Slc26a1 is not essential for spermatogenesis and male fertility in mice
- Published
- Accepted
- Received
- Academic Editor
- Sonia Oliveira
- Subject Areas
- Biochemistry, Genomics, Molecular Biology, Histology
- Keywords
- Slc26a1, Spermatogenesis, Fertility, Gene compensation, Testis
- Copyright
- © 2023 Meng et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2023. Slc26a1 is not essential for spermatogenesis and male fertility in mice. PeerJ 11:e16558 https://doi.org/10.7717/peerj.16558
Abstract
Thousands of genes are expressed in the testis of mice. However, the details about their roles during spermatogenesis have not been well-clarified for most genes. The purpose of this study was to examine the effect of Slc26a1 deficiency on mouse spermatogenesis and male fertility. Slc26a1-knockout (KO) mice were generated using CRISPR/Cas9 technology on C57BL/6J background. We found no obvious differences between Slc26a1-KO and Slc26a1-WT mice in fertility tests, testicular weight, sperm concentrations, or morphology. Histological analysis found that Slc26a1-KO mouse testes had normal germ cell types and mature sperm. These findings indicated that Slc26a1 was dispensable for male fertility in mice. Our results may save time and resources by allowing other researchers to focus on genes that are more meaningful for fertility studies. We also found that mRNAs of two Slc26a family members (Slc26a5 and Slc26a11) were expressed on higher mean levels in Slc26a1-KO total mouse testes, compared to Slc26a1-WT mice. This effect was not found in mouse GC-1 and GC-2 germ cell lines with the Slc26a1 gene transiently knocked down. This result may indicate that a gene compensation phenomenon was present in the testes of Slc26a1-KO mice.
Introduction
The solute carrier 26A (SLC26A) family member is a highly conserved membrane protein that regulates transport of various anions (e.g., chloride (Cl−), bicarbonate (HCO3−), sulfate (SO42−), iodide (I−), formate (HCOO−), and oxalate (C2O42−)) across the plasma membrane of epithelial cells, regulating the composition and pH of body secretions (El Khouri et al., 2018). SLC26A-related proteins are present in a variety of organisms, including bacteria, yeast, algae, plants (SulP/Sultr proteins), and non-mammalian vertebrates (Mount & Romero, 2004). The SLC26A family members are encoded 11 identified genes, but SLC26A10 is a pseudogene in humans (Alper & Sharma, 2013). In humans, SLC26A family members are located in different tissues and have important roles in maintenance of ion balance and pH values (Bernardino et al., 2019). Mutations in these genes lead to unique clinical diseases related to their specific distribution: kidney stones (SLC26A1), maldevelopment (SLC26A2), congenital chloride diarrhea (SLC26A3), Pendred syndrome and goiter (SLC26A4), deafness (SLC26A5), and male infertility (SLC26A3, SLC26A8) (Dawson & Markovich, 2005; El Khouri & Touré, 2014; Everett & Green, 1999).
The effects of the SLC26A family members on male fertility remain poorly described. SLC26A8 (i.e., testicular anion transporter 1, TAT1) was first identified in 2001 and specifically expressed in spermatocytes and spermatids (Lohi et al., 2002; Toure et al., 2001). Generation of Slc26a8 knockout (KO) mice results in male infertility due to decreased sperm motility, but mouse viability is not affected. This result indicates that SLC26A8 is a key factor in sperm differentiation and sperm motility (Touré et al., 2007). In addition to induction of congenital chloride-deficient diarrhea (CLD), the SLC26A3 gene is expressed in the male reproductive tract and sperm cells (Chávez et al., 2012). Previous studies that analyzed male reproductive parameters and functions in Slc26a3-KO mice found that Slc26a3 deletion is associated with severe lesions and abnormal cell structure in the epididymis and defects in sperm number, morphology, and function; these effects jointly impair male fertility (El Khouri et al., 2018). SLC26A4 and SLC26A7 are present in the testis, epididymis, seminiferous duct, and ejaculatory duct (Pierucci-Alves, Akoyev & Schultz, 2015). Taken together, these study findings indicate that it is necessary to further understand the effects of the SLC26A families on male fertility.
