Abstract
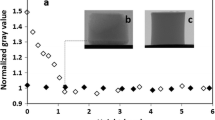
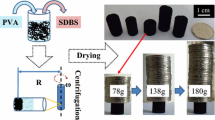
We fabricated two types of single wall carbon nanotube (SWCNT) gels prepared by freeze-drying (cryogels) and air-drying (xerogels) from the dispersed SWCNT hydrogels. The comparative pore structure analysis using N2 adsorption at 77 K and H2O adsorption at 298 K was applied to these SWCNT gels. The N2 adsorption isotherms of both SWCNT gels have a steep initial uptake, low-pressure adsorption hysteresis and adsorption hysteresis above P/P0 = 0.9, indicating the presence of pore mouth structures in addition to micropores and mesopores. The mesoporosity of the SWCNT cryogels was about twice higher than that of the SWCNT xerogels, while their microporosities were similar. The H2O adsorption isotherms of both SWCNT gels differ from each other along the whole P/P0 range. The H2O adsorption isotherm of the SWCNT xerogels shows an explicit adsorption below P/P0 = 0.1 and marked low-pressure adsorption hysteresis; the pore mouth volume of the SWCNT xerogels from the initial uptake of H2O was nearly three times larger than that of the SWCNT cryogels. The H2O adsorption isotherms of our SWCNTs gels give similar micropore volumes, being by ≈ 40% larger than that of N2 adsorption isotherms. The excess micropore volume evaluated by H2O adsorption comes from the pore mouth structures in which N2 cannot access. This work demonstrates that H2O adsorption and N2 adsorption analysis are crucial for elucidation of the pore mouth structure.







Similar content being viewed by others
References
Srinivas, G., Krungleviciute, V., Guo, Z.-X., Yildirim, T.: Exceptional CO2 capture in a hierarchically porous carbon with simultaneous high surface area and pore volume. Energy Environ. Sci. 7, 335–342 (2014). https://doi.org/10.1039/C3EE42918K
Wang, S., Tristan, F., Minami, D., Fujimori, T., Cruz-Silva, R., Terrones, M., Takeuchi, K., Teshima, K., Rodríguez-Reinoso, F., Endo, M., Kaneko, K.: Activation routes for high surface area graphene monoliths from graphene oxide colloids. Carbon 76, 220–231 (2014). https://doi.org/10.1016/j.carbon.2014.04.071
Barroso-Bogeat, A., Alexandre-Franco, M., Fernández-González, C., Macías-García, A., Gómez-Serrano, V.: Electrical conductivity of activated carbon–metal oxide nanocomposites under compression: a comparison study. Phys. Chem. Chem. Phys. 16, 25161–25175 (2014). https://doi.org/10.1039/C4CP03952A
Futamura, R., Iiyama, T., Takasaki, Y., Gogotsi, Y., Biggs, M.J., Salanne, M., Ségalini, J., Simon, P., Kaneko, K.: Partial breaking of the Coulombic ordering of ionic liquids confined in carbon nanopores. Nat. Mater. 16, 1225–1232 (2017). https://doi.org/10.1038/nmat4974
Salanne, M., Rotenberg, B., Naoi, K., Kaneko, K., Taberna, P.-L., Grey, C.P., Dunn, B., Simon, P.: Efficient storage mechanisms for building better supercapacitors. Nat. Energy 1, 16070 (2016). https://doi.org/10.1038/nenergy.2016.70
Chen, J.P., Wu, S.: Acid/Base-treated activated carbons: characterization of functional groups and metal adsorptive properties. Langmuir 20, 2233–2242 (2004). https://doi.org/10.1021/la0348463
Ajayan, P.M., Ebbesen, T.W., Ichihashi, T., Iijima, S., Tanigaki, K., Hiura, H.: Opening carbon nanotubes with oxygen and implications for filling. Nature 362, 522–525 (1993). https://doi.org/10.1038/362522a0
Kuznetsova, A., Mawhinney, D.B., Naumenko, V., Yates, J.T., Liu, J., Smalley, R.E.: Enhancement of adsorption inside of single-walled nanotubes: opening the entry ports. Chem. Phys. Lett. 321, 292–296 (2000). https://doi.org/10.1016/S0009-2614(00)00341-9
