Spatial variability of Middle East respiratory syndrome coronavirus survival rates and mortality hazard in Saudi Arabia, 2012–2019
- Published
- Accepted
- Received
- Academic Editor
- Richard Maude
- Subject Areas
- Epidemiology, Infectious Diseases, Public Health, Respiratory Medicine
- Keywords
- MERS-COV, Survival rates, Mortality, Risk factors, Epidemiology, GIS, Saudi Arabia
- Copyright
- © 2020 Al-Ahmadi et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2020. Spatial variability of Middle East respiratory syndrome coronavirus survival rates and mortality hazard in Saudi Arabia, 2012–2019. PeerJ 8:e9783 https://doi.org/10.7717/peerj.9783
Abstract
About 83% of laboratory-confirmed Middle East respiratory syndrome coronavirus (MERS-CoV) cases have emerged from Saudi Arabia, which has the highest overall mortality rate worldwide. This retrospective study assesses the impact of spatial/patient characteristics for 14-and 45-day MERS-CoV mortality using 2012–2019 data reported across Saudi regions and provinces. The Kaplan–Meier estimator was employed to estimate MERS-CoV survival rates, Cox proportional-hazards (CPH) models were applied to estimate hazard ratios (HRs) for 14-and 45-day mortality predictors, and univariate local spatial autocorrelation and multivariate spatial clustering analyses were used to assess the spatial correlation. The 14-day, 45-day and overall mortality rates (with estimated survival rates) were 25.52% (70.20%), 32.35% (57.70%) and 37.30% (56.50%), respectively, with no significant rate variations between Saudi regions and provinces. Nationally, the CPH multivariate model identified that being elderly (age ≥ 61), being a non-healthcare worker (non-HCW), and having an underlying comorbidity were significantly related to 14-day mortality (HR = 2.10, 10.12 and 4.11, respectively; p < 0.0001). The 45-day mortality model identified similar risk factors but with an additional factor: patients aged 41–60 (HR = 1.44; p < 0.0001). Risk factors similar to those in the national model were observed in the Central, East and West regions and Riyadh, Makkah, Eastern, Madinah and Qassim provinces but with varying HRs. Spatial clusters of MERS-CoV mortality in the provinces were identified based on the risk factors (r2 = 0.85–0.97): Riyadh (Cluster 1), Eastern, Makkah and Qassim (Cluster 2), and other provinces in the north and south of the country (Cluster 3). The estimated HRs for the 14-and 45-day mortality varied spatially by province. For 45-day mortality, the highest HRs were found in Makkah (age ≥ 61 and non-HCWs), Riyadh (comorbidity) and Madinah (age 41–60). Coming from Makkah (HR = 1.30 and 1.27) or Qassim province (HR = 1.77 and 1.70) was independently related to higher 14-and 45-day mortality, respectively. MERS-CoV patient survival could be improved by implementing appropriate interventions for the elderly, those with comorbidities and non-HCW patients.
Introduction
Middle East respiratory syndrome coronavirus (MERS-CoV), first reported in Saudi Arabia in 2012 (Zaki et al., 2012), is an infectious viral respiratory disease with high mortality and morbidity in humans. From 2012 through 30 April 2019, 27 countries reported a total of 2,428 MERS-CoV laboratory-confirmed cases to WHO, of which 83% were reported by Saudi Arabia (WHO, 2019). The largest cluster outside the Arabian Peninsula was reported from South Korea in 2015 (Chen et al., 2017). MERS-CoV mortality rates vary by region: 34.5–45% worldwide (Ahmed, 2018; Chen et al., 2017; Penttinen et al., 2013; WHO, 2019), 10–65% in Saudi Arabia (Ahmed, 2017b; Al Ghamdi et al., 2016; Alqahtani et al., 2018; Assiri et al., 2016, 2013a; Coleman et al., 2017; El Bushra et al., 2017; Feikin et al., 2015; Lessler et al., 2016; Mohd, Al-Tawfiq & Memish, 2016; Noorwali et al., 2015; Oboho et al., 2015; Korea Centers for Disease Control & Prevention, 2015; Saad et al., 2014; Sherbini et al., 2017) and 19.9–63.6% in South Korea (Kim et al., 2019; Mizumoto et al., 2015; Nam et al., 2017; Korea Centers for Disease Control & Prevention, 2015). The highest MERS-CoV mortality rates reported for Saudi Arabia (Assiri et al., 2013a) and South Korea (Nam et al., 2017) were linked to nosocomial outbreaks. Some studies have estimated mortality rates between 26.6% and 59.4% (Breban, Riou & Fontanet, 2013; Liu et al., 2015; Sha et al., 2017) using data from multiple regions, including the Middle East, South Korea and China. Due to its high mortality rate and a lack of effective antiviral treatment, preventive vaccine, or prophylactic therapy, MERS-CoV remains a public health risk (Mohd et al., 2016).
Middle East respiratory syndrome coronavirus is an emergent zoonotic virus and camel–human transmission is linked to direct or indirect exposure to infected dromedary camels (Camelus dromedarius) and their products (Azhar et al., 2014). Although dromedary camels have been recognized as intermediate hosts of MERS-CoV, human–human virus transmission has been identified as nosocomial transmission in healthcare facilities among patients and healthcare workers (HCWs) (Hui et al., 2018). It has also been identified within Saudi households (Drosten et al., 2014), raising concerns about an epidemic risk. The risk assessment of MERS-CoV mortality is crucial to improving clinical patient outcomes by implementing measures and strategies that improve patients’ chances of survival and provide effective early treatment.
Primary factors related to MERS-CoV mortality are old age (Ahmed, 2017b, 2018; Alqahtani et al., 2018; Chen et al., 2017; Majumder et al., 2015) and underlying comorbidities (Ahmed, 2017b, 2018; Alqahtani et al., 2018; Assiri et al., 2013a; Majumder et al., 2015; Nam et al., 2017; Sha et al., 2017; Sherbini et al., 2017), including diabetes (Alqahtani et al., 2018; Assiri et al., 2013b; Sherbini et al., 2017), cardiac diseases (Alqahtani et al., 2018; Assiri et al., 2013b), chronic renal disease (Alqahtani et al., 2018; Assiri et al., 2013b), kidney failure (Sherbini et al., 2017), and respiratory disease (Alqahtani et al., 2018; Nam et al., 2017). The severity of the illness for patients receiving vasopressor therapy (Almekhlafi et al., 2016), requiring invasive mechanical ventilation (Almekhlafi et al., 2016), and with higher chest radiographic scores (Das et al., 2015) is also associated with mortality risk resulting from MERS-CoV (Adegboye, Gayawan & Hanna, 2017; Ahmed, 2017a). Other symptoms associated with MERS-CoV deaths include demonstrated gastrointestinal symptoms (Sherbini et al., 2017), leucocytosis (Nam et al., 2017), respiratory symptoms, abnormal renal function (Nam et al., 2017), and lower blood pressures (Sherbini et al., 2017). Some researchers have found that being an HCW is associated with higher mortality rates (Alsahafi & Cheng, 2016), but, conversely, other studies have linked being a non-healthcare worker (non-HCW) with higher mortality rates (Adegboye, Gayawan & Hanna, 2017; Ahmed, 2017b, 2018; Rivers, Majumder & Lofgren, 2016; Sha et al., 2017). Another study found that exposure to camels and nosocomial and unknown infection sources demonstrated a higher mortality risk compared to exposure to household infection (Ahmed, 2018). However, another study reported no significant association between MERS-CoV survival and exposure to camels (Sha et al., 2017). Mortality risk factors also include a longer period from onset to laboratory confirmation (Sha et al., 2017), a longer time from onset to hospitalization (Sha et al., 2017), a longer duration of symptoms (Sherbini et al., 2017), and a shorter incubation period (Cowling et al., 2015). In the Middle East, extended times from the onset of clinical signs to laboratory confirmation (4–8 days) have been reported compared to South Korea (4–5 days) (Sha et al., 2017). Saudi Arabia has reported a shorter incubation period (5.2 days) compared to South Korea (6 days) (Assiri et al., 2013a; Sha et al., 2017). The risk of mortality for MERS-CoV patients in Saudi Arabia was 4.1 times higher after 14 days and 5 times higher after 45 days compared to South Korea and other countries (Ahmed, 2018).