SLC26A1/SAT1 cDNA was first cloned from rat liver tissue; it is a Na+-independent transporter (Bissig et al., 1994). Human SLC26A1 shows moderate cross-species conservation, with 78% homology to rat and 77% homology to mouse cDNA (Regeer, Lee & Markovich, 2003). Mice with a Slc26a1 deficiency exhibit urolithiasis, hyperoxaluria, hyperoxalemia, hyposulfatemia, hypersulfaturia, and increased susceptibility to hepatotoxicity (Dawson et al., 2010). SLC26A1 is expressed in the testis, as well as in the kidney and liver (Yin et al., 2017). However, no studies on the role of Slc26a1 in mouse fertility and no human infertility caused by SLC26A1 mutation have been reported. In this study, we examined the role of Slc26a1 in spermatogenesis and male fertility. We generated Slc26a1-deficient mice, investigated the effects of Slc26a1-deficiency on the murine testis and epididymis using histological and immunohistochemical staining, and assessed quality of spermatozoa using a computer-assisted sperm analyzer. We found that male mice with Slc26a1-KO displayed normal fertility parameters. These results suggested that the effect of Slc26a1 deficiency on male mouse fertility is dispensable. We speculate that these results can be explained by genetic redundancy.
Materials and Methods
Generation of Slc26a1−/− mice using CRISPR/Cas9 technology
C57BL/6J healthy mice were purchased from Cyagen Biosciences Inc and raised at the Experimental Animal Center of Nanjing Medical University under conditions of 30–70% humidity and 26 °C. The mice were allowed free access to food and water throughout the experiment. The food contains 20% protein and 4% fat and was provided Xietong Pharmaceutical Bio-engineering (Nanjing, China). All the cages were exactly of the same size and material. Animal house was specific pathogen-free (Murray et al., 2021) and 12 h light-12 h darkness-cycles. At the end of the study, the mice were anesthetized with carbon dioxide.
This study was approved by the Animal Ethics and Welfare Committee of Nanjing Medical University (No. IACUC-2004020) and was conducted in accordance with the Guide for the Care and Use of Laboratory Animals. Slc26a1 KO mice were generated using CRISPR/Cas9 genome editing technology, as previously described (Shen et al., 2014; Wang et al., 2013). Single guide (sg)RNA target sequences for Slc26a1 are as follows: 5′-CATGGTGGTCCACACATGGT-3′. The combination of sgRNA and Cas9mRNA was microinjected into the fertilized eggs of C57BL/6J mice and then immediately transplanted into the oviduct of pseudopregnant females of the same strain. The mutant mice have been registered in the MGI database as Slc26a1<em1Njrml> (MGI:7529731).
Genotype identification
Slc26a1−/− mutation was identified by Sanger sequencing. Polymerase chain reaction (PCR) and Sanger sequencing were performed using the forward primer, 5′-GGCTGGGCTTCGTGTCTACCTA-3′, and the reverse primer, 5′-GCTCTTGGTTGGCACTGACAGA-3′.
Fertility test
Slc26a1 (WT/KO) adult males were respectively mated with WT females at a ratio of 1:2 for 3 months. The mice were raised at 26 °C. Female mice, whether pregnant or not, were replaced once a week. The numbers of litters and born mice per litter were recorded. The genetic background of WT controls was C57BL/6J (from the same batch as KO mice but not littermates).