Tsang, S.C., Harris, P.J.F., Green, M.L.H.: Thinning and opening of carbon nanotubes by oxidation using carbon dioxide. Nature 362, 520–522 (1993). https://doi.org/10.1038/362520a0
Arai, M., Kanamaru, M., Matsumura, T., Hattori, Y., Utsumi, S., Ohba, T., Tanaka, H., Yang, C.M., Kanoh, H., Okino, F., Touhara, H., Kaneko, K.: Pore characterization of assembly-structure controlled single wall carbon nanotube. Adsorption 13, 509–514 (2007). https://doi.org/10.1007/s10450-007-9055-z
Collins, P.G., Keith, B., Ishigami, M., Zettl, A.: Extreme oxygen sensitivity of electronic properties of carbon nanotubes. Science 287, 1801–1804 (2000). https://doi.org/10.1126/science.287.5459.1801
Maniwa, Y., Matsuda, K., Kyakuno, H., Ogasawara, S., Hibi, T., Kadowaki, H., Suzuki, S., Achiba, Y., Kataura, H.: Water-filled single-wall carbon nanotubes as molecular nanovalves. Nat. Mater. 6, 135–141 (2007). https://doi.org/10.1038/nmat1823
Romero, H.E., Bolton, K., Rosén, A., Eklund, P.C.: Atom collision-induced resistivity of carbon nanotubes. Science 307, 89–93 (2005). https://doi.org/10.1126/science.1102004
Zhao, J., Buldum, A., Han, J., Lu, J.P.: Gas molecule adsorption in carbon nanotubes and nanotube bundles. Nanotechnology 13, 195–200 (2002). https://doi.org/10.1088/0957-4484/13/2/312
Zhong, J., Chiou, J., Dong, C., Glans, P.-A., Pong, W.-F., Chang, C., Wu, Z., Guo, J.: Interfacial interaction of gas molecules and single-walled carbon nanotubes. Appl. Phys. Lett. 100, 201605 (2012). https://doi.org/10.1063/1.4718421
Meyyappan, M.: Carbon nanotube-based chemical sensors. Small 12, 2118–2129 (2016). https://doi.org/10.1002/smll.201502555
Zhang, T., Mubeen, S., Myung, N.V., Deshusses, M.A.: Recent progress in carbon nanotube-based gas sensors. Nanotechnology 19, 332001 (2008). https://doi.org/10.1088/0957-4484/19/33/332001
Honda, H., Yang, C.-M., Kanoh, H., Tanaka, H., Ohba, T., Hattori, Y., Utsumi, S., Kaneko, K.: Choking effect of single-wall carbon nanotubes on solvent adsorption in radial breathing mode. J. Phys. Chem. C 111, 3220–3223 (2007). https://doi.org/10.1021/jp067856w
Battie, Y., Ducloux, O., Thobois, P., Dorval, N., Lauret, J.S., Attal-Trétout, B., Loiseau, A.: Gas sensors based on thick films of semi-conducting single walled carbon nanotubes. Carbon 49, 3544–3552 (2011). https://doi.org/10.1016/j.carbon.2011.04.054
Khoerunnisa, F., Morelos-Gomez, A., Tanaka, H., Fujimori, T., Minami, D., Kukobat, R., Hayashi, T., Hong, S.Y., Choi, Y.C., Miyahara, M., Terrones, M., Endo, M., Kaneko, K.: Metal–semiconductor transition like behavior of naphthalene-doped single wall carbon nanotube bundles. Faraday Discuss. 173, 145–156 (2014). https://doi.org/10.1039/C4FD00119B
Kong, J., Frankin, N.R., Zhou, C., Chapline, M.G., Peng, S., Cho, K., Dai, H.: Nanotube molecular wires as chemical sensors. Science 287, 622–625 (2000). https://doi.org/10.1126/science.287.5453.622
Snow, E.S., Perkins, F.K., Houser, E.J., Badescu, S.C., Reinecke, T.L.: Chemical detection with a single-walled carbon nanotube capacitor. Science 307, 1942–1945 (2005). https://doi.org/10.1126/science.1109128
Star, A., Joshi, V., Skarupo, S., Thomas, D., Gabriel, J.-C.P.: Gas sensor array based on metal-decorated carbon nanotubes. J. Phys. Chem. B 110, 21014–21020 (2006). https://doi.org/10.1021/jp064371z
Dumlich, H., Gegg, M., Hennrich, F., Reich, S.: Bundle and chirality influences on properties of carbon nanotubes studied with van der Waals density functional theory. Phys. Status Solidi 248, 2589–2592 (2011). https://doi.org/10.1002/pssb.201100212
