/10.1038/nprot.2016.190
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.190
This article is cited by
-
1H-detected characterization of carbon–carbon networks in highly flexible protonated biomolecules using MAS NMR
Journal of Biomolecular NMR (2023)
-
Cell-free synthesis of amyloid fibrils with infectious properties and amenable to sub-milligram magic-angle spinning NMR analysis
Communications Biology (2022)
-
Characterizing proteins in a native bacterial environment using solid-state NMR spectroscopy
Nature Protocols (2021)
-
Protein resonance assignment by solid-state NMR based on 1H-detected 13C double-quantum spectroscopy at fast MAS
Journal of Biomolecular NMR (2021)
-
Multi-receiver solid-state NMR using polarization optimized experiments (POE) at ultrafast magic angle spinning
Journal of Biomolecular NMR (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.