Cell culture and transfection
Mouse germ cell lines GC-1spg (GC-1) and GC-2(spd)ts (GC-2) were purchased from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in DMEM medium containing 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA) under the conditions of 37 °C and 5% CO2. SLC26A1 and negative control small interfering RNAs (si-NC) were synthesized by GenePharma (Suzhou, China). GC-1 and GC-2 cells were transfected with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). At 6 h post-transfection, cells were transferred to culture medium; at 48 h post-transfection, cells were collected for analysis. The sequences of the small interfering RNAs (siRNAs) were:
si-NC:5′-CACUCAAGAUUGUCAGCAA-3′;
si-Slc26a1 #1:5′-GGAAUACCUAGCAGGUGAU-3′;
si-Slc26a1 #2:5′-GCCAAUACCCACAGAGUUA-3′
Cell viability and migration analysis
After transfection with siRNA, GC-1 and GC-2 cells were seeded in 96-well plates at a density of 4 × 103 cells/well. Cell viability was evaluated using a cell counting kit-8 (CCK-8; Beyotime Institute of Biotechnology, Nantong, China), as previously described (Chen et al., 2022a; Xue et al., 2022; Yu et al., 2022). In the colony formation assay, 1,000 transfected cells were placed in 6-well plates; 2 mL DMEM containing 10% FBS was added to each well and changed after 6 d. After 2 weeks, the cells were fixed with methanol and stained with 0.1% crystal violet (Beyotime, Jiangsu, China) for 30 min, and the visible proliferating clumps were counted under a microscope (Carl Zeiss, Oberkochen, Germany).
In the migration assay, 300 μL serum-free DMEM was added to the top of a culture chamber with an 8-mm membrane (14831; Corning, Corning, NY, USA), and the cells were then added; 700 μL DMEM containing 10% FBS was added to the lower part of the chamber. After 24–48 h, the cells below the membrane were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. Finally, photographs were taken under a microscope (Carl Zeiss, Oberkochen, Germany).
Western blot
The proteins of transfected GC-1 and GC-2 cells were extracted using RIPA buffer (RIPA, Beyotime, Jiangsu, China) and denatured at 100 °C. The denatured proteins were then separated on 10% sodium dodecyl sulfate-polyacrylamide gels, transferred to a polyvinylidene difluoride membrane, and sealed with 5% skim milk at room temperature for 1 h. The primary antibodies were anti-SLC26A1 (1:1,000, NBP1-84897; Novus, St. Charles, MO, USA) and anti-tubulin antibody (1:1,000, MA1-91878, Thermo Fisher Science, Waltham, MA, USA). The membranes were then incubated with secondary antibody (1:3,000, A0208, Beyotime, Jiangsu, China) for 1 h at room temperature. Finally, a quantitative analysis was performed using a SuperSignal West Femto chemiluminescence substrate detection system (Thermo Fisher Scientific, Waltham, MA, USA).
RNA extraction and reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from testicular tissue and transfected GC-1 and GC-2 cells using TRIZOL™ reagent (Vazyme, Nanjing, China) according to the manufacturer’s instructions. The RNA was then reverse transcribed into complementary cDNA by using a PrimeScript RT reagent Kit (Vazyme, Nanjing, China) and quantified using a real-time PCR system (Applied Biosystems, Foster City, CA, USA). The reaction system was set up as follows: SYBR Green Mix (10 μL), forward primer (0.4 μL), reverse primer (0.4 μL), cDNA template (1.0 μL), ddH2O (8.2 μL), resulting in a total volume of 20 μL. 18S rRNA was used as an internal control. Target gene expression was calculated using the 2−ΔΔCT method: ΔΔCT = (CTTarget − CT18srRNA)Sample − (CTTarget − CT18srRNA)Control. The primers used are summarized in Table S1.
