Abstract
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a serious global issue. To prevent viral transmission, it is important to disinfect contaminated environmental surfaces and aerosols. We previously demonstrated that nano-sized electrostatic atomized water particles (NEAWPs) inactivate SARS-CoV-2. Herein, we focused on the underlying mechanisms. Morphological observation by transmission electron microscopy revealed that compared with NEAWPs-untreated virus, the shapes of particles corresponding to the size of SARS-CoV-2 particles were distorted significantly when exposed to NEAWPs. The amounts of viral RNA and protein in NEAWPs-treated SARS-CoV-2 showed a significantly greater decline than those in viruses unexposed to NEAWPs. Furthermore, much less NEAWPs-treated SARS-CoV-2 than NEAWPs-untreated virus bound to host cells. These results strongly suggest that NEAWPs damage the viral envelope, as well as viral protein and RNA, thereby impairing the ability of the virus to bind to host cells. Reactive oxygen species in NEAWPs may be involved in the inactivating effects on SARS-CoV-2.
Similar content being viewed by others
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19), which is characterized by fever, cough, pneumonia, and severe respiratory distress. SARS-CoV-2 has spread rapidly worldwide, leading to an estimated 276 million confirmed cases and 5.37 million deaths by December 2021 (World Health Organization n.d). Although there are numerous vaccines for SARS-CoV-2 (8.6 billion doses were administered worldwide by December 2021) (World Health Organization n.d), viral spread continues and new variants have emerged (World Health Organization n.d). SARS-CoV-2 is transmitted by exposure to infectious respiratory fluids, deposition of respiratory droplets onto exposed mucous membranes, and by touching mucous membranes with hands soiled either by respiratory fluids or by touching contaminated surfaces (Center for Disease Control and Prevention 2021). In addition, there is growing evidence of airborne transport of the virus in aerosol particles under favorable conditions (Fathizadeh et al. 2020; Fears et al. 2020; Jarvis 2020). Furthermore, SARS-CoV-2 can remain viable up to 16 h in aerosols (Fears et al. 2020), for up to 3 days on plastic and stainless steel surfaces (Doremalen et al. 2020), and for up to 7 days on the outer layer of a medical mask (Chin et al. 2020). Therefore, to prevent viral transmission, it is crucial to disinfect contaminated surfaces and aerosols, in addition to hand hygiene and use of medical masks.
Various types of biocidal agent, including hydrogen peroxide, alcohols, sodium hypochlorite, or benzalkonium chloride are used worldwide for disinfection in healthcare settings (Kampf et al. 2020). Alcohols, chlorine-based products, and hydrogen peroxide are, in particular, recommended for use on environmental surfaces to inactivate SARS-CoV-2 (Fathizadeh et al. 2020; World Health Organization 2020). By contrast, there is no effective inactivation strategy for contaminated aerosols. The WHO does not recommend routine application of disinfectants via spraying or fogging because (i) spraying as a primary disinfection strategy is ineffective at removing contaminants outside of the direct spray zone, and (ii) sprayed or fogged chemicals cause adverse health effects in humans, such as eye and skin irritation, bronchospasm, nausea, and vomiting (World Health Organization 2020). Another inactivation technology, based on UV irradiation, has been designed for use in healthcare settings to inactivate pathogens on environmental surfaces and in aerosols (World Health Organization 2020). Although UV irradiation inactivates SARS-CoV-2 (Inagaki et al. 2020; Kitagawa et al. 2021), the efficacy of UV irradiation is affected by factors related to the device (e.g., irradiation dose, wavelength, and exposure time) and, moreover, by environmental factors (e.g., distance from the device, the presence of obstacles, and the presence of organic components) (World Health Organization 2020; Rutala and Weber 2008). Furthermore, UV irradiation can be used only in unoccupied spaces because it is a direct hazard to humans (Jarvis 2020; World Health Organization 2020).
Nano-sized electrostatic atomized water particles (NEAWPs) are generated using an electrospray device. The device collects water by condensing atmospheric water vapor on an electrode and then electrostatically atomizes the condensed moisture by applying high voltage to the discharge electrode (Nomura et al. 2017; Panasonic. N.d). NEAWPs have a unique structure: an electron rich water shell of 5 to 20 nm in diameter, which contains reactive oxygen species (ROS) such as hydroxy radicals generated during the electrospray process. In general, ROS tend to react easily with other substances and have a short life. However, the ROS in NEAWPs are longer-lived and can be delivered to wider areas (Pyrgiotakis et al. 2014a). Several reports showed that NEAWPs inactivate several bacterial species on surfaces (Pyrgiotakis et al. 2014a, b, 2012). Furthermore, a recent study by Vaze et al. (Vaze et al. 2019) revealed that NEAWPs inactivate airborne influenza virus. In a previous paper, we demonstrated that NEAWPs inactivate SARS-CoV-2 on surfaces under closed experimental conditions (Yasugi et al. 2020). NEAWPs, therefore, have the potential to inactivate airborne pathogens and pathogens on environmental surfaces; however, the mechanisms underlying pathogen inactivation have not been fully understood, particularly with respect to viruses. The aim of this study was to identify the mechanisms by which NEAWPs inactivate SARS-CoV-2.
