Abstract
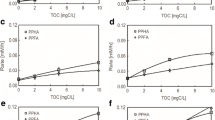
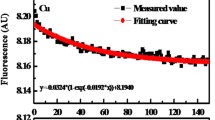
Little is known of potential reactivity and redox properties of reduced dissolved organic matter (DOM), although DOM in anoxic environments, e.g., groundwater, peat soils, or lake sediments, can be expected to differ from DOM of oxidized environments. We therefore investigated the impact of electrochemical and wet chemical [hydrogen (H2)/Pd catalyst] reduction in Sigma-Aldrich humic acid (HA) as a model DOM for high salinity, high ionic strength, or iron-rich systems on its reactivity toward sulfide. Mediated electrochemical measurement showed that the reactivity of HA toward sulfide decreased in the order non-reduced HA > electrochemically reduced (−0.1 V) HA > H2/Pd-reduced HA > electrochemically reduced (−0.4 V) HA. Results indicated that measured initial values of electron-accepting capacities of HA had a strongly positive correlation with the sulfide transformation, except for the H2/Pd treatment of HA. This latter treatment obviously changed HA structures and lead to a different reactivity toward sulfide, limiting a direct comparison to electrochemically reduced organic matter. Our result confirmed that reduced HA was still reactive toward sulfide, although to a lower extent compared with oxidized HA. Compared to electrochemical reduction, H2/Pd pre-treatment of HA alters redox properties and reactivity of organic matter and may therefore lead to results that cannot be transferred to natural systems.



Similar content being viewed by others
References
Adam P, Schneckenburger P, Schaeffer P, Albrecht P (2000) Clues to early diagenetic sulfurization processes from mild chemical cleavage of labile sulfur-rich geomacromolecules. Geochim Cosmochim Acta 64(20):3485–3503
Aeschbacher M, Sander M, Schwarzenbach RP (2010) Novel electrochemical approach to assess the redox properties of humic substances. Environ Sci Technol 44(1):87–93
Aeschbacher M, Vergari D, Schwarzenbach RP, Sander M (2011) Electrochemical analysis of proton and electron transfer equilibria of the reducible moieties in humic acids. Environ Sci Technol 45(19):8385–8394
Aeschbacher M, Graf C, Schwarzenbach RP, Sander M (2012) Antioxidant properties of humic substances. Environ Sci Technol 46(9):4916–4925
Bauer I, Kappler A (2009) Rates and extent of reduction of Fe(III) compounds and O2 by humic substances. Environ Sci Technol 43(13):4902–4908
Bauer M, Heitmann T, Macalady DL, Blodau C (2007) Electron transfer capacities and reaction kinetics of peat dissolved organic matter. Environ Sci Technol 41(1):139–145
Benz M, Schink B, Brune A (1998) Humic acid reduction by Propionibacterium freudenreichii and other fermenting bacteria. Appl Environ Microbiol 64(11):4507–4512
Brown KA (1986) Formation of organic sulphur in anaerobic peat. Soil Biol Biochem 18:131–140
Cline JD (1969) Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr 14(3):454–458
Ferdelman TG, Church TM, Luther GW III (1991) Sulfur enrichment of humic substances in a Delaware salt marsh sediment core. Geochim Cosmochim Acta 55(4):979–988
Heitmann T, Blodau C (2006) Oxidation and incorporation of hydrogen sulfide by dissolved organic matter. Chem Geol 235(1–2):12–20
Heitmann T, Goldhammer T, Beer J, Blodau C (2007) Electron transfer of dissolved organic matter and its potential significance for anaerobic respiration in a northern bog. Glob Change Biol 13(8):1771–1785
Henneke E, Luther GW III, De Lange GJ, Hoefs J (1997) Sulphur speciation in anoxic hypersaline sediments from the eastern Mediterranean Sea. Geochim Cosmochim Acta 61(2):307–321
Hoffmann M, Mikutta C, Kretzschmar R (2012) Bisulfide reaction with natural organic matter enhances arsenite sorption: insights from X-ray absorption spectroscopy. Environ Sci Technol 46(21):11788–11797
Jiang J, Kappler A (2008) Kinetics of microbial and chemical reduction of humic substances: implications for electron shuttling. Environ Sci Technol 42(10):3563–3569
Jørgensen BB (1982) Mineralization of organic matter in the sea bed—the role of sulphate reduction. Nature 296(5858):643–645
Kappler A, Haderlein BS (2003) Natural organic matter as reductant for chlorinated aliphatic pollutants. Environ Sci Technol 37(12):2714–2719
Kappler A, Benz M, Schink B, Brune A (2004) Electron shuttling via humic acids in microbial iron(III) reduction in a freshwater sediment. FEMS Microbiol Ecol 47(1):85–92
Karlsson T, Persson P (2010) Coordination chemistry and hydrolysis of Fe(III) in a peat humic acid studied by X-ray absorption spectroscopy. Geochim Cosmochim Acta 74(1):30–40
