Abstract
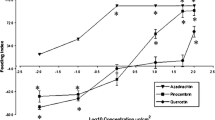
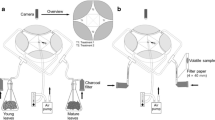
Larvae of the lycaenid butterfly Polyommatus bellargus were reared on leaves of Coronilla varia that are rich in flavone C-glycosides. Six flavonoids including isovitexin and isoorientin, as well as several of their congeners were isolated and identified by spectroscopic means. Comparative HPLC analysis of the host plant and of larvae, pupae, and imagines of P. bellargus indicated selective uptake of isovitexin versus isoorientin derivatives. Isovitexin-2″-O-xyloside was the major flavonoid detected in pupae and in imagines of P. bellargus. Several minor components were tentatively identified as quercetin- and kaempferol-O-glycosides based on their on line-UV spectra and by comparison with known standards. Since leaves of the host plant accumulate exclusively flavones, the flavonol glycosides are considered to be biotransformation products that are formed by the insects and/ or by symbiotic bacteria. Imagines of P. bellargus caught in the wild exhibited similar flavonoid patterns compared to imagines reared in the laboratory. Within the imagines, approximately 80% of all flavonoids are stored in the wings (especially in the orange submarginal lunules), whereas the remaining 20% resides in the bodies. Female butterflies show a significantly higher flavonoid concentration than males. It is suggested that the sequestered flavonoids are involved in visual mate recognition.
Similar content being viewed by others
REFERENCES
BERNARD, G. D., and REMINGTON, C. L. 1991. Color vision in Lycaena butterflies: Spectral tuning of receptor arrays in relation to behavioral ecology. Proc. Natl. Acad. Sci. U.S.A. 88:2783–2787.
BURGHARDT, F., FIEDLER, K., and PROKSCH, P. 1995. Wirtspflanzenabhängige Flavonoidmuster im Bläuling Polyommatus icarus (Lycaenidae). Verh. Dtsch. Zool. Ges. 88:256.
DOUWES, P. 1976. Mating behaviour in Heodes virgaureae with particular references to the stimuli from the female (Lepidoptera: Lycaenidae). Entomol. Gen. 2:232–241.
EBERT, G., and RENNWALD, E. (eds.) 1991. Die Schmetterlinge Baden-Württembergs. Band 1, Tagfalter 2. E. Ulmer, Stuttgart, 535 pp.
FIEDLER, K., and SAAM, C. 1994. Does ant-attendance influence development in 5 European Lycaenidae butterfly species? Nota Lepid. 17:5–24.
FORD, E. B. 1941. Studies on the chemistry of pigments in the Lepidoptera with reference to their bearing on systematics. 1. The anthoxanthins. Proc. R. Entomol. Soc. London Ser. A. 16:65–90.
HARBORNE, J. B. 1991. Flavonoid pigments, pp. 389–429, in G. A. Rosenthal and M. R. Berenbaum (eds.). Herbivores: Their Interaction with Secondary Plant Metabolites, 2nd. ed., Vol. 1. Academic Press, San Diego.
HATTORI, M., SHU, Y.-Z., EL-SEDAWY, A. I., NAMBA, T., KOBASHI, K., and TOMIMORI, T. 1988. Metabolism of homoorientin by human intestinal bacteria. J. Nat. Prod. 51:874–878.
HESSELBARTH, G., VAN OORSCHOT, H., and WAGENER, P. 1995. Die Tagfalter der Türkei unter Berücksichtigung der angrenzenden Länder, 3 vols. S. Wagener Verlag, Bocholt.
HOPKINS, T. L., and AHMAD, S. A. 1991. Flavonoid wing pigments in grasshoppers. Experientia 47:1089–1091.
MABRY, T. J., MARKHAM, K. R., and THOMAS, M. B. 1970. The Systematic Identification of Flavonoids. Springer, New York.
MEYER-ROCHOW, V. B. 1991. Differences in ultraviolet wing patterns in the New Zealand lycaenid butterflies Lycaena salustius, L. rauparaha and L. feredayi as a likely isolating mechanism. J. R. Soc. N.Z. 21:169–177.
MORRIS, S. H., and THOMSON, R. H. 1963. The flavonoid pigments of the marbled white butterfly (Melanargia galathea Seltz). J. Insect Physiol. 9:391–399.
SCHURIAN, K. G. 1989. Revision der Lysandra-Gruppe des Genus Polyommatus Latr. (Lepidoptera: Lycaenidae). Neue Entomol. Nachr. 24:1–181.
SHERWOOD, R. T., SHAMMA, M., MONIOT, J. L., and KROSCHEWSKY, J. R. 1973. Flavone C-glycosides from Coronilla varia. Phytochemistry 12:2275–2278.
SILBERGLIED, R. E. 1979. Communication in the ultraviolet. Annu. Rev. Ecol. Syst. 10:373–398.
SILBERGLIED, R. E. 1984. Visual communication and sexual selection among butterflies, pp. 207–223, in R. I. Vane-Wright and P. R. Ackery (eds.). The Biology of Butterflies. Academic Press, London.
THOMAS, J. A. 1983. The ecology and conservation of Lysandra bellargus (Lepidoptera: Lycaenidae) in Britain. J. Appl. Ecol. 20:59–83.
WIESEN, B., KRUG, E., FIEDLER, K., WRAY, V., and PROKSCH, P. 1994. Sequestration of host-plant-derived flavonoids by lycaenid butterfly Polyommatus icarus. J. Chem. Ecol. 20:2523–2538.
WILSON, A. 1985. Flavonoid pigments of butterflies in the genus Melanargia. Phytochemistry 24:1685–1691.
WILSON, A. 1986. Flavonoid pigments in swallowtail butterflies. Phytochemistry 25:1309–1313.
WILSON, A. 1987. Flavonoid pigments in chalkhill blue (Lysandra coridon Poda) and other lycaenid butterflies. J. Chem. Ecol. 13:473–493.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Geuder, M., Wray, V., Fiedler, K. et al. Sequestration and Metabolism of Host-Plant Flavonoids by the Lycaenid Butterfly Polyommatus bellargus . J Chem Ecol 23, 1361–1372 (1997). https://doi.org/10.1023/B:JOEC.0000006469.00557.69
Issue Date:
DOI: https://doi.org/10.1023/B:JOEC.0000006469.00557.69