Materials and methods
NEAWPs
NEAWPs were generated by an electrostatic atomizing device (Panasonic, Osaka, Japan) (enlarged diagram in Fig. 1). Within the device, an electric current is applied to the Peltier element, which is thermally connected to the ground electrode; this cools the ground electrode and causes condensation of atmospheric water. Then, by applying a high voltage between the ground electrode and the positive electrode, the dew condensation water is charged and moves to the tip of the ground electrode to form a conical shape called a Taylor cone. Charge is concentrated at the tip of the Taylor cone, and the electrostatic force acting from the ground electrode to the positive electrode exceeds the surface tension and gravity, thereby discharging the water at the tip into the air as a minute droplet. Afterwards, the fine droplet undergoes Rayleigh fission (Taylor 1964; Mora 1992) due to the repulsive force of the charges, finally being discharged into the space as a nano-sized water particle.
Schematic diagram of NEAWPs. An electrostatic atomizing device is placed, facing downward, at a height of 15 cm above the viral sample in a 45 L sealed container. Within the device, the ground electrode is cooled by a Peltier module to condense water on the electrode. When a high voltage is applied to the condensed water, negatively charged water molecules gather at the tip and form a conical shape called a Taylor cone. The charge is concentrated at the tip of the Taylor cone. Because the electrostatic force acting from the ground electrode toward the positive electrode exceeds the surface tension and gravity, the water at the tip is discharged into the air as a droplet. This droplet is broken repeatedly by the repulsive force created by the electric charge. Finally, it fragments to yield fine water particles of 5 to 20 nm in diameter
The size of the generated water particles was measured using a scanning mobility particle sizer (SMPS) (3936N76; TSI, Shoreview, MN, USA). Particles at a point 10 mm away from the discharge point of the NEAWPs generator were sucked in to the SMPS device. ROS contained in the NEAWPs were measured by electron spin resonance (ESR) analysis (Nakai 2005; Oowada et al. 2012) using an ELEXSYS E500 (Bruker, Billerica, MA, USA); stabilized radicals were captured by exposing NEAWPs to the spin trapping agent 5-(2,2-dimethyl-1,3-propoxycyclophosphoryl)-5-methyl-1-pyrroline N-oxide (CYPMPO; Mikuni Pharmaceutical Industrial, Osaka, Japan) at a distance of 50 mm. ROS in NEAWPs were then identified by comparing the obtained spectra with theoretical spectra of ROS such as hydroxy radicals and superoxide radicals.
Cells
The African green monkey kidney cell line VeroE6 and VeroE6 cells expressing the transmembrane serine protease TMPRSS2 (VeroE6/TMPRSS2) (Matsuyama et al. 2020) were purchased from the National Institutes of Biomedical Innovation, Health and Nutrition, Osaka, Japan. VeroE6 cells were cultured in Eagle’s minimal essential medium (Sigma, St. Luis, MO, USA) supplemented with 10% fetal bovine serum (FBS) and antibiotics. VeroE6/TMPRSS2 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 5% FBS and 1 mg/ml G418 (Nacalai, Kyoto, Japan). Cells were grown at 37˚C in a CO2 incubator.
Virus
All experiments with live SARS-CoV-2 were conducted at the bio-safety level 3 laboratory in Osaka Prefecture University after obtaining the permission from the biorisk committee of Osaka Prefecture University. SARS-CoV-2 strain JPN/TY/WK-521 was provided by the National Institute of Infectious Diseases, Tokyo, Japan. Viruses were propagated in monolayers of VeroE6/TMPRSS2 cells in DMEM supplemented with 2% FBS and 1 mg/ml G418 at a multiplicity of infection of 0.01. For some experiments, the virus was concentrated using polyethylene glycol (PEG) as described previously (McSharry and Benzinger 1970), with slight modifications. Briefly, viruses were mixed with one-third volume of PEG buffer (32% PEG8000, 2.4% NaCl) and incubated overnight at 4˚C. After the mixture was centrifuged at 15,000 × g for 20 min, the pellets were resuspended in phosphate buffered saline (PBS).
Virus titration
Quantitative PCR (qPCR) is used for diagnosis of SARS-CoV-2 in healthcare settings. However, qPCR detects both live and dead viruses (Matson et al. 2020). The purpose of the present study was to assess whether NEAWPs inactivate infectious (live) viruses. Virus infectivity was therefore measured by titration to obtain the 50% tissue culture infectious dose (TCID50) as described previously (Cavanagh 2008; Kratzel et al. 2020). Briefly, viruses were serially diluted tenfold in DMEM supplemented with 2% FBS and 1 mg/ml G418 and infected to the confluent VeroE6/TMPRSS2 cells for 72 h. The cells were fixed with methanol and stained with 0.5% methylene blue. Viral titers were calculated using the Reed-Muench method (Ramakrishnan 2016).
Treatment with NEAWPs
SARS-CoV-2 was exposed to NEAWPs as described previously (Yasugi et al. 2020), but with slight modifications. Briefly, 300 µl of virus (3.16 × 106 TCID50/ml) was placed on a sterilized gauze (1 × 2 cm) on a sterilized petri dish and incubated for 30 min at room temperature before the virus was exposed to NEAWPs. The electrostatic atomizing device generating NEAWPs was placed, facing downward, 15 cm above the samples in a 45 L (340 × 344.5 × 387 mm) sealed container (Fig. 1). Generated NEAWPs were applied continuously to the samples for 1 to 3 h. After the virus was exposed to NEAWPs, the gauzes were immersed in 500 µl of DMEM supplemented with 2% FBS and 1 mg/ml G418. After incubation overnight at 4˚C, the virus was titrated. For experiments using concentrated virus (1 × 109 TCID50/ml), 40 µl of virus was placed on a gauze (0.5 × 1 cm) and incubated for 15 min before exposure to NEAWPs. After exposure for 1 h, the samples were immersed in 100 µl PBS overnight at 4˚C. As a control, viruses were exposed to a fan placed in the container instead of the electrostatic atomizing device. Air volume flow from the fan was adjusted such that it was similar to that from the electrostatic atomizing device. All experiments were conducted at 21–27˚C and 51–69% relative humidity.