Histology and sperm phenotyping
Testes and epididymides from Slc26a1-WT and Slc26a1-KO mice aged 8–12 weeks were immediately fixed in modified Davidson’s solution for 48 h, dehydrated in a series of graded ethanol solutions, embedded in paraffin, and cut into sections (thickness, 4 μm). The sections were rehydrated and stained for histological analysis using hematoxylin-eosin (H&E) or periodic acid Schiff (PAS) stain, as previously described (Shen et al., 2021; Wu et al., 2022; Yu et al., 2021). For sperm malformation analysis, we selected and quantified the head and tail deformities of sperm, respectively. Then, we performed computer-assisted sperm analysis (CASA). Cauda epididymides were suspended in human tubal fluid culture medium (InVitroCare, Inc., Frederick, MD, USA), incubated at atmospheric pressure and 37 °C for 10 min, and analyzed for semen quality using a Ceros™II sperm analysis system (Hamilton Thorne, Beverly, MA, USA).
Immunofluorescence
Immunofluorescence staining of mouse testis was performed as previously described (Gao et al., 2020; Zhao et al., 2019). The paraffin sections were boiled in 10 mM citrate buffer (pH 6.0), soaked in 3% hydrogen peroxide, blocked with bovine serum albumin, and incubated with primary antibody (Table S2) at 4 °C. Subsequently, the slides were rinsed three times with phosphate-buffered saline, then incubated with Alexa-Fluor secondary antibody (Thermo Fisher Scientific, Waltham, USA) at 37 °C and stained with 4′,6-diaminodiphenylindole. Fluorescent staining images of all sections were obtained under a confocal microscope (Zeiss, Oberkochen, Germany). Images of 50 tubules per male were used for quantification.
Terminal deoxynucleotidyl transferase-dUTP nick-end labeling (TUNEL) assay
Apoptosis was detected using the TUNEL assay kit (Vazyme, Nanjing, Jiangsu, China), according to the manufacturer’s instructions. Paraffin sections were rehydrated and treated with proteinase K, then reacted with TUNEL labeling mix buffer at 37 °C. Images were obtained under a confocal microscope (Zeiss, Oberkochen, Germany). Fifty tubules per male were analyzed.
Statistical analysis
All relevant statistical analyses were performed in triplicate. Unpaired student’s t-tests or one-way ANOVA were used to verify differences between Slc26a1-WT and Slc26a1-KO mice. Data are the mean ± Standard Deviation (SD). A P-value < 0.05 was considered to indicate a significant difference.
Results
Generation of Slc26a1-KO mice
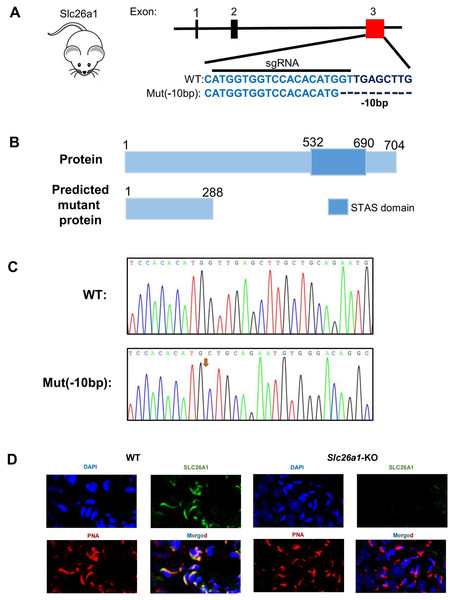
To investigate the effect of SLC26A1 on male fertility, CRISPR/Cas9 gene editing technology was used to introduce a mutation consisting of a 10 bp deletion in exon 3 of Slc26a1 (Fig. 1A), which is predicted to lead to frame-shift mutations. This mutation causes a complete deletion of the Sulfate Transport Anti-Sigma antagonist (STAS) domain (Fig. 1B). PCR and Sanger sequencing were used to confirm the changes (Fig. 1C). Immunofluorescence staining of sperm showed that SLC26A1 was specific localized in the acrosome of spermatids of WT mice, but not in the Slc26a1-KO spermatids (Fig. 1D). Localization of Slc26a1 in mice suggested that Slc26a1 may affect acrosome during spermatogenesis.
Figure 1: Generation of Slc26a1-KO mice.