Previous studies have provided useful information on the mortality risk for MERS-CoV patients. These studies exemplify particular patient clusters, such as critical care units (Almekhlafi et al., 2016), patients in a single hospital (Saad et al., 2014), area-specific groups within Saudi Arabia (Assiri et al., 2013a; Noorwali et al., 2015; Oboho et al., 2015; Sherbini et al., 2017), and outbreak-specific groups in light of the South Korean outbreak (Cowling et al., 2015; Kim et al., 2019; Majumder et al., 2015; Mizumoto et al., 2015). Viral and host factors, such as the virus’s replication and mutation rates, and local medical expertise also affect MERS-CoV mortality rates (Feikin et al., 2015). Thus, mortality risk factors vary according to regional characteristics, such as the strategies and measures implemented by healthcare systems, hospitalization practices, and accessibility to healthcare centers (Ahmed, 2018; Feikin et al., 2015). MERS-CoV mortality risk factors may vary geographically (Banik et al., 2016). Significant spatiotemporal variations and clusters of MERS-CoV incidence have been identified in Saudi Arabia (Al-Ahmadi, Alahmadi & Al-Zahrani, 2019). A spatial modeling approach (Adegboye, Gayawan & Hanna, 2017) used a geoadditive regression model to examine the risk of mortality from MERS-CoV in the Arabian Peninsula. However, this study did not consider risk factors by province. Using publicly available national MERS-CoV data for Saudi Arabia, (Banik et al., 2016) assessed risk factors related to severity and mortality using a relatively large dataset; however, this study lacked multivariate risk modeling. One study (Ahmed, 2017b) applied multivariate analysis to estimate the survival rate of MERS-CoV mortality in Saudi Arabia; however, it did not examine risk factors by region or province.
To the authors’ knowledge, the risk factors for MERS-CoV mortality at the regional level in Saudi Arabia have not been examined, including variations between mortality risk factors in different geographical areas (both regions and provinces). It is not evident whether the mortality risk factors for MERS-CoV vary by region in Saudi Arabia. It is essential to perform multi-region and multi-province recognition to compare MERS-CoV patients in relation to their clinical outcomes; this could highlight variances in survival rates by region. This might also support the healthcare system, public health control measures, and clinical practices to advance the clinical outcomes in healthcare facilities by prioritizing high-risk regions and patient groups. This study aimed to estimate the survival rates and hazard ratios (HRs) for MERS-CoV mortality for 14-and 45-day post-symptom onset periods and assess spatial variations by both Saudi Arabian regional and patient characteristics between 2012 and 2019.
Materials and Methods
MERS-CoV data
All publicly available laboratory-confirmed cases of MERS-CoV reported between 13 June 2012 and 30 April 2019 were compiled by the Saudi Ministry of Health via their Command and Control Center (CCC) website (Ministry of Health of Saudi Arabia, 2019) and WHO (WHO, 2019). With the emergence of MERS-CoV in Saudi Arabia, the CCC was launched in 2014 to take immediate proactive action against future public health challenges by way of continuous coordination and monitoring. For the current study, the MERS-CoV data was thoroughly reviewed, and a range of checks was performed to ensure the data were consistent, complete, and fit for the study. A data dictionary was developed for the variables for each case of MERS-CoV, including gender, age, underlying comorbidity, exposure to camels, healthcare status, outcome, date of symptom onset, date of death, city of residence, province and region. The variables were categorized according to gender (male/female), age (<20, 20–40, 41–60, or ≥61), comorbidity (yes/no), exposure to camels (yes/no), HCW (yes/no), regions (five categories as shown below), provinces (13 categories as shown below) and outcome (alive/dead). The data contained no reported comorbidity variable for 58 patients and no exposure to camel variable for 939 patients (713 were unknown or missing a source of infection and 226 were primary or secondary cases without camel exposure status). The MERS-CoV data had been reported by Saudi city, so the data were aggregated for the following 13 provinces, comprising five regions: Riyadh and Qassim (Central region); Tabuk, Jouf and Hail (North region); Assir, Baha, Jazan and Najran (South region); the Eastern province (East region); and Makkah and Madinah (West region). A spatial database of MERS-CoV incidence and mortality in Saudi Arabia was created using geographical information systems (GIS).
Statistical analysis
A comparative epidemiological analysis using a Chi-square test was undertaken to determine the significant differences between the demographic and clinical characteristics of the survival and mortality rates of MERS-CoV cases at the regional level in Saudi Arabia. The variables used in the analysis included age and gender and whether the patients were HCWs, demonstrated underlying comorbidities, and had been exposed to camels. The statistical analyses were performed using MedCalc software, Version 19.0.6 and a P-value < 0.05 was considered statistically significant.
Survival analysis and hazard modeling
Survival analysis aims to model and analyze time-to-event data, that is, a dataset of the time that an event occurred as its endpoint. In the current study, two MERS-CoV parameters, the time of symptom onset and the date of death, were evaluated for 14 days and 45 days after the appearance of symptoms. The incubation period of MERS-CoV, the elapsed time between contracting the virus and displaying symptoms, ranged from 2 to 14 days (Ahmed, 2018). The 14-day period is medically important because it indicates the point at which most MERS-CoV patients typically experience symptoms. The 45-day period was chosen to exhibit the progression of MERS-CoV for an advanced period. The 14-day and 45-day MERS-CoV survival rates were estimated using the Kaplan–Meier estimator at both the national and regional levels in Saudi Arabia. The log-rank test was used to compare survival curves according to the demographic and clinical factors affecting MERS-CoV patients. However, the log-rank test delivers a statistical but not clinical appraisal of the effect of the factors (Bradburn et al., 2003). Thus, the Cox proportional-hazards (CPH) model was applied to assess the survival of MERS-CoV patients regarding several concurrent factors and estimate the extent of the influence of each constituent factor. The CPH model is broadly applicable and is the most widely used multivariate approach for survival analysis (Bradburn et al., 2003). The CPH model examines the relationships of covariates to the time-to-event as expressed by the hazard function. The CPH model is interpreted using HRs, which are expressed as the ratio of the estimated hazard function under two different values of a covariate (George, Seals & Aban, 2014). An HR above one indicates that the event (in this case, death resulting from MERS-CoV) has a higher likelihood of occurring and that the covariate is positively related to the event probability and negatively related to the duration of survival. An HR less than one indicates that an event has a lower probability of occurring, and an HR of one indicates that the covariate does not influence the hazard of an event.