Transmission electron microscopy
Morphological observation of SARS-CoV-2 by transmission electron microscope (TEM) was performed by Tokai Electron Microscopy, Aichi, Japan. Samples were fixed in an equivalent volume of 4% glutaraldehyde (pH 7.4) and negative staining was performed as follows: samples were absorbed onto formvar film-coated copper grids and stained for 1 min with 2% phospho-tungstic acid solution (pH 7.0). The grids were observed by a TEM (JEM-1400Plus; JEOL, Tokyo, Japan) at an acceleration voltage of 100 kV. Digital images (3296 × 2472 pixels) of particles with a diameter of 60 to 80 nm were taken by a CCD camera (EM-14830RUBY2; JEOL). More than 50 particles per sample per experiment were observed.
To assess distortion of each particle, the command ‘solidity’ in ImageJ software was used (ImageJ User Guide n.d). Solidity, also known as convexity, is calculated as the area of a particle divided by its convex hull area, and indicates less concavity. It can therefore differentiate cells with protrusions or an irregular shape from those that are generally round. Particle solidity was calculated automatically by the software and presented as a percentage of solidity.
RNA extraction
Viral RNA was extracted from virus culture medium using a Qiaquick viral RNA extraction kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Total RNA was extracted from virus-attached cells using Trizol (Sigma) according to the manufacturer’s instructions.
RT and qPCR
Extracted RNA (300 ng in each sample) was reverse transcribed using superscript III reverse transcriptase (Thermo Fisher Scientific) and random primers. Synthesized cDNA was subjected to a SYBR green real-time PCR assay (Thermo Fisher Scientific) using the Applied Biosystems StepOne real-time PCR system (Thermo Fisher Scientific). The primers used were as follows: SARS-CoV-2-nucleocapsid gene primer set (forward, 5′-AAATTTTGGGGACCAGGAAC-3′; reverse, 5′-TGGCAGCTGTGTAGGTCAAC-3′) and GAPDH gene primer set (forward, 5′-ACCACAGTCCATGCCATCAC-3′; reverse, 5′-TCCACCACCCTGTTGCTGTA-3′).
Western blotting and Coomassie brilliant blue (CBB) staining
Virus culture medium or infected cells were mixed with a SDS sample loading buffer containing 2-mercaptoethanol and loaded to a polyacrylamide gel for SDS-PAGE. After the gel was electrophoresed, proteins were blotted onto a PVDF membrane. The membrane was blocked using 5% skim milk and then probed for 1 h at 37˚C with a rabbit anti-SARS-CoV-2-nucleocapsid antibody (Thermo Fisher Scientific) or a mouse anti-ß actin antibody (Sigma), followed by incubation with HRP-conjugated IgG for 1 h at 37˚C. Blots were visualized using an enhanced chemiluminescence system (Immobilon Western; Merck, Burlington, MA, USA).
Virus culture medium in SDS sample loading buffer containing 2-mercaptoethanol was subjected to SDS-PAGE as described above. After the gel was electrophoresed, it was fixed and stained with 0.25% CBB R-250 (Sigma).
Binding assay
Viral endocytosis and virus-cell fusion are prevented when virus-attached cells are kept at 4˚C (Bradley et al. 2011; Cheng et al. 2004). Viral samples were therefore absorbed to confluent VeroE6 cells in 12-well plates at 4˚C for 1 h. The cells were then washed three times with PBS and collected for further analysis.
Statistical analysis
Statistical analyses were performed using Student’s t-test or the Mann–Whitney U test. The statistical significance of multiple comparisons was determined by one-way ANOVA, followed by the Tukey–Kramer test. P < 0.05 was considered significant.
Results
Physicochemical characterization of NEAWPs
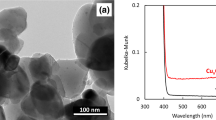
We first confirmed physiological and chemical characterization of water particles generated by an electrostatic atomizing device. The size of the water particles ranged from 5 to > 60 nm in diameter, the most common size being 11 nm in diameter (Fig. 2A). More than 75% of all particles were 5 to 20 nm in diameter. These results indicate that water particles generated by the device were nano-sized particles. Next, we examined ROS contained in NEAWPs because NEAWPs are generated in the space in which the discharge is occurring. ESR spectra obtained from NEAWPs generated by the device were merged with those of hydroxyl radicals and superoxide radicals (Fig. 2B). We found that the ESR spectra of NEAWPs were almost the same as those of hydroxyl radicals. The spectra of NEAWPs were also compared with those of previously reported t-butoxyl radicals, t-butyl peroxyl radicals, and methyl radicals (Oowada et al. 2012). However, none of these ESR spectra were consistent with those of NEAWPs. These results strongly suggest that the NEAWPs contained hydroxyl radicals.