(A) CRISPR/Cas9-mediated gene editing strategy for Slc26a1. (B) Predictive analysis of mutant proteins in Slc26a1-KO mice. (C) Sanger sequencing of Slc26a1-WT and Slc26a1-KO mice. (D) Immunostaining of SLC26A1 and PNA in the sperm of WT and Slc26a1-KO mice. PNA served as an acrosome marker. The SLC26A1 antibody recognition site is at the C-terminal, the antibody recognizes only the WT form, not the mutant protein. Scale bar, 20 μm. SLC26A1, solute carrier 26A; WT, wild type; DAPI, 4′,6-diamidino-2-phenylindole; PNA, peanut agglutinin.Slc26a1 KO mice are fertile
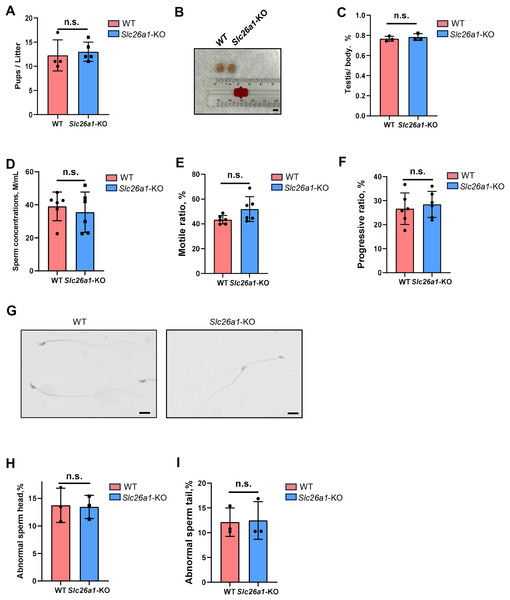
We subsequently performed fertility tests to study the effects of Slc26a1 on mouse fertility. The pups and litters of the experimental and control groups were counted. The results suggested that the difference in fertility between the Slc26a1-WT and Slc26a1-KO mice was not statistically significant (Fig. 2A). We also examined testicular morphology and testicular/body weight ratios of Slc26a1-KO male mice; compared with the control group, the difference was not statistically significant (Figs. 2B and 2C). The CASA results suggested that compared with WT mice, Slc26a1-KO mice had normal sperm concentrations, and percentage of sperm with motility and with progressive motility (Figs. 2D–2F). H&E staining of sperm found that the abnormal sperm of Slc26a1-KO mice was not significantly different from that of Slc26a1-W mice (Figs. 2G–2I). Therefore, Slc26a1 was not essential for mouse fertility.
Figure 2: Normal fertility in Slc26a1-KO mice.
(A) Fertility test of Slc26a1-WT and Slc26a1-KO mice; to five males (n) per group P > 0.05. (B) Representative examples of testicular morphology of 8-week-old Slc26a1-WT and Slc26a1-KO mice. Scale bar = 5 mm. (C) Testis/body weight ratio of the control group and the experimental group; n = 6 per group, P > 0.05. (D–F) Sperm concentrations (D), motility (E), and progressive motility (F) in Slc26a1-WT and Slc26a1-KO mice. M, millions; n = 6 per group, P > 0.05. (G) Examples of H&E staining of sperm from Slc26a1-WT