The probability of the endpoint (i.e., time to death) is termed the hazard, and the hazard is modeled as a group of predictor variables. The predictors (risk factor) are dichotomous and coded as 1 if present (died) and 0 if absent (alive). The value of the hazard ratio can then be inferred as the instant relative risk of an event at any time for a patient with the risk factor present compared to a patient with the risk factor absent, given both patients are equivalent for all other predictors. A variable is removed from the model if its related significance level is larger than the P-value (0.05). Survival time is the variable of the time to reach the event of interest (the outcome, in our case, death). The endpoint variable represents the occurrence of the event (i.e., death) and comprises patient cases that have reached the endpoint (coded as 1) or cases that have not reached the endpoint (coded as 0) as they are removed from the study (in our case, at 14 and 45 days). The predictor variables are the variables we expect to predict survival time (gender, age, underlying comorbidity, exposure to camels, healthcare status, region and province) (MedCalc, 2020). When building our model, we applied a filter for particular regions and provinces.
Using MedCalc software, the CPH model was applied to estimate the HRs and 95% confidence intervals (CIs) for the predictors of the two primary endpoints: 14-and 45-day mortality. Both univariate and multivariate analyses were applied using the CPH model. The former describes the MERS-CoV survival rate regarding the factor under examination but disregards effects from any other factors; whereas, the latter simultaneously considers several covariates that may affect patients’ prognoses to enable better clinical assessment. The variables used in the CPH model included a patient’s age and gender and whether the patient was an HCW, had a pre-existing illness, and had been exposed to camels.
The MERS-CoV mortality risk factors for the 14-and 45-day post-symptom periods were estimated for national, regional and provincial levels in Saudi Arabia, and the spatial variations were assessed according to region, province and mortality hazard factors using both univariate and multivariate CPH model analyses. For each post-symptom period, ten CPH models were built: national, each region and province separately, incorporating regions as a covariate, and incorporating provinces as a covariate for both the univariate and multivariate model analyses. The regional and provincial factors characterized the geographical representation, following a previously used approach (Ayele, Zewotir & Mwambi, 2017) for a survival analysis of mortality using the CPH model.
Spatial analysis
The spatial variations of the risk factors associated with MERS-CoV mortality were assessed using univariate local spatial autocorrelation and multivariate spatial clustering analyses. Spatial autocorrelation measures the association within values across locations (Getis, 2008). It is the dependance of a particular variable’s values on the values of the same variable recorded at nearby locations (Fortin & Dale, 2005). We applied the Getis–Ord Gi* statistic, a local spatial autocorrelation that detects the locations of statistically significant hot spots (the clustering of high values) and cold spots (the clustering of low values) within the setting of nearby features (Ord & Getis, 1995) using Esri ArcGIS 10.7.1. Given a set of provinces and associated risk factor values, the Gi* statistic assesses whether the expressed pattern is high-value cluster or low-value cluster (Mitchell, 2005). To consider the spatial interaction between the outcome and covariates examined in this study, we applied the Bivariate Local Moran Index via GeoDa package. In principle, this index identifies the association between the value for one variable at a location and the average of the neighboring values for another variable, that is, its spatial lag (Anselin, Syabri & Kho, 2006).
Clustering is an unsupervised machine learning approach that involves determining natural groupings within data (Duque, Ramos & Suriñach, 2007), and the K-means algorithm is one of the most popular methods for clustering data (Spielman & Thill, 2008). We used multivariate spatial clustering analysis to identify clusters of Saudi provinces according to patients’ characteristics variables for MERS-CoV mortality using the K-means algorithm via Esri ArcGIS. The resulting maps created distinct clusters of variables (demographic, occupational and clinical patient characteristics for MERS-CoV mortality); whereby, the provinces that were part of a cluster were as similar as possible and the provinces between clusters were as dissimilar as possible. Each variable’s contribution, evaluated using r2 values, was used to differentiate the provinces, which revealed how much of the variation in the original data was kept after the clustering process: the higher the r2 value for a specific variable, the better that variable was for discerning the provinces (Pimpler, 2017).
Results
Characteristics of MERS-CoV patients
The analysis of the relationship of MERS-CoV mortality to patients’ characteristics is shown in Table 1. A total of 2,037 laboratory-confirmed cases of human infection with MERS-CoV were reported in Saudi Arabia between 13 June 2012 and 30 April 2019. Among these, 760 cases died (37.30%), with an overall crude mortality rate of 2.37 per 100,000 population. A higher number of deaths resulted from MERS-CoV cases involving males (n = 558; 73.42%) compared to females (n = 202; 26.58%); a statistically significant difference (p = 0.0007). The overall median age of the deceased cases was 62 years (interquartile range = 50–73), ranging from 2 to 114 years, while the mean age of the cases was 60.36, with a standard deviation of ±17.99.
| Factors | Vital status | Total (n = 2,037) (%) |
P | |
|---|---|---|---|---|
| Alive (n = 1,277, 62.70%) | Dead (n = 760, 37.30%) | |||
| Gender | 0.0007 | |||
| Male | 846 (66.24%) | 558 (73.42%) | 1404 (68.92%) | |
| Female | 431 (33.76%) | 202 (26.58%) | 633 (31.08%) | |
| Age (years) | < 0.0001 | |||
| <20 | 39 (3.05%) | 10 (1.31%) | 49 (2.40%) | |
| 20–40 | 439 (34.37%) | 82 (10.78%) | 521 (25.57%) | |
| 41–60 | 529 (41.42%) | 234 (30.78%) | 763 (37.45%) | |
| ≥61 | 270 (21.16%) | 434 (57.13%) | 704 (34.58%) | |
| Healthcare worker | < 0.0001 | |||
| Yes | 345 (27.01%) | 31 (4.07%) | 376 (18.45%) | |
| No | 932 (72.99%) | 729 (95.93%) | 1,661 (81.55%) | |
| Comorbidity | < 0.0001 | |||
| Yes | 780 (62.90%) | 696 (94.18%) | 1,476 (74.58%) | |
| No | 460 (37.10%) | 43 (5.82%) | 503 (25.42%) | |
| Exposure to camels | 0.3651 | |||
| Yes | 157 (22.81%) | 84 (20.48%) | 241 (21.94%) | |
| No | 531 (77.19%) | 326 (79.52%) | 857 (78.05%) | |
| Region | 0.7706 | |||
| Central | 641 (50.20%) | 366 (48.16%) | 1,007 (49.44%) | |
| East | 150 (11.75%) | 98 (12.98%) | 248 (12.17%) | |
| West | 358 (28.03%) | 220 (28.95%) | 578 (28.38%) | |
| North | 51 (3.99%) | 35 (4.16%) | 86 (4.22%) | |
| South | 77 (6.03%) | 41 (5.39%) | 118 (5.79%) | |
The greatest number of deaths occurred among elderly patients (age ≥ 61), representing 57.13% (n = 434) of the total deceased cases, which was statistically significant (p < 0.0001). MERS-CoV mortality was higher among non-HCWs (n = 729; 95.93%) than among HCWs (n = 31; 4.07%), and this difference was statistically significant (p < 0.0001). MERS-CoV patients with underlying comorbidities had a higher risk for mortality compared to those without (n = 696 (94.18%) vs. n = 43 (5.82%); p < 0.0001). A lower mortality rate was found among those exposed to camels compared to those not exposed, although this difference was not statistically significant (p = 0.3651). The Central region reported 366 deaths resulting from MERS-CoV, accounting for 48.16% of the total number of MERS-CoV deaths, followed by the West region (n = 220, with 28.95% mortality) and the East region (n = 98, with 12.89% mortality), but the variation across regions was not statistically significant (p = 0.7706).