Karlsson T, Persson P (2012) Complexes with aquatic organic matter suppress hydrolysis and precipitation of Fe(III). Chem Geol 322–323:19–27
Kertesz MA (2000) Riding the sulfur cycle-metabolism of sulfonates and sulfate esters in gram-negative bacteria. FEMS Microbiol Rev 24(2):135–175
Klüpfel L, Piepenbrock A, Kappler A, Sander M (2014) Humic substances as fully regenerable electron acceptors in recurrently anoxic environments. Nat Geosci 7:195–200
Knorr K-H (2013) DOC-dynamics in a small headwater catchment as driven by redox fluctuations and hydrological flow paths—are DOC exports mediated by iron reduction–oxidation cycles. Biogeosciences 10:891–904
Knorr K-H, Lischeid G, Blodau C (2009) Dynamics of redox processes in a minerotrophic fen exposed to a water table manipulation. Geoderma 153(3–4):379–392
Lovley DR, Coates JD, Blunt-Harris EL, Phillips EJP, Woodward JC (1996) Humic substances as electron acceptors for microbial respiration. Nature 382:445–448
Novák M, Wieder RK (1992) Inorganic and organic sulfur profiles in nine Sphagnum peat bogs in the United States and Czechoslovakia. Water Air Soil Pollut 65(3):353–369
Passier HF, Luther GW III, de Lange GJ (1997) Early diagenesis and sulphur speciation in sediments of the Oman Margin, northwestern Arabian Sea. Deep Sea Res Part II 44(6–7):1361–1380
Peretyazhko T, Sposito G (2006) Reducing capacity of terrestrial humic acids. Geoderma 137(1–2):140–146
Perlinger JA, Kalluri VM, Venkatapathy R, Angst W (2002) Addition of hydrogen sulfide to juglone. Environ Sci Technol 36(12):2663–2669
Pester M, Knorr K-H, Friedrich MW, Wagner M, Loy A (2012) Sulfate-reducing microorganisms in wetlands—fameless actors in carbon cycling and climate change. Front Microbiol 3:72
Piepenbrock A, Schröder C, Kappler A (2014) Electron transfer from humic substances to biogenic and abiogenic Fe(III) oxyhydroxide minerals. Environ Sci Technol 48(3):1656–1664
Ratasuk N, Nanny MA (2007) Characterization and quantification of reversible redox sites in humic substances. Environ Sci Technol 41(22):7844–7850
Scott DT, McKnight DM, Blunt-Harris EL, Blunt-Harris EL, Kolesar SE, Lovley DR (1998) Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ Sci Technol 32(19):2984–2989
Tipping E, Rey-Castro C, Bryan SE, Hamilton-Taylor J (2002) Al(III) and Fe(III) binding by humic substances in freshwaters, and implications for trace metal speciation. Geochim Cosmochim Acta 66(18):3211–3224
Urban NR, Ernst K, Bernasconi S (1999) Addition of sulfur to organic matter during early diagenesis of lake sediments. Geochim Cosmochim Acta 63(6):837–853
Vairavamurthy A, Mopper K (1987) Geochemical formation of organosulphur compounds (thiols) by addition of H2S to sedimentary organic matter. Nature 329(6140):623–625
Van Dongen BE, Schouten S, Baas M, Geenevasen JAJ, Sinninghe Damstéa JS (2003) An experimental study of the low-temperature sulfurization of carbohydrates. Org Geochem 34(8):1129–1144
Wolf M, Kappler A, Jiang J, Meckenstock RU (2009) Effects of humic substances and quinones at low concentrations on ferrihydrite reduction by Geobacter metallireducens. Environ Sci Technol 43(15):5679–5685
Yu Z, Peiffer S, Goettlicher J, Knorr K-H (2015) Electron transfer budgets and kinetics of abiotic oxidation and incorporation of aqueous hydrogen sulfide by dissolved organic matter. Environ Sci Technol 49(9):5441–5449
Yücel M, Konovalov SK, Moore TS, Janzen CP, Luther GW III (2010) Sulfur speciation in the upper Black Sea sediments. Chem Geol 269(3–4):364–375
Zak D, Gelbrecht J (2007) The mobilisation of phosphorus, organic carbon and ammonium in the initial stage of fen rewetting (a case study from NE Germany). Biogeochemistry 85(2):141–151
Acknowledgments
We gratefully acknowledge student assistants Daniela Braun and Sarah Hofmann for laboratory work support, and also thank Zhengrong Xue, Jutta Eckert, and Silke Hammer for technical support. The study was funded by the German Research Foundation, research group ‘electron transfer processes in anoxic aquifers.’ (FOR 580, KN 929/2-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, ZG., Orsetti, S., Haderlein, S.B. et al. Electron Transfer Between Sulfide and Humic Acid: Electrochemical Evaluation of the Reactivity of Sigma-Aldrich Humic Acid Toward Sulfide. Aquat Geochem 22, 117–130 (2016). https://doi.org/10.1007/s10498-015-9280-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10498-015-9280-0