and Slc26a1-KO mice. Scale bar, 20 μm. (H) Percentages of abnormal sperm head in Slc26a1-WT and Slc26a1-KO mice; n = 3 per group, 500 sperm cells were counted in each group. P > 0.05. (I) Percentages of abnormal sperm tail in Slc26a1-WT and Slc26a1-KO mice; n = 3 per group, 500 sperm cells were counted in each group. P > 0.05.Spermatogenesis is normal in Slc26a1-KO mice
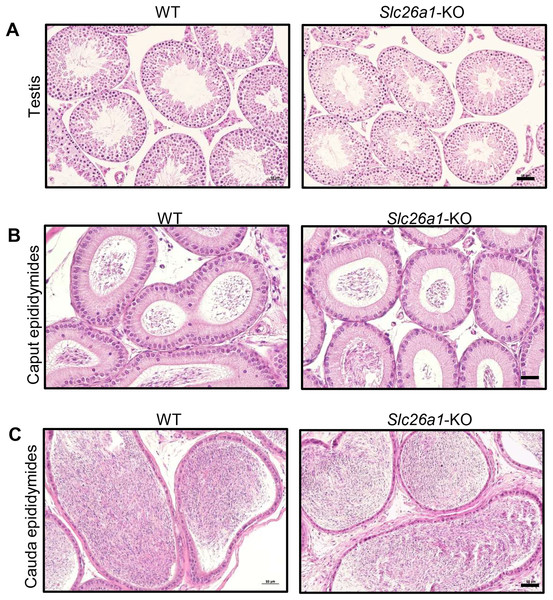
H&E staining results for testes suggested that Slc26a1-KO mice had intact seminiferous tubules and spermatogenic cells at all stages (Fig. 3A). H&E staining of the epididymis found that there were no significant histological differences between the epididymides of Slc26a1-WT and Slc26a1-KO mice and that they were filled with sperm in both groups of animals (Figs. 3B and 3C).
Figure 3: Histological analysis of testes and epididymides from 8-week-old mice.
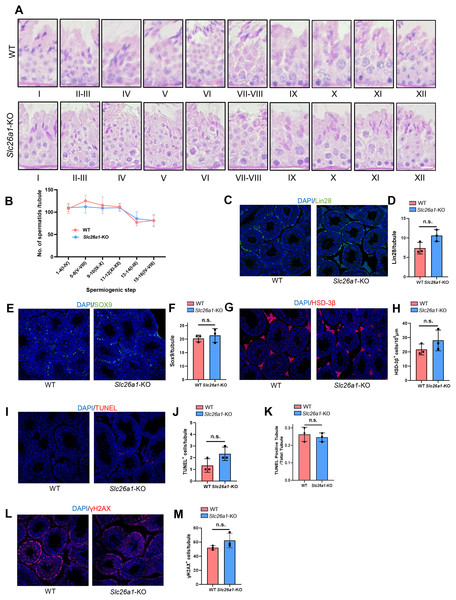
(A) Representative H&E staining of testicular sections from Slc26a1-WT and Slc26a1-KO mice, scale bar = 50 μm. (B) Examples of H&E staining of caput epididymal sections from Slc26a1-WT and Slc26a1-KO mice, scale bar = 50 μm. (C) H&E staining of cauda epididymis sections from Slc26a1-WT and Slc26a1-KO mice, scale bar = 50 μm.Different spermatogenic cells are arranged in the seminiferous tubules according to special cell connections. Spermatogenesis can be divided into the three processes of spermatogonial stem cell (SSC) proliferation and differentiation, spermatocyte meiosis, and spermiogenesis. To examine the differences in spermatogenesis between Slc26a1-WT and Slc26a1-KO mice, we first analyzed the nuclei and acrosomes of the spermatogenic cells in seminiferous tubules using PAS staining. The results indicated there were no obvious morphological differences (Fig. 4A). We quantitatively analyzed spermatids in spermatogenic tubules by H&E-stained mouse testicular sections (Fig. 4B), the results suggest that there is no significant difference in the numbers of spermatids between WT and KO mice.
Figure 4: Normal spermatogenesis in Slc26a1-KO mice.