Physicochemical characterization of NEAWPs. (A) Particle size of NEAWPs measured using the scanning mobility particle sizer. (B) Electron spin resonance spectra of NEAWPs. The spectra of NEAWPs (red) were merged with those of hydroxyl radicals (•OH) (black in upper panel) or superoxide radicals (O2–•) (black in lower panel)
Inactivation of SARS-CoV-2 by NEAWPs
We next confirmed the inactivation potential of NEAWPs, as shown in our previous study (Yasugi et al. 2020). After exposure to NEAWPs for 1 h, the titer of SARS-CoV-2 was significantly lower than that after exposure to control conditions (exposure to a fan only as described in the Materials and methods section). The amount of live virus in samples exposed to NEAWPs for 2 and 3 h was 100 and 1000 times lower, respectively, than that after exposure to control conditions for 2 and 3 h (white bars in Fig. 3A). We wondered whether the rate of reduction in the viral titer would increase if the amount of ROS contained in NEAWPs was increased. We thus prepared the modified electrostatic atomizing device which generates NEAWPs containing 100-fold amounts of ROS compared with the original device. Exposure to NEAWPs from the modified device for 1 to 3 h led to a significant fall in the amount of live virus compared with the control (gray bars in Fig. 3A). However, the difference of viral titers was not significant between the original and modified device. Guidance on the biocidal products regulation issued by the European chemical agency states that a 4 log10 reduction in viral titer is required if a product is to be recognized as an effective biocidal agent (European Chemicals Agency 2022). We therefore extrapolated the time required to achieve a 4 log10 reduction in SARS-CoV-2 titer in our devices, and found that it would take 4.3 h and 3.6 h to obtain such a reduction using the original and modified devices, respectively (Figure S1).
Inactivation of SARS-CoV-2 by NEAWPs. (A) Viruses suspended in DMEM supplemented with FBS and G418 were exposed to NEAWPs from the original device (white) or from the modified device (generating NEAWPs containing 100-fold amounts of ROS compared with the original device) (gray) for 1 to 3 h. And then they were titrated by TCID50 and compared with samples after exposure to control conditions (a fan only) (black). (B) After viruses in PBS were exposed to NEAWPs for 1 h, viral titers (TCID50) were measured and compared with the control. All data represent the means + SD from three independent experiments. # indicates that the viral titers were below the detection thresholds. **, P < 0.01 indicate significant differences between the two samples
The data in Fig. 3A were generated using viruses in culture medium (DMEM supplemented with FBS and G418); therefore, viruses were precipitated using PEG and resuspended in PBS to remove organic components. When viruses in PBS were exposed to NEAWPs for 1 h, the titers were 1000 times lower than those of the control (Fig. 3B). These results indicate that NEAWPs inactivated SARS-CoV-2, and that the efficiency of inactivation was affected by components in culture medium containing FBS.
NEAWPs-mediated damage to SARS-CoV-2
We next focused on the mechanisms by which NEAWPs inactivate SARS-CoV-2. Pyrgiotakis et al. (2014a) used membrane permeability tests and TEM imaging to show that NEAWPs inactivate Serratia marcescens by destroying the bacterial cell membrane. We thus used TEM to examine changes in viral morphology after exposure to NEAWPs. Viruses were concentrated prior to the experiments because high amounts of live virus were needed for TEM analysis. We confirmed that viral titers in the NEAWPs-exposed group were 1000 times lower than those in the control after 1-h exposure. All particles measuring 60 to 80 nm in diameter (corresponding to that of SARS-CoV-2) observed under TEM were analyzed for distortion of the particles using the command ‘solidity’ in ImageJ software as described in the Materials and methods section; this is an indirect measure of integrity of viral envelope. Particles exposed to NEAWPs were significantly more distorted than those exposed to control conditions (Fig. 4), suggesting that NEAWPs-exposed particles were damaged. Given that NEAWPs destroyed bacterial cell membranes (Pyrgiotakis et al. 2014a), there is a possibility that NEAWPs-exposed virus has lost the integrity of the viral envelope; however, a limitation of these experiments is that the particles examined were not confirmed to be SARS-CoV-2.
Analysis of viral morphology by TEM. Concentrated virus was treated with (exposure) or without (control) NEAWPs for 1 h. Samples were fixed and negative staining was performed. Digital images of particles of 60 to 80 nm in diameter were taken. The 114 particles in the exposure samples and 143 particles in the control samples from two independent experiments were analyzed for morphological distortion using the command ‘solidity’ in ImageJ software. Dots and horizontal bars denote the percentage solidity of each particle and the mean value, respectively. The viral particles in images (a) to (d) correspond with the dots (a) to (d) (in red on the graph). Scale bar, 100 nm
We next examined whether viral RNA and protein are damaged by exposure to NEAWPs. Concentrated virus was exposed to NEAWPs for 1 h and more than 3 log10 reduction in titer compared with the control samples was confirmed. The amount of viral RNA (the nucleocapsid gene) in NEAWPs-exposed virus was significantly lower than that after exposure to control conditions (Fig. 5A). These results suggest that viral RNA was degraded upon exposure to NEAWPs. We next performed RNA electrophoresis to identify whether viral genomic RNA (not only nucleocapsid RNA) is degraded; however, there were no significant differences between the control and exposure groups (data not shown). It is likely that carrier RNAs used for viral RNA extraction masked any differences. We next examined NEAWPs-mediated damage to viral protein. When the same volume in each sample was loaded onto the gels, the amount of protein detected was much lower after NEAWPs exposure than after control exposure (Fig. 5B). No aggregated proteins or short peptides were detected by SDS-PAGE and CBB staining of samples from the NEAWPs exposure group (Figure S2). We then confirmed that the amounts of virus-specific protein (nucleocapsid protein) were lower after exposure to NEAWPs than after exposure to control conditions (Fig. 5C). These results suggest that viral protein was degraded upon exposure to NEAWPs.