Khoerunnisa, F., Fujimori, T., Itoh, T., Urita, K., Hayashi, T., Kanoh, H., Ohba, T., Hong, S.Y., Choi, Y.C., Santosa, S.J., Endo, M., Kaneko, K.: Enhanced CO2 adsorptivity of partially charged single walled carbon nanotubes by methylene blue encapsulation. J. Phys. Chem. C 116, 11216–11222 (2012). https://doi.org/10.1021/jp303630m
Hu, G., Cheng, M., Ma, D., Bao, X.: Synthesis of carbon nanotube bundles with mesoporous structure by a self-assembly solvothermal route. Chem. Mater. 15, 1470–1473 (2003). https://doi.org/10.1021/cm0209362
Kim, D.Y., Yang, C.-M., Noguchi, H., Yamamoto, M., Ohba, T., Kanoh, H., Kaneko, K.: Enhancement of H2 and CH4 adsorptivities of single wall carbon nanotubes produced by mixed acid treatment. Carbon 46, 611–617 (2008). https://doi.org/10.1016/j.carbon.2008.01.008
Yang, C.-M., Kim, D.Y., Lee, Y.H.: Formation of densely packed single-walled carbon nanotube assembly. Chem. Mater. 17, 6422–6429 (2005). https://doi.org/10.1021/cm0507157
Kamijyou, Y., Stevic, D., Kukobat, R., Urita, K., Chotimah, N., Hattori, Y., Futamura, R., Vallejos-Burgos, F., Moriguchi, I., Utsumi, S., Sakai, T., Kaneko, K.: Mesoscopic cage-like structured single-wall carbon nanotube cryogels. Microporous Mesoporous Mater. 293, 109814 (2020). https://doi.org/10.1016/j.micromeso.2019.109814
Thommes, M., Kaneko, K., Neimark, A.V., Olivier, J.P., Rodriguez-Reinoso, F., Rouquerol, J., Sing, K.S.W.: Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 87, 1051–1069 (2015). https://doi.org/10.1515/pac-2014-1117
Kobori, R., Ohba, T., Suzuki, T., Iiyama, T., Ozeki, S., Inagaki, M., Nakamura, A., Kawai, M., Kanoh, H., Kaneko, K.: Fine pore mouth structure of molecular sieve carbon with GCMC-assisted supercritical gas adsorption analysis. Adsorption 15, 114–122 (2009). https://doi.org/10.1007/s10450-009-9162-0
Ohba, T., Kanoh, H., Kaneko, K.: Affinity transformation from hydrophilicity to hydrophobicity of water molecules on the basis of adsorption of water in graphitic nanopores. J. Am. Chem. Soc. 126, 1560–1562 (2004). https://doi.org/10.1021/ja038842w
Ohba, T., Kanoh, H., Kaneko, K.: Structures and stability of water nanoclusters in hydrophobic nanospaces. Nano Lett. 5, 227–230 (2005). https://doi.org/10.1021/nl048327b
Thommes, M., Morell, J., Cychosz, K.A., Fröba, M.: Combining nitrogen, argon, and water adsorption for advanced characterization of ordered mesoporous carbons (CMKs) and periodic mesoporous organosilicas (PMOs). Langmuir 29, 14893–14902 (2013). https://doi.org/10.1021/la402832b
Silvestre-Albero, J., Silvestre-Albero, A., Rodríguez-Reinoso, F., Thommes, M.: Physical characterization of activated carbons with narrow microporosity by nitrogen (77.4K), carbon dioxide (273K) and argon (87.3K) adsorption in combination with immersion calorimetry. Carbon 50, 3128–3133 (2012). https://doi.org/10.1016/j.carbon.2011.09.005
Kukobat, R., Minami, D., Hayashi, T., Hattori, Y., Matsuda, T., Sunaga, M., Bharti, B., Asakura, K., Kaneko, K.: Sol–gel chemistry mediated Zn/Al-based complex dispersant for SWCNT in water without foam formation. Carbon 94, 518–523 (2015). https://doi.org/10.1016/j.carbon.2015.07.025
Kaneko, K., Ishii, C.: Superhigh surface area determination of microporous solids. Colloids Surf. 67, 203–212 (1992). https://doi.org/10.1016/0166-6622(92)80299-H
Kaneko, K., Ishii, C., Ruike, M., Kuwabara, H.: Origin of superhigh surface area and microcrystalline graphitic structures of activated carbons. Carbon 30, 1075–1088 (1992). https://doi.org/10.1016/0008-6223(92)90139-N