(A) Representative images of PAS staining of spermatogenic cells (epithelial stages I–XII) from Slc26a1-WT and Slc26a1-KO mice, scale bar = 20 μm. (B) The number of spermatids in each spermatogenic tubule were counted in H&E-stained mouse testicular sections. (C) Representative immunostaining of LIN28 in testicular sections of 8-week-old Slc26a1-WT and Slc26a1-KO mice. Scale bar = 50 μm. (D) Comparative quantification of LIN28-positive cells (spermatogonial stem cells, SSCs) per tubule (based on (C), n = 3 per group, P > 0.05. (E) Immunostaining of SOX9 (served as a Sertoli cell marker) in 8-week-old Slc26a1-WT and Slc26a1-KO mice. Scale bar = 50 μm. (F) Quantification of (E), n = 3 per group, P > 0.05. (G) Immunostaining of 3β-HSD (served as a Leydig cell marker) in 8-week-old Slc26a1-WT and Slc26a1-KO mice. Scale bar = 50 μm. (H) Quantification of (G), n = 3 per group, P > 0.05. (I) TUNEL assay of testes from Slc26a1-WT and Slc26a1-KO mice, scale bar = 50 μm. (J) Quantification of apoptotic cells (based on I), n = 3 per group, P > 0.05. (K) Percentage of TUNEL-positive tubules (apoptotic cells) in total spermatogenic tubules, 50 tubules were counted in each group. (L) Immunostaining of γH2AX (served as a spermaticyte marker) in testes of Slc26a1-WT and Slc26a1-KO 8-week-old mice, scale bar = 50 μm. (M) Quantification of primary spermatocytes (base on L), n = 3 per group, P > 0.05. Scale bar = 50 μm.We also used lin-28 homolog (LIN28), SRY-box 9 (SOX9), hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 1(HSD-3β), TUNEL, and H2AX variant histone (γ-H2AX) immunostaining. These five marker genes are respectively located in SSCs (Figs. 4C and 4D), Sertoli cells (Figs. 4E and 4F), Leydig cells (Figs. 4G and 4H), apoptotic cells (Figs. 4I–4K), and spermatocytes (Figs. 4L and 4M). Then we quantified the number of positive cells in the testicular sections; there were no significant between-group differences. Taken together, these results suggested that Slc26a1 was not essential for spermatogenesis in mice.
Possible functional compensation from paralogs in Slc26a1-KO mice
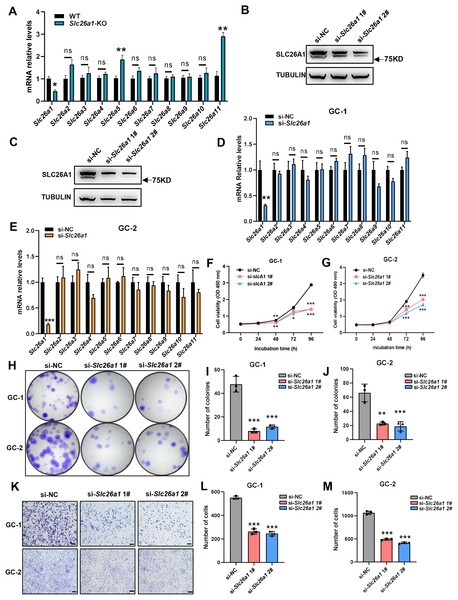
We extracted total RNA from the testes of Slc26a1-WT and Slc26a1-KO mice. Relative transcript levels of eleven Slc26a family members were detected using RT-qPCR. The mean expression of Slc26a5 and Slc26a11 in the testes of Slc26a1-KO mice was higher than that of Slc26a1-WT mice (Fig. 5A). These results may suggest that paralog-associated functional compensation was present in the testes of Slc26a1-KO mice.
Figure 5: Putative compensation in Slc26a1-KO mice.