Damages to viral RNA and protein by NEAWPs. Concentrated virus was treated with (exposure) or without (control) NEAWPs for 1 h. (A) RT-qPCR was performed using 300 ng of RNA in each sample to amplify the SARS-CoV-2 nucleocapsid genomic RNA. The Y-axis indicates fold changes in the amount of nucleocapsid gene in NEAWPs-exposed group relative to that in the control group. All data represent the means + SD from three independent experiments. (B) (C) The same volume in each sample was loaded to gels and subjected to SDS-PAGE followed by CBB staining (B) or western blotting to detect the nucleocapsid protein (C). M indicates molecular markers. Images are representative of two (B) or three (C) independent experiments
Functional damage to SARS-CoV-2 by NEAWPs
We finally focused on viral function, in particular the ability of the virus to bind to host cells. The amounts of viral RNA extracted from viral particles bound to cells were significantly lower after 3-h exposure in NEAWPs than those after 3-h exposure to control conditions (Fig. 6A). The amount of nucleocapsid protein extracted from viruses bound to cells was also lower after exposure to NEAWPs (Fig. 6B). These data suggest that NEAWPs inhibited the ability of the virus to attach to host cells.
Binding of NEAWPs-exposed virus. Viruses with (exposure) or without (control) NEAWPs exposure for 3 h were absorbed onto VeroE6 cells for 1 h at 4 °C. Next, the amount of virus bound to cells was measured by qPCR (A) and western blotting (B). (A) RT-qPCR was performed to amplify the viral genomic RNA (nucleocapsid gene). Data were normalized to the internal control gene (GAPDH). The Y-axis indicates fold changes in the amount of nucleocapsid gene in NEAWPs-exposed group relative to that in the control group. All data represent the means + SD from three independent experiments. (B) Western blotting was performed to detect nucleocapsid protein (upper panel) and ß-actin (internal control; lower panel). M indicates molecular marker. Images are representative of three independent experiments. Upper band in upper panel, nucleocapsid protein; lower band in upper panel, undetermined (it may be a cleaved form of the nucleocapsid protein or a non-specific protein)
Discussion
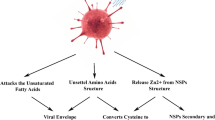
The data presented herein suggested that NEAWPs damage the SARS-CoV-2 envelope, as well as viral protein and RNA, thereby impairing its ability to bind to host cells (Fig. 7). To the best of our knowledge, this is the first report describing possible mechanisms by which NEAWPs inactivate viruses. NEAWPs have a unique structure: an electron rich water shell containing ROS generated during the electrospray process (Nomura et al. 2017; Pyrgiotakis et al. 2012; Yamauchi et al. 2007). Although previous reports showed that NEAWPs inactivate bacterial pathogens by damaging the bacterial membrane, the exact mechanisms were not investigated (Pyrgiotakis et al. 2012; Kobayashi et al. 2012). Pygiotakis et al. (Pyrgiotakis et al. 2014b) firstly demonstrated that NEAWPs induce bacterial membrane lipid peroxidation, a process by which oxidants such as ROS attack lipids (Ayala et al. 2014). They therefore suggested that ROS are one of the dominant pathways of inactivation of bacteria exposed to NEAWPs (Pyrgiotakis et al. 2014b). In the present study, exposure of SARS-CoV-2 in culture medium to NEAWPs for 3 h led to a 3 log10 reduction in titer compared with the control samples (Fig. 3A). By contrast, it took 1 h to lead to a 3 log10 reduction in titer when viruses suspended in PBS were exposed to NEAWPs (Fig. 3B). These results indicate that components in culture medium (DMEM supplemented with FBS and G418) affect the inactivation efficacy of NEAWPs. Susceptibility of pathogens to ROS such as ozone varies according to the pH of the medium, temperature, and humidity (Kim et al. 1999). In particular, oxidizable organic components and reductants can easily consume ROS (Seki et al. 2017; Urban et al. 2019). Kim et al. (Kim et al. 1999) showed that inactivation of viruses in waste water requires a longer contact time and a higher ozone concentration than inactivation in ozone demand-free systems; this is due to the presence of oxidizable materials in the medium. Taken together, our results suggest that ROS in NEAWPs were consumed by oxidizable organic components in culture medium (DMEM supplemented with FBS and G418), which affects the inactivation efficacy against SARS-CoV-2. Therefore, ROS in NEAWPs are likely involved in the mechanism underlying inactivation of SARS-CoV-2.
NEAWPs induce lipid peroxidation in Gram-positive Mycobacterial membranes (Pyrgiotakis et al. 2014b). Given that gram-positive bacterial membrane consists of a lipid bilayer, made up of two layers of phospholipids containing proteins and glycolipids (Rajagopal and Walker 2017; Silhavy et al. 2010), and that its structure and composition are consistent with those of the viral envelope (including that of SARS-CoV-2) (Abu-Farha et al. 2020; Simon et al. 2021), NEAWPs may induce lipid peroxidation of the viral envelope. NEAWPs contained ROS such as hydroxy radicals (Fig. 2B). ROS mediate lipid peroxidation by reacting with lipids. During lipid peroxidation, free radicals oxidize an unsaturated lipid chain, which gives rise to toxic breakdown products such as hydroxynonenal and malondialdehyde, leading to alteration of the membrane structure, thereby affecting its fluidity and damaging its integrity (Juan et al. 2021). Murray et al. (Murray et al. 2008) demonstrated that the lipid bilayer of the viral envelope was peroxidized by ozone-mediated ROS, which results in damage, destruction, and (ultimately) inactivation of enveloped viral species. Taken together, these data suggest a possibility that the distortion of viral particles by NEAWPs observed in this study (Fig. 4) may be mediated by ROS-induced lipid peroxidation of the viral envelope.