Spatial pattern of MERS-CoV survival
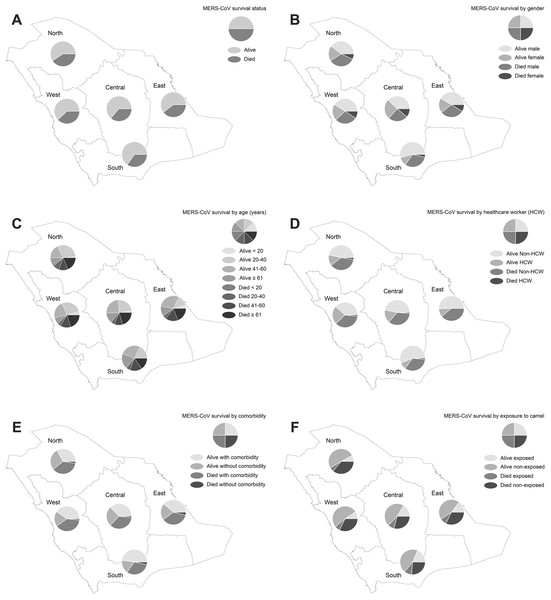
Figure 1 displays the results of the spatial pattern of MERS-CoV survival in Saudi regions. Approximately 59.30–65.25% of MERS-CoV cases across the Saudi regions were not fatal, but 34.75–40.70% of the cases resulted in death, as shown in Fig. 1A. The distribution of demographic, occupational, and clinical characteristics of MERS-CoV survival is shown in Figs. 1B–1F. MERS-CoV mortality was more frequent in males compared to females across all regions (ranging from 1.42% to 12.47% for males and 0.20–5.50% for females) out of the total number of MERS-CoV cases, as shown in Fig. 1B. MERS-CoV mortality was significantly higher among elderly patients aged ≥61 for all regions (ranging from 0.79% in the North region to 9.97% in the Central region), as shown in Fig. 1C. MERS-CoV mortality was higher among non-HCWs (1.57–17.33%) than HCWs (0.10–0.64%) across all regions (see Fig. 1D). A greater number of patients with underlying comorbidities (1.52–17.53%) died compared to those without (0.10–0.96%) across all regions (see Fig. 1E). A higher mortality rate was found among MERS-CoV patients who had not been exposed to camels (2.46–23.32%) than those who had been exposed (0.36–6.56%) for all regions, as shown in Fig. 1F.
Figure 1: Spatial distribution of MERS-CoV cases in Saudi regions between 2012 and 2019 by (A) vital status, (B) gender, (C) age group, (D) healthcare worker, (E) comorbidity and (F) exposure to camels.
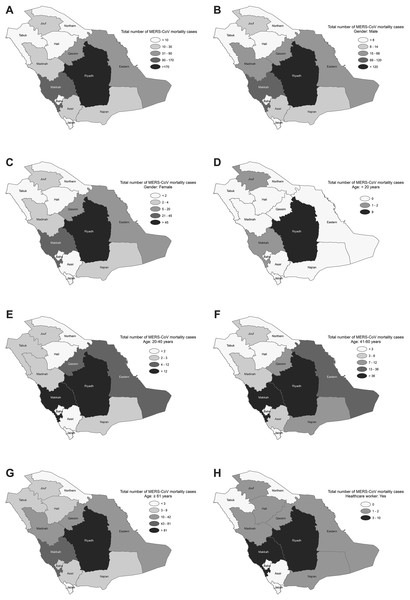
The spatial distribution of the total MERS-CoV mortality cases varied across the provinces, with most cases reported from Riyadh and Makkah followed by the Eastern and Qassim provinces (see Fig. 2A). The spatial variation patterns of the risk factors, based on the demographic, occupational, and clinical characteristics of the MERS-CoV patients, also indicated spatially varied effects and were mainly elevated in Riyadh, Makkah, Eastern and Qassim provinces but were degraded in the northern and southern provinces (see Figs. 2B–2H and Appendix Figs. 1A–1E). The univariate local spatial autocorrelation Getis–Ord Gi* analysis suggested that there were statistically significant hot spots in Riyadh province for all risk factors except for age <20, for which there were no hot spots across the entire country. Madinah province was a hot spot for the age 20–40 factor, and Riyadh and Madinah provinces were hot spots for the exposed to camel factor. Statistically significant cold spots were observed in Jouf province for all risk factors and detected for age <20, age 20–40, and patients without comorbidities in Jazan province. The bivariate spatial analysis with Bivariate Local Moran Index showed no statistically significant spatial autocorrelation between the examined covariates and MERS-CoV mortality attributable to <20 years and non-comorbidity in all provinces. However, the identification of such an autocorrelation for all the other covariates was observed in Riyadh province. The multivariate spatial clustering analysis applied here optimized within-cluster similarities and between-cluster differences for Saudi provinces based on the risk factors with subcategories (12 variables) for demographic, occupational and clinical patient characteristics of MERS-CoV mortality. The results identified three spatial clusters of provinces, with the r2 for variables ranging between 0.85 and 0.97. Clusters were found in Riyadh province (Cluster 1); Eastern, Makkah and Qassim provinces (Cluster 2); and the remaining provinces in the north and south of the country (Cluster 3), see Appendix Fig. 1F.
Figure 2: Spatial distribution of MERS-CoV mortality in Saudi provinces between 2012 and 2019 by (A) overall, (B) male, (C) female, (D) age < 20, (E) age 20–40, (F) age 41–60, (G) age ≥ 61 and (H) healthcare worker.