Marsh, H.: Adsorption methods to study microporosity in coals and carbons—a critique. Carbon 25, 49–58 (1987). https://doi.org/10.1016/0008-6223(87)90039-X
Ohba, T., Kaneko, K.: GCMC study on relationship between DR plot and micropore width distribution of carbon. Langmuir 17, 3666–3670 (2001). https://doi.org/10.1021/la001463l
Neimark, A.V., Lin, Y., Ravikovitch, P.I., Thommes, M.: Quenched solid density functional theory and pore size analysis of micro-mesoporous carbons. Carbon 47, 1617–1628 (2009). https://doi.org/10.1016/j.carbon.2009.01.050
Jorio, A., Pimenta, M.A., Filho, A.G.S., Saito, R., Dresselhaus, G., Dresselhaus, M.S.: Characterizing carbon nanotube samples with resonance Raman scattering. New J. Phys. 5, 139–139 (2003). https://doi.org/10.1088/1367-2630/5/1/139
Silvestre-Albero, A.M., Juárez-Galán, J.M., Silvestre-Albero, J., Rodríguez-Reinoso, F.: Low-pressure hysteresis in adsorption: an artifact? J. Phys. Chem. C 116, 16652–16655 (2012). https://doi.org/10.1021/jp305358y
Stevic, D., Furuse, A., Vallejos-Burgos, F., Kukobat, R., Kaneko, K.: Cu-phthalocyanine-mediated nanowindow production on single-wall carbon nanohorn. Mol. Phys. (2020). https://doi.org/10.1080/00268976.2020.1815883
Setoyama, N., Suzuki, T., Kaneko, K.: Simulation study on the relationship between a high resolution αs-plot and the pore size distribution for activated carbon. Carbon 36, 1459–1467 (1998). https://doi.org/10.1016/S0008-6223(98)00138-9
Wang, S., Vallejos-Burgos, F., Furuse, A., Yoshikawa, Y., Tanaka, H., Kaneko, K.: The subtracting pore effect method for an accurate and reliable surface area determination of porous carbons. Carbon 175, 77–86 (2021). https://doi.org/10.1016/j.carbon.2020.12.075
Kaneko, K., Hanzawa, Y., Iiyama, T., Kanda, T., Suzuki, T.: Cluster-mediated water adsorption on carbon nanopores. Adsorption 5, 7–13 (1999). https://doi.org/10.1023/A:1026471819039
Miyawaki, J., Kanda, T., Kaneko, K.: Hysteresis-associated pressure-shift-induced water adsorption in carbon micropores. Langmuir 17, 664–669 (2001). https://doi.org/10.1021/la000175m
Vallejos-Burgos, F., Coudert, F.-X., Kaneko, K.: Air separation with graphene mediated by nanowindow-rim concerted motion. Nat. Commun. 9, 1812 (2018). https://doi.org/10.1038/s41467-018-04224-6
Nakamura, M., Ohba, T., Branton, P., Kanoh, H., Kaneko, K.: Equilibration-time and pore-width dependent hysteresis of water adsorption isotherm on hydrophobic microporous carbons. Carbon 48, 305–308 (2010). https://doi.org/10.1016/j.carbon.2009.09.008
Rouquerol, J., Rouquerol, F., Sing, K.S.W., Llewellyn, P., Maurin, G.: Adsorption by powders and porous solids, principles, methhodology and applications. Academic Press, Cambridge (2014)
Ohba, T.: Size-dependent water structures in carbon nanotubes. Angew. Chemie Int. Ed. 53, 8032–8036 (2014). https://doi.org/10.1002/anie.201403839
Futamura, R., Iiyama, T., Hamasaki, A., Ozeki, S.: Negative thermal expansion of water in hydrophobic nanospaces. Phys. Chem. Chem. Phys. 14, 981–986 (2012). https://doi.org/10.1039/C1CP22954K
Acknowledgements
This work was supported by JST-OPERA program (JPMJOP 1722). KK was greatly stimulated by adsorption science studies by Dr. Shivaji Sircar when KK started his adsorption research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kamijyou, Y., Kukobat, R., Sakai, T. et al. Nanopore structure analysis of single wall carbon nanotube xerogels and cryogels. Adsorption 27, 673–681 (2021). https://doi.org/10.1007/s10450-021-00315-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-021-00315-x