Our results showed that the amount of viral protein was much lower after NEAWPs exposure than after control exposure (Fig. 5B). Proteins that react with ROS are oxidized; this involves oxidation of amino acid residues, protein aggregation, and cleavage of peptide bonds, leading to structural damage and loss of function (Juan et al. 2021). Cleavage of the peptide backbone is initiated by abstraction of the α-carbon hydrogen atom, leading to formation of a carbon-centered radical. Subsequent reactions with oxygen or ROS decompose the peptide to an N-terminal carbonyl compound and a C-terminal isocyanate via the diamide pathway, or to a peptide amide and an α-ketoacyl peptide via α-amidation (Hawkins and Davies 2019; Sajapin and Hellwig 2020). Species such as hydroxyl radicals can attack a range of different cleavage sites along a protein backbone, which is detected as a smear on protein gels (Hawkins and Davies 2019). Furthermore, Uchida et al. (Uchida and Kawakishi 1988) revealed that oxidized bovine serum albumin was initially fragmented to short peptides and eventually became undetectable by SDS-PAGE. Given that NEAWPs contain ROS, in particular hydroxy radicals (Fig. 2B), and that the aggregated proteins and short peptides were not detected by SDS-PAGE analysis after NEAWPs exposure (Figure S2), we believe that the viral protein may be fragmented due to ROS-induced oxidation and cannot therefore be detected in SDS-PAGE gels.
The data also suggested that viral RNA is degraded by exposure to NEAWPs (Fig. 5A). In addition to oxidation of polyunsaturated fatty acids and amino acids, ROS also induce oxidation of nucleic acids, which leads to single strand breaks and nucleic base modifications, ultimately causing loss of function (Siddiqui et al. 2016). DNA fragmentation caused by oxidative damage to DNA-deoxyribose has been well investigated (Juan et al. 2021). Reaction of deoxyribose with oxygen leads to several transposition reactions resulting in expansion of the ring, which subsequently degrades to different products. Ultimately, hydroxy acetal derivatives are formed by addition of water molecules to the carbon, leading to fragmentation to generate the acrylaldehyde-derived base. In another pathway, the radical evolves to the oxonium cation in deoxyribose, leading to nucleophilic attack by a water molecule, which results in decomposition of the nucleotide to the free base and various other fragments (Juan et al. 2021). In contrast to DNA, little is known about degradation/fragmentation of RNA after oxidative damage. However, given that RNA is a single-strand molecule and does not possess protective histones (Martinet et al. 2004), RNA oxidation may give rise to RNA-ribose oxidation and subsequent fragmentation, as well as DNA. Actually, there are several reports describing ROS-inducing RNA degradation/fragmentation. Martinet et al. (Martinet et al. 2004) revealed that purified total human RNAs are degraded by exposure to ROS-releasing compounds. Roy et al. (Roy et al. 1981) demonstrated that polioviral RNA did not sediment as a single band; rather, broad bands were observed by treatment of ozone (one of ROS), which implied that the viral RNA was damaged due to ozonation, and possibly fragmented into a number of short chains. Taken together, the data from the present study suggest that ROS in NEAWPs may contribute to RNA degradation. It is not clear which ROS molecule degrades the viral RNA examined in the present study. It is possible that ROS themselves induce oxidation of viral RNA as follows: ROS in NEAWPs first oxidize the viral envelope (lipids and proteins) and alter the envelope structure, thereby affecting its fluidity and damaging its integrity (Juan et al. 2021); this results in direct contact between ROS and viral RNA. Another possibility is that the contribution of secondary reactive species generated as a consequence of direct interactions between ROS and chemical subunits may play a role in the mechanisms underlying viral RNA degradation (Bayarri et al. 2021). Taken together, in addition to impairing virus binding to host cells, NEAWPs may disrupt viral replication by degrading viral RNA, although we provide no direct evidence for this possibility. Further studies are needed to elucidate the precise mechanisms by which NEAWPs inactivate SARS-CoV-2.