Survival analysis of MERS-CoV
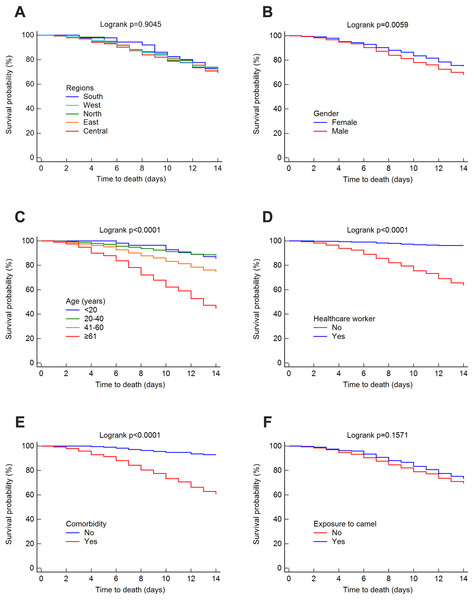
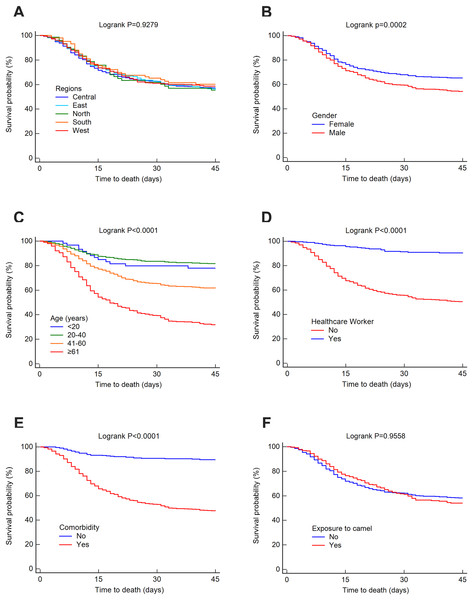
The mortality rate was 25.52% at 14 days, 32.35% at 45 days and 37.30% overall. The survival rates for MERS-CoV at 14 and 45 days were estimated using the Kaplan–Meier method. These estimated survival rates were 70.20% (at 14 days), 57.70% (at 45 days) and 56.50% (overall). Figs. 3 and 4 present the Kaplan–Meier curves for regional, demographic, occupational and clinical factors for 14-and 45-day mortalities, respectively. The Central region (69.30%) had a lower 14-day survival rate than the East (70.10%), north (70.20%), west (71.40%) and south (72.70%) regions, as shown in Fig. 3A. For 45 days, the East region (56%) had a lower survival rate than the Central (57.30%), west (58.70%), south (60.30%) and north (70.20%) regions (see Fig. 4A). However, the differences in survival rates across all Saudi regions were not statistically significant (p = 0.9045 and 0.9279 for 14-day and 45-day survival, respectively). Lower survival probabilities were found among males compared to females (see Figs. 3B and 4B) (14 days = 67.80% vs. 75%, respectively, and 45 days = 54.10% vs. 65.20%, respectively). Similarly, lower survival probabilities were found among the elderly compared to younger people, see Figs. 3C and 4C. Survival rates were also lower among non-HCWs than HCWs (see Figs. 3D and 4D): 14 days = 63.60% vs. 96.20%, respectively, and 45 days = 50.30% vs. 91.30%, respectively. Survival rates were lower among patients with comorbidities than among those without (see Figs. 3E and 4E): 14 days = 60.60% vs. 93%, respectively, and 45 days = 47.50% vs. 89.60%, respectively, and for patients who were not exposed to camels compared to those who were exposed (see Figs. 3F and 4F): 14 days = 69.40% vs. 73.10%, respectively, and 45 days = 54% vs. 58.20%, respectively.
Figure 3: Survival rates for MERS-CoV for 14-day mortality at the Saudi national level between 2012 and 2019 by (A) region, (B) gender, (C) age group, (D) healthcare worker, (E) comorbidity, and (F) exposure to camels.
Figure 4: Survival rates for MERS-CoV for 45-day mortality at the Saudi national level between 2012 and 2019 by (A) region, (B) gender, (C) age group, (D) healthcare worker, (E) comorbidity and (F) exposure to camels.
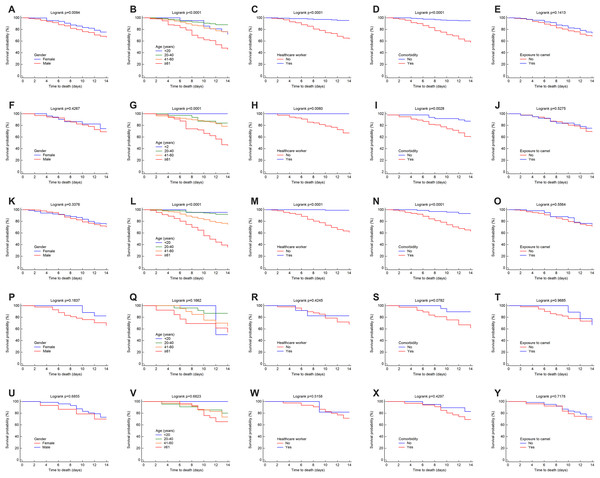
Overall, the 14-day Kaplan–Meier curves for the factors of gender, age, HCW, presence of comorbidities, and having been exposed to camels for each region showed relatively similar patterns to those at the national level but with variations in the survival probabilities (see Figs. 5A–5Y).
Figure 5: Survival rates for MERS-CoV for 14-day mortality at the Saudi regional level between 2012 and 2019 by gender, age group, healthcare worker, comorbidity and exposure to camels for the (A–E) central, (F–J) east, (K-O) west, (P–T) north and (U–Y) south regions.
Risk assessment of MERS-CoV mortality
The MERS-CoV mortality risk factors for 14-and 45-day periods were estimated for the national level, each of the regions, and each of the provinces using both CPH modeled univariate and multivariate analyses. We also built models that incorporated regions and provinces as covariates.
The univariate risk factors for 14-and 45-day mortality are shown in Appendix Table 1. At the national level, during the 14-day mortality period, MERS-CoV survival was impaired according to the following HRs with varying statistical significance: males (1.34), age 41–60 (2.20), age ≥ 61 (5.83), non-HCWs (17.61) and underlying comorbidities (7.21). During the 45-day mortality period, similar risk factors were found compared to the 14-day period, but with relatively higher HRs for males and patients aged 41–60 and lower HRs for the other risk factors. At the regional level, during the 14-day period and in all regions except the North and South regions, patients aged ≥61 (HR = 2.56–11.39) and those with underlying comorbidities (HR = 4.30–10.95) were associated with MERS-CoV mortality. In the Central (HR = 9.47) and West regions (HR = 55.77), non-HCWs were linked to higher 14-day mortality rates, whereas being male (HR = 1.46) was only significant in the Central region. Overall, the univariate analysis for 45-day mortality identified similar risk factors as for the 14-day mortality, with relatively lower HRs. However, non-HCWs (HR = 5.93) in the East region, males (HR = 1.40) in the West region, and patients aged ≥61 (HR = 2.43) in the North region were at higher risk for the 45-day period but not for the 14-day period. Overall, the univariate analysis at the provincial level identified similar risk factors as for the regional level (Appendix Table 2). When regions and provinces were used as covariates in the CPH univariate models, only coming from Qassim province was significantly associated with MERS-CoV mortality at 14 days (HR = 2.12) (Appendix Tables 3 and 4).
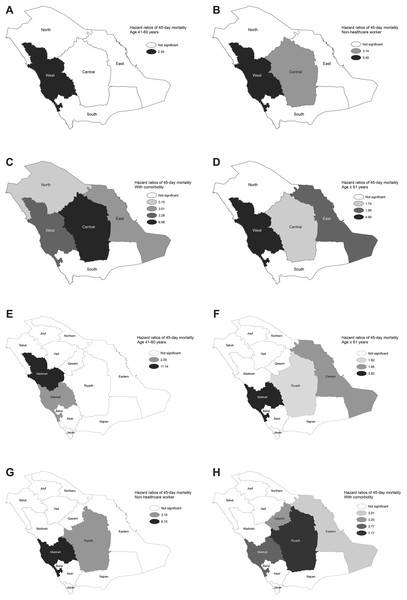
The results of the multivariate analysis for the national level and each of the regions are shown in Table 2. The national model revealed that people aged ≥61 (HR = 2.10), non-HCWs (HR = 10.12), and those with underlying comorbidities (HR = 4.11) were independently related to higher 14-day mortality (p < 0.0001). The model for 45-day mortality identified similar risk factors to those of the 14-day model, but with an additional risk factor: patients aged 41–60 (HR = 1.44). The regions showed variations in the mortality hazard models. Those aged ≥61 (HR = 1.86–5.39) and patients with underlying comorbidities (HR = 2.69–6.69) were independently related to higher 14-day mortality in all regions except for the North and South regions. Non-HCWs were significantly associated with mortality in the West (HR = 29.34) and Central (HR = 4.55) regions, while those aged 41–60 were at risk only in the West region (HR = 2.44). During the 45-day mortality period, by region, similar risk factors were identified as for those in the 14-day models, but with relatively lower HRs. The spatial distribution of the HRs for the MERS-CoV risk factors for 14-and 45-day mortality based on multivariate analysis for each of the regions and provinces are shown in Fig. 6 and Appendix Fig. 2, respectively. The estimated HRs for 14- and 45-day mortality varied spatially by province. For the former, the highest HRs were found in Makkah (HR = 2.1 for age 41–60 and HR = 27.03 for non-HCWs), in Riyadh (HR = 8.24 for having comorbidity) and in Madinah (HR = 8.59 for age ≥ 61), while for the latter, the highest HRs were found in Makkah (HR = 3.52 for age ≥ 61 and HR = 6.15 for non-HCWs), in Riyadh (HR = 7.17 for having comorbidity) and in Madinah (HR = 11.14 for age 41–60) (p < 0.0001). Overall, the multivariate analysis at the provincial level identified similar risk factors as for the regional levels, as shown in Table 3, with the assistance to discover the specific risk factor for each province. The results of the multivariate CPH modeling that incorporated regions and provinces as covariates in addition to the other risk factors are shown in Tables 4 and 5. Regionality was not significantly associated with 14-and 45-day mortality, while for the provinces, coming from Makkah (HRs = 1.30 and 1.27) or Qassim (HRs = 1.77 and 1.70) provinces were independently related to higher 14-and 45-day mortality, respectively (p < 0.0001).
| Regions/Factors | 14-day mortality | 45-day mortality | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI for HR | P | HR | 95% CI for HR | P | |
| National | <0.0001 | <0.0001 | ||||
| Age 41–60 years | NV | NV | NV | 1.44 | [1.12–1.86] | 0.0040 |
| Age ≥ 61 years | 2.10 | [1.72–2.56] | <0.0001 | 2.47 | [1.93–3.17] | <0.0001 |
| Non-healthcare worker | 10.12 | [4.75–21.52] | <0.0001 | 4.45 | [2.90–6.81] | <0.0001 |
| With comorbidity | 4.11 | [2.75–6.14] | <0.0001 | 3.55 | [2.56–4.93] | <0.0001 |
| Exposed to camel | 0.73 | [0.56–0.93] | 0.0134 | 0.82 | [0.67–0.99] | 0.0450 |
| Central | <0.0001 | <0.0001 | ||||
| Age ≥ 61 years | 1.86 | [1.41–2.46] | <0.0001 | 1.74 | [1.38–2.19] | <0.0001 |
| Non-healthcare worker | 4.55 | [1.99–10.38] | 0.0003 | 3.14 | [1.73–5.68] | 0.0002 |
| With comorbidity | 6.69 | [3.49–12.80] | <0.0001 | 6.08 | [3.57–10.36] | <0.0001 |
| East | <0.0001 | <0.0001 | ||||
| Age ≥ 61 years | 2.67 | [1.46–4.87] | 0.0013 | 1.95 | [1.24–3.05] | 0.0034 |
| With comorbidity | 2.69 | [1.00–7.23] | 0.0485 | 3.01 | [1.40–6.44] | 0.0044 |
| West | <0.0001 | <0.0001 | ||||
| Age 41–60 years | 2.44 | [1.28–4.62] | 0.0062 | 2.34 | [1.45–3.79] | 0.0005 |
| Age ≥ 61 years | 5.39 | [2.91–9.96] | <0.0001 | 4.80 | [2.97–7.74] | <0.0001 |
| Non-healthcare worker | 29.34 | [4.05–212.40] | 0.0008 | 5.40 | [2.72–10.74] | <0.0001 |
| With comorbidity | 3.76 | [1.62–8.71] | 0.0019 | 3.29 | [1.77–6.13] | 0.0002 |
| Exposed to camel | 0.52 | [0.29–0.92] | 0.0254 | 0.67 | [0.45–0.98] | 0.0436 |
| North | 0.0009 | |||||
| Without comorbidity | NV | NV | NV | 0.15 | [0.03–0.65] | 0.0109 |
Notes:
NV, no variables were retained in this model.
No variables were retained in this model for the South region.
Figure 6: Spatial distribution of hazard ratios for MERS-CoV mortality risk factors based on multivariate analysis for each region between 2012 and 2019 for 45-day mortality by (A) age 41–60, (B) age ≥ 61, (C) non-healthcare worker and (D) comorbidity. For 45-day mortality by (E) age 41–60, (F) age ≥ 61, (G) non-healthcare worker and (H) comorbidity.
| Provinces/Factors | 14-day mortality | 45-day mortality | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI for HR | P | HR | 95% CI for HR | P | |
| Riyadh | <0.0001 | <0.0001 | ||||
| Age ≥ 61 years | 2.07 | [1.53–2.81] | <0.0001 | 1.92 | [1.49–2.46] | <0.0001 |
| Non-healthcare worker | 3.80 | [1.65–8.71] | 0.0016 | 3.15 | [1.65–6.04] | 0.0005 |
| With comorbidity | 8.24 | [3.81–17.80] | <0.0001 | 7.17 | [3.87–13.30] | <0.0001 |
| Makkah | <0.0001 | <0.0001 | ||||
| Age 41–60 years | 2.11 | [1.08–4.11] | 0.0283 | 2.06 | [1.24–3.41] | 0.0048 |
| Age ≥ 61 years | 3.92 | [2.06–7.47] | <0.0001 | 3.52 | [2.12–5.82] | <0.0001 |
| Non-healthcare worker | 27.03 | [3.71–196.76] | 0.0011 | 6.15 | [2.81–13.42] | <0.0001 |
| With comorbidity | 4.09 | [1.76–9.50] | 0.0010 | 3.77 | [1.96–7.25] | 0.0001 |
| Madinah | <0.0001 | <0.0001 | ||||
| Age ≥ 61 years | 8.59 | [3.05–24.17] | <0.0001 | 11.14 | [4.58–27.13] | <0.0001 |
| Exposed to camel | NV | NV | NV | 0.40 | [0.17–0.93] | 0.0346 |
| Eastern | <0.0001 | <0.0001 | ||||
| Age ≥ 61 years | 2.67 | [1.46–4.87] | 0.0013 | 1.95 | [1.24–3.05] | 0.0034 |
| With comorbidity | 2.69 | [1.00–7.23] | 0.0485 | 3.01 | [1.40–6.44] | 0.0044 |
| Qassim | P = 0.0061 | P = 0.0096 | ||||
| With comorbidity | 3.99 | [1.21–13.13] | 0.0224 | 3.20 | [1.14–8.98] | 0.0266 |
Notes:
NV, no variables were retained in this model.