References
Abu-Farha M, Thanaraj TA, Qaddoumi MG, Hashem A, Abubaker J, Al-Mulla F (2020) The role of lipid metabolism in COVID-19 virus infection and as a drug target. Int J Mol Sci 21(10). https://doi.org/10.3390/ijms21103544
Ayala A, Munoz MF, Arguelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014:360438. https://doi.org/10.1155/2014/360438
Bayarri B, Cruz-Alcalde A, Lopez-Vinent N, Mico MM, Sans C (2021) Can ozone inactivate SARS-CoV-2? A review of mechanisms and performance on viruses. J Hazard Mater 415:125658. https://doi.org/10.1016/j.jhazmat.2021.125658
Bradley KC, Galloway SE, Lasanajak Y, Song X, Heimburg-Molinaro J, Yu H et al (2011) Analysis of influenza virus hemagglutinin receptor binding mutants with limited receptor recognition properties and conditional replication characteristics. J Virol 85(23):12387–12398. https://doi.org/10.1128/JVI.05570-11
Cavanagh D (2008) SARS- and other coronaviruses. Methods Mol Biol 454:v–vi. https://doi.org/10.1007/978-1-59745-181-9
Center for Disease Control and Prevention (2021) SARS-CoV-2 transmission. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/index.html. Accessed 10 May 2022
Cheng HY, Lin TC, Yang CM, Wang KC, Lin LT, Lin CC (2004) Putranjivain A from Euphorbia jolkini inhibits both virus entry and late stage replication of herpes simplex virus type 2 in vitro. J Antimicrob Chemother 53(4):577–583. https://doi.org/10.1093/jac/dkh136
Chin AWH, Chu JTS, Perera MRA, Hui KPY, Yen HL, Chan MCW et al (2020) Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 1(1):e10. https://doi.org/10.1016/S2666-5247(20)30003-3
de la Mora JF (1992) The effect of charge emission from electrified liquid cones. J Fluid Mech 243:561–574. https://doi.org/10.1017/S0022112092002829
European Chemicals Agency (2022) Guidance on the biocidal products regulation. Volume II efficacy-assessment and evaluation (Parts B+C). https://echa.europa.eu/guidance-documents/guidance-on-biocides-legislation. Accessed 10 May 2022
Fathizadeh H, Maroufi P, Momen-Heravi M, Dao S, Kose S, Ganbarov K et al (2020) Protection and disinfection policies against SARS-CoV-2 (COVID-19). Infez Med 28(2):185–191
Fears AC, Klimstra WB, Duprex P, Hartman A, Weaver SC, Plante KS et al (2020) Persistence of severe acute respiratory syndrome coronavirus 2 in aerosol suspensions. Emerg Infect Dis 26(9):2168–2171. https://doi.org/10.3201/eid2609.201806
Hawkins CL, Davies MJ (2019) Detection, identification, and quantification of oxidative protein modifications. J Biol Chem 294(51):19683–19708. https://doi.org/10.1074/jbc.REV119.006217
ImageJ User Guide (n.d.) https://imagej.nih.gov/ij/docs/guide/146.html
Inagaki H, Saito A, Sugiyama H, Okabayashi T, Fujimoto S (2020) Rapid inactivation of SARS-CoV-2 with deep-UV LED irradiation. Emerg Microbes Infect 9(1):1744–1747. https://doi.org/10.1080/22221751.2020.1796529
Jarvis MC (2020) Aerosol transmission of SARS-CoV-2: physical principles and implications. Front Public Health 8:590041. https://doi.org/10.3389/fpubh.2020.590041
Juan CA, Perez de la Lastra JM, Plou FJ, Perez-Lebena E (2021) The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int J Mol Sci 22(9). https://doi.org/10.3390/ijms22094642
Kampf G, Todt D, Pfaender S, Steinmann E (2020) Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect 104(3):246–251. https://doi.org/10.1016/j.jhin.2020.01.022
Kim JG, Yousef AE, Dave S (1999) Application of ozone for enhancing the microbiological safety and quality of foods: a review. J Food Prot 62(9):1071–1087. https://doi.org/10.4315/0362-028x-62.9.1071
Kitagawa H, Nomura T, Nazmul T, Omori K, Shigemoto N, Sakaguchi T et al (2021) Effectiveness of 222-nm ultraviolet light on disinfecting SARS-CoV-2 surface contamination. Am J Infect Control 49(3):299–301. https://doi.org/10.1016/j.ajic.2020.08.022
Kobayashi I, Kanayama A, Imai T, Suda H, Asano Y (2012) [Bactericidal effectiveness of an electrostatic atomizing device against antimicrobial-resistant organisms]. Bokin Bobai 40(11) (in Japanese).
Kratzel A, Todt D, V’Kovski P, Steiner S, Gultom M, Thao TTN et al (2020) Inactivation of severe acute respiratory syndrome coronavirus 2 by WHO-recommended hand rub formulations and alcohols. Emerg Infect Dis 26(7):1592–1595. https://doi.org/10.3201/eid2607.200915
Martinet W, de Meyer GR, Herman AG, Kockx MM (2004) Reactive oxygen species induce RNA damage in human atherosclerosis. Eur J Clin Invest 34(5):323–327. https://doi.org/10.1111/j.1365-2362.2004.01343.x
Matson MJ, Yinda CK, Seifert SN, Bushmaker T, Fischer RJ, van Doremalen N et al (2020) Effect of environmental conditions on SARS-CoV-2 stability in human nasal mucus and sputum. Emerg Infect Dis 26(9):2276–2278. https://doi.org/10.3201/eid2609.202267
Matsuyama S, Nao N, Shirato K, Kawase M, Saito S, Takayama I et al (2020) Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Natl Acad Sci U S A 117(13):7001–7003. https://doi.org/10.1073/pnas.2002589117
McSharry J, Benzinger R (1970) Concentration and purification of vesicular stomatitis virus by polyethylene glycol “precipitation.” Virology 40(3):745–746. https://doi.org/10.1016/0042-6822(70)90219-9
Murray BK, Ohmine S, Tomer DP, Jensen KJ, Johnson FB, Kirsi JJ et al (2008) Virion disruption by ozone-mediated reactive oxygen species. J Virol Methods 153(1):74–77. https://doi.org/10.1016/j.jviromet.2008.06.004
Nakai Y (2005) Basics and applications of ESR. J Jpm Soc Colour Mater 78(11):539–545 (in Japanese)
Nomura M, Aiamla-Or S, Tanaka S, Shigyo M, Masuda Y, Yamauchi N (2017) Effect of reactive oxygen species on quality maintenance of broccoli florets with electrostatic atomized water particle treatment. Food Chem 237:749–755. https://doi.org/10.1016/j.foodchem.2017.05.145
Oowada S, Endo N, Kameya H, Shimmei M, Kotake Y (2012) Multiple free-radical scavenging capacity in serum. J Clin Biochem Nutr 51(2):117–121. https://doi.org/10.3164/jcbn.11-113
Panasonic: Nano-sized electrostatic atmized water particles, hydroxy radicals contained in water. https://www.panasonic.com/global/consumer/clean/hydroxyl.html Accessed.