No variables were retained in this model for the other provinces.
| Factors | 14-day mortality | 45-day mortality | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI for HR | P | HR | 95% CI for HR | P | |
| <0.0001 | <0.0001 | |||||
| Age 41–60 years | NV | NV | NV | 1.44 | [1.12–1.86] | 0.0040 |
| Age ≥ 61 years | 2.10 | [1.72–2.56] | <0.0001 | 2.47 | [1.93–3.17] | <0.0001 |
| Non-healthcare worker | 10.12 | [4.75–21.52] | <0.0001 | 4.45 | [2.90–6.81] | <0.0001 |
| With comorbidity | 4.11 | [2.75–6.14] | <0.0001 | 3.55 | [2.56–4.93] | <0.0001 |
| Exposed to camel | 0.73 | [0.56–0.93] | 0.0134 | 0.82 | [0.67–0.99] | 0.0450 |
Notes:
NV, no variables were retained in this model.
No variables were retained in this model for the region factor.
| Factors | 14-day mortality | 45-day mortality | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI for HR | P | HR | 95% CI for HR | P | |
| <0.0001 | <0.0001 | |||||
| Age 41–60 years | 1.39 | [1.01–1.91] | 0.0400 | 1.47 | [1.14–1.89] | 0.0028 |
| Age ≥ 61 years | 2.61 | [1.93–3.55] | <0.0001 | 2.49 | [1.95–3.20] | <0.0001 |
| Non-healthcare worker | 9.98 | [4.68–21.28] | <0.0001 | 4.60 | [3.00–7.06] | <0.0001 |
| With comorbidity | 3.78 | [2.51–5.68] | <0.0001 | 3.52 | [2.54–4.88] | <0.0001 |
| Exposed to camel | 0.72 | [0.56–0.93] | 0.0121 | 0.82 | [0.67–0.99] | 0.0043 |
| Makkah province | 1.30 | [1.22–2.55] | 0.0226 | 1.27 | [1.06–1.52] | 0.0081 |
| Qassim province | 1.77 | [0.56–0.93] | 0.0023 | 1.70 | [1.24–2.33] | 0.0009 |
Discussion
Saudi Arabia’s MERS-CoV mortality rate is the highest in the world (Ahmed, 2018; Park et al., 2018; WHO, 2019) and has been examined by several Saudi Arabian studies (Assiri et al., 2013a; Mohd, Al-Tawfiq & Memish, 2016). Nevertheless, little is known about the spatial variation of the survival rate and risk factors associated with MERS-CoV mortality across Saudi regions. This study first assessed whether there was a significant difference in MERS-CoV mortality related to demographic, occupational and clinical characteristics. The survival rates and HRs for MERS-CoV mortality were then estimated for 14-and 45-day post-symptom mortality periods, and the variations were assessed in relation to regions and risk factors.
The overall mortality rate in this study for all MERS-CoV patients in Saudi Arabia between 2012 and 2019 was estimated to be 37.30%, similar to the previously reported 37% (Al Ghamdi et al., 2016) and comparable with previous studies estimating 36.5% or 38% (Coleman et al., 2017; Oboho et al., 2015). This rate is higher than the 10–34% rates (Mohd et al., 2016; Sherbini et al., 2017) and lower than the 40–65% rates reported by other studies (Assiri et al., 2016; El Bushra et al., 2017; Feikin et al., 2015; Noorwali et al., 2015). These disparities might partially relate to sample size because most studies considered hospital-based cases reported by a specific hospital or referral hospital in a particular region. These differences can also be explained by the source of the MERS-CoV infection; some studies considered nosocomial outbreaks (Assiri et al., 2013b), while others considered household (Drosten et al., 2014) or overall cases (Alqahtani et al., 2018; Sherbini et al., 2017). At the Saudi regional level, variations in mortality rates ranged from 34.75% to 40.70%, although the differences were not significant. In South Korea, where the largest number of MERS-CoV outbreaks outside the Arabian Peninsula occurred (Kim et al., 2019), one study that considered all MERS-CoV patients in South Korea described a mortality rate of 20.4% (Korea Centers for Disease Control & Prevention, 2015), lower than that of Saudi Arabia.
Although no vaccine or specific antiviral agents are currently available that can effectively prevent or treat MERS-CoV, MERS-CoV mortality rates in Saudi Arabia have recently declined from 33.81% in 2016 to 6.40% in 2019. This might be attributable to the extensive control measures implemented by the Saudi healthcare system in response to the MERS-CoV outbreak.
In this study, the estimated national survival rates for 14 days, 45 days, and overall were 70.20%, 57.70% and 56.50%, respectively, while survival rates worldwide were 83.67%, 65.9% and 69.04%, respectively (Ahmed, 2018), higher than those of Saudi Arabia. There are marginal variations in survival rates across Saudi regions for the 14-day and 45-day periods (70.10–72.70% and 57.30–70.20%, respectively), but these were not statistically significant. Similar survival rates across Saudi regions, particularly for the 14-day period, could be ascribed to the simultaneous reporting of MERS-CoV symptoms and receiving a MERS-CoV diagnosis.
According to MERS-CoV patient characteristics, at the national level and among all regions, mortality rates were higher among males, elderly patients aged ≥61, patients with underlying comorbidities, non-HCWs, and those not exposed to camels. Therefore, these groups increase the odds of mortality resulting from MERS-CoV in Saudi Arabia and should be closely monitored.
The male–female ratio was estimated to be 2.76:1 for fatal MERS-CoV cases in Saudi Arabia. The predominance of MERS-CoV cases among males in the current study is consistent with previous studies from Saudi Arabia and South Korea (Chen et al., 2017; Sha et al., 2017). Our findings suggest that there is also a statistically significant difference between male and female mortality at the national level. This conforms with some previous research (Banik et al., 2016; Sherbini et al., 2017) but conflicts with other research (Ahmed, 2017b; Alqahtani et al., 2018) that reported that males did not have a significantly higher risk of dying from MERS-CoV. One study even suggested that survival rates were higher among males (Al Ghamdi et al., 2016), although this study used a very small data sample. In the current study, MERS-CoV mortality cases were found to be more frequent in males than females at the national level and for all regions, and there was a statistically significant difference in the 14-and 45-day survival rates between males and females at the national level and in the Central region but not in other regions. The univariate analysis showed that the hazard of 14-and 45-day mortality in MERS-CoV male patients was higher than that for females only at the national level and in the Central and West regions. This can be attributed to the high prevalence of MERS-CoV among males who interact with camel-related activities in these regions (Gossner et al., 2016). In contrast, the multivariate analysis could not find a significant gender effect at either the national or regional level. Therefore, it is essential to examine the impact of gender on 14-and 45-day survival and HRs across Saudi regions.
The analyses of the age distribution of MERS-CoV mortality showed that patients aged ≥61 most often died and had the lowest survival rates at the national and regional levels. According to the univariate and multivariate analyses, being elderly was associated with a higher risk of 14-and 45-day mortality in Saudi Arabia in general and in the Central, West and East regions in particular compared to those aged <61. These findings are consistent with previous studies (Adegboye, Gayawan & Hanna, 2017; Ahmed, 2017b, 2018; Alqahtani et al., 2018; Alsahafi & Cheng, 2016; Noorwali et al., 2015; Sherbini et al., 2017). The higher mortality rate of MERS-CoV in aged patients might be explained by age-associated impairment of the immune system and the presence of senescence-associated immune vulnerability among the elderly. This could amplify a person’s susceptibility to and the progression of severe infections, increasing the likelihood of death (Aly et al., 2017). It may also be explained by the social and cultural customs that result in suboptimal immune reactions following an exposure risk in which the elderly are involved, for example, camel-related activities and interactions (Omrani et al., 2013). A large proportion of aged patients also have comorbidities that may also impair immune functioning, thus affecting the likelihood of death from MERS-CoV (Webb et al., 2004).
In the current study and several others (Ahmed, 2017b, 2018; Alqahtani et al., 2018; Assiri et al., 2013b; Majumder et al., 2015; Nam et al., 2017; Sha et al., 2017; Sherbini et al., 2017), mortality from MERS-CoV was found to be significantly associated with underlying comorbidities. This is shown in the lower survival probability and higher HRs at 14 and 45 days among patients with comorbidities than in those without both at the national level and in most Saudi regions. The higher risk of death among patients with underlying diseases can partly be explained by higher viral loads in the respiratory tract and a longer duration of viral shedding compared to patients without such conditions (Sha et al., 2017). However, as the MERS-CoV data in this study were collected from publicly available sources, this study lacks information on the underlying causes of death and details of pre-existing conditions. Such information would be valuable for recognizing specific pre-existing conditions related to higher mortality rates for implementing measures that most effectively reduce fatalities.
It was also found that non-HCWs were linked to higher mortality rates, which is consistent with other studies (Adegboye, Gayawan & Hanna, 2017; Ahmed, 2018; Rivers, Majumder & Lofgren, 2016; Sha et al., 2017). Lower survival rates and higher HRs for mortality for 14-and 45-day post-symptom periods were also found among non-HCWs at the national level and in the Central, East and West regions. The lower survival rates and higher HRs for mortality among non-HCWs could be explained by a lack of public awareness about the clinical signs and symptoms of the disease. The early identification of MERS-CoV symptoms could result in early diagnosis and timely treatment, leading to better rates of survival (Ahmed, 2017a; Zumla, Hui & Perlman, 2015). Higher MERS-CoV survival rates among HCWs could be attributed to levels of knowledge, early diagnosis and timely treatment, access to healthcare services, and adherence to control and prevention guidelines.
Dromedary camels are likely a natural host for MERS-CoV. Transmission to humans can occur through sporadic zoonotic infection after direct or indirect exposure to infected dromedary camels or their products (Azhar et al., 2014). However, this study’s findings suggest that MERS-CoV patients with a history of exposure to camels were not significantly associated with lower survival at the national level or in the Saudi regions. One possible explanation for this unexpected finding is non-reported data for the status of exposure to camels in the early stage of infection. However, similarly, another study (Sha et al., 2017) found no effect of exposure to camels on survival, either in the nosocomial or sporadic MERS-CoV cases in Saudi Arabia or South Korea.
The current study has revealed spatial variations in the risk factors for MERS-CoV mortality among regions and provinces. However, the bivariate spatial analysis discovered statistically significant spatial autocorrelation of high MERS-CoV mortality with high values for all covariates excluding <20 years and non-comorbidity in Riyadh province. Although the spatial pattern of each risk factor varies among the provinces, when they are combined based on the demographic, occupational and clinical characteristics of MERS-CoV patients, three spatial clusters were identified: Riyadh (Cluster 1), Makkah, Eastern and Qassim (Cluster 2), and other provinces (Cluster 3). This implies that the impact of the patients’ characteristics is comparable for particular provinces. The spatial pattern of the HRs for the significant risk factors was also elevated in certain provinces, such as Riyadh, Makkah, Eastern and Qassim. A possible explanation for this could be differing provincial characteristics, such as healthcare systems, quality of hospitalization practices, and accessibility to healthcare centers (Ahmed, 2018; Feikin et al., 2015). It can also be attributed to the spatial distribution at the provincial level for other social, environmental, and health factors. The Riyadh (25%, 23% and 25%), Makkah (26%, 14% and 31%) and Eastern (15%, 18% and 13%) provinces accounted for the highest proportions in Saudi Arabia in terms of the total human population (General Authority for Statistics of Saudi Arabia, 2010) and the numbers of dromedary camels (General Authority for Statistics of Saudi Arabia, 2015) and patients aged ≥15 who suffer from chronic disease (General Authority for Statistics of Saudi Arabia, 2017), respectively. Although the spatial levels of the analyses in this study included national, regional (n = 5) and provincial (n = 13) levels, further research should be conducted at lower spatial levels (e.g., cities n = 250 and governorates n = 140) and take into account spatial correlation effects.
Conclusions
The MERS-CoV mortality rate in Saudi Arabia is the highest worldwide. However, no significant variations in MERS-CoV survival rates were found between Saudi regions and provinces. Being elderly, being a patient with underlying medical conditions, or being a non-HCW were identified as risk factors for MERS-CoV mortality at the national level and in most Saudi regions and some provinces. The spatial pattern of the HRs for the significant risk factors was elevated in certain regions and provinces, and the reasons for this should be studied further. Detailed investigations at lower spatial levels should be undertaken to incorporate spatial correlation and dependency using an extended spatial hazard model for survival analysis, as reported by (Li, Hanson & Zhang, 2015) and (Zhou, Hanson & Zhang, 2020). The survival of MERS-CoV patients could be improved by raising public awareness about the importance of early-symptom notification and providing appropriate interventions, especially in aged MERS-CoV patients and those with underlying conditions. Further investigation of specific pre-existing conditions linked to MERS-CoV mortality is also needed.
Supplemental Information
Appendex Figure 1.
Spatial distribution of MERS-CoV mortality in Saudi provinces between 2012 and 2019 by (A) non-healthcare worker, (B) with comorbidity, (C) without comorbidity, (D) exposed to camels, (E) not exposed to camels, (F) multivariate spatial clusters for MERS-CoV mortality, (G) MERS-CoV 14-day mortality, and (H) MERS-CoV 45-day mortality.
Appendix Figure 2.
Spatial distribution of hazard ratios for MERS-CoV mortality risk factors based on multivariate analysis for each region between 2012 and 2019 for 14-day mortality by (A) age 41–60, (B) age ≥ 61, (C) non-healthcare worker, and (D) comorbidity. For 45-day mortality by (E) age 41–60, (F) age ≥ 61, (G) non-healthcare worker, and (H) comorbidity.
Appendix Table 1.
Risk factors and estimated hazard ratios for 14- and 45-day mortality for MERS-CoV patients at the national level and each of the regions between 2012 and 2019 from univariate Cox proportional-hazards (CPH) modeling.
Appendix Table 2.
Risk factors and estimated hazard ratios of 14- and 45-day mortality for MERS-CoV patients for each of the provinces between 2012 and 2019 from univariate Cox proportional-hazards (CPH) modeling.
Appendix Table 3.
Estimated hazard ratios for regions as covariates for 14- and 45-day mortality for MERS-CoV patients between 2012 and 2019 from univariate Cox proportional-hazards (CPH) modeling.
Appendix Table 4.
Estimated hazard ratios for provinces as covariates for 14- and 45-day mortality for MERS-CoV patients between 2012 and 2019 from univariate Cox proportional-hazards (CPH) modeling