Pyrgiotakis G, McDevitt J, Bordini A, Diaz E, Molina R, Watson C et al (2014a) A chemical free, nanotechnology-based method for airborne bacterial inactivation using engineered water nanostructures. Environ Sci Nano 1:15–26. https://doi.org/10.1039/C3EN00007A
Pyrgiotakis G, McDevitt J, Gao Y, Branco A, Eleftheriadou M, Lemos B et al (2014b) Mycobacteria inactivation using engineered water nanostructures (EWNS). Nanomedicine 10(6):1175–1183. https://doi.org/10.1016/j.nano.2014.02.016
Pyrgiotakis G, McDevitt J, Yamauchi T, Demokritou P (2012) A novel method for bacterial inactivation using electrosprayed water nanostructures. J Nanopart Res 14:1027. https://doi.org/10.1007/s11051-012-1027-x
Rajagopal M, Walker S (2017) Envelope structures of Gram-positive bacteria. Curr Top Microbiol Immunol 404:1–44. https://doi.org/10.1007/82_2015_5021
Ramakrishnan MA (2016) Determination of 50% endpoint titer using a simple formula. World J Virol 5(2):85–86. https://doi.org/10.5501/wjv.v5.i2.85
Roy D, Wong PK, Engelbrecht RS, Chian ES (1981) Mechanism of enteroviral inactivation by ozone. Appl Environ Microbiol 41(3):718–723. https://doi.org/10.1128/aem.41.3.718-723.1981
Rutala WA, Weber DJ (2019) Guideline for disinfection and sterilization in healthcare facilities. Center for Diseases Control and Prevention. https://www.cdc.gov/infectioncontrol/guidelines/disinfection/. Accessed 10 May 2022
Sajapin J, Hellwig M (2020) Studies on the synthesis and stability of alpha-ketoacyl peptides. Amino Acids 52(10):1425–1438. https://doi.org/10.1007/s00726-020-02902-8
Seki M, Ishikawa T, Terada H, Nashimoto M (2017) Microbicidal effects of stored aqueous ozone solution generated by nano-bubble technology. In Vivo 31(4):579–583. https://doi.org/10.21873/invivo.11097
Siddiqui T, Zia MK, Ali SS, Rehman AA, Ahsan H, Khan FH (2016) Reactive oxygen species and anti-proteinases. Arch Physiol Biochem 122(1):1–7. https://doi.org/10.3109/13813455.2015.1115525
Silhavy TJ, Kahne D, Walker S (2010) The bacterial cell envelope. Cold Spring Harb Perspect Biol 2(5):a000414. https://doi.org/10.1101/cshperspect.a000414
Simon M, Veit M, Osterrieder K, Gradzielski M (2021) Surfactants - compounds for inactivation of SARS-CoV-2 and other enveloped viruses. Curr Opin Colloid Interface Sci 55:101479. https://doi.org/10.1016/j.cocis.2021.101479
Taylor GI (1964) Disintegration of water drops in an electroic field. Proc Roy Soc London A280:383–397. https://doi.org/10.1098/rspa.1964.0151
Uchida K, Kawakishi S (1988) Selective oxidation of tryptophan and histidine residues in protein through the copper-catalyzed autoxidation of L-ascorbic acid. Agric Biol Chem 52(6):1529–1535. https://doi.org/10.1271/bbb1961.52.1529
Urban MV, Rath T, Radtke C (2019) Hydrogen peroxide (H2O2): a review of its use in surgery. Wien Med Wochenschr 169(9–10):222–225. https://doi.org/10.1007/s10354-017-0610-2
van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN et al (2020) Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 382(16):1564–1567. https://doi.org/10.1056/NEJMc2004973
Vaze N, Pyrgiotakis G, McDevitt J, Mena L, Melo A, Bedugnis A et al (2019) Inactivation of common hospital acquired pathogens on surfaces and in air utilizing engineered water nanostructures (EWNS) based nano-sanitizers. Nanomedicine 18:234–242. https://doi.org/10.1016/j.nano.2019.03.003
World Health Organization (2020) Cleaning and disinfection of environmental surfaces in the context of COVID-19: interim guidance. WHO/2019-nCoV/Disinfection/2020.1. https://apps.who.int/iris/handle/10665/332096. Accessed 10 May 2022
World Health Organization (n.d.) Coronavirus (COVID-19) dashboard. https://www.who.int/. Accessed 10 May 2022
Yamauchi T, Suda H, Matsui Y (2007) Development of home appliances using electrostatic atomization. J Aerosol Res 22(1):5–10. https://doi.org/10.11203/jar.22.5
Yasugi M, Komura Y, Ishigami Y (2020) Inactivation of newly emerged crona virus (SARS-CoV-2) by nano-sized electrostatic atomized water particles. Indoor Environ 23(3):241–246. https://doi.org/10.7879/siej.23.241 (in Japanese)
Acknowledgements
We thank the National Institute of Infectious Diseases for providing the viral strain.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was performed in collaboration with Panasonic Corporation Living Appliances and Solutions Company, and was financially supported by the company.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yasugi, M., Komura, Y. & Ishigami, Y. Mechanisms underlying inactivation of SARS-CoV-2 by nano-sized electrostatic atomized water particles. J Nanopart Res 24, 99 (2022). https://doi.org/10.1007/s11051-022-05485-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-022-05485-5