(A) Relative mRNA levels of eleven Slc26a family genes in total testes of Slc26a1-WT and Slc26a1-KO mice, analyzed using RT-qPCR. *P < 0.05, six males per group. (B and C) Western blot analysis revealed that Slc26a1 was knocked down in Slc26a1-specific-siRNA-treated GC-1 (B) and GC-2 (C) cells; tubulin was used as an internal reference. (D and E) Relative mRNA levels of Slc26a family genes in GC-1 (D) and GC-2 (E) cells, using RT-qPCR. *P < 0.05, **P < 0.01, ***P < 0.001. (F and G) Viability of GC-1 (F) and GC-2 (G) cells assessed using CCK-8 assay 48 h after transfection, n = 3 per group. (H–J) Colony formation assay performed to determine proliferation capacity of GC-1 (I) and GC-2 (J) cells 48 h after transfection, n = 3 per group. (K–M) Migration ability of GC-1 (L) and GC-2 (M) cells assessed using transwell assay 48 h after transfection.To test whether transient knockdown of the Slc26a1 gene also had a similar effect, we transiently transfected GC-1 and GC-2 cells with si-Slc26a1 #1 and #2; western blot analysis revealed reduced expression of SLC26A1 (Figs. 5B and 5C). We also extracted total RNA from GC-1 and GC-2 cells and performed RT-qPCR. The PCR results indicated there was no significant difference in relative transcription levels of Slc26a family members between Slc26a1-WT and Slc26a1-KO mice (Figs. 5D and 5E).
Since there was no compensation in these two cell lines, we assessed whether the knockdown of Slc26a1 affects their phenotype. The results of the CCK-8 and colony formation assays revealed that proliferation of GC-1 and GC-2 cells decreased with downregulation of Slc26a1 expression (Figs. 5F–5J). Transwell assays further found that downregulation of Slc26a1 inhibited cell migration (Figs. 5K–5M).
Taken together, these results indicated that the deletion of Slc26a1 could be compensated for by other Slc26a family members in some testicular cells, but transient gene knockdown in GC-1 and GC-2 cells had no such effect.
Discussion
As anion transporters, SLC26A family members have important roles in pH homeostasis, sperm maturation, and capacitation in the male reproductive tract. Previous studies found that the SLC26A family members (e.g., SLC26A3 and SLC26A8 proteins) are involved in the regulation of germ cells (Touré, 2019). However, the role of Slc26a1 in mouse spermatogenesis and fertility has remained unknown. In this study, we examined SLC26A1 and obtained Slc26a1-KO mice using CRISPR/Cas9 technology. We found no significant differences in spermatogenesis or fertility of Slc26a1-KO mice. This result indicated that Slc26a1 was dispensable for mouse fertility. However, in the Slc26a1 knockdown model, proliferation and migration ability of mouse GC-1 and GC-2 cells were decreased. Therefore, there were phenotypic differences between the KD and KO models. Similar to the findings of previous studies on Rai14 (Wu et al., 2021) and Fank1 (Zhang et al., 2019), this difference might have been due to functional compensation of homologs in the KO model in some germ cells.
Genetic robustness refers to the ability of living organisms to maintain viability and adaptability under conditions of genetic variation (including disturbance; Waddington, 1959). When there are small differences in genetic composition or environmental conditions, organisms need buffer systems to ensure similar developmental outcomes; this process is known as robustness (Waddington, 1959). As previously reported (White et al., 2013), this genetic robustness may come from a redundant gene. Gene loss can be compensated for by other genes with overlapping functions and the same gene expression patterns (Bouché & Bouchez, 2001; Kok et al., 2015; White et al., 2013).
We speculate that the minimal effect of Slc26a1 on mouse fertility was due to functional redundancy. Fertility is an important function, and there may be redundant genes with overlapping functions to ensure stability of individual fertility. Asb-1, 2, 3, 4, 5, 7, 8, 9, 11, 14, 15, 17, and 18 genes are expressed at significantly higher levels in Asb12-KO mice than in Asb12-WT mice (Zhang et al., 2022). The Slc26a family is highly conserved, with 11 members in mice (SLC26A10 is a pseudogene in humans). Similarly, possible upregulation of two Slc26a family members was found in Slc26a1-KO mice and base on results from the short-term mRNA suppression, we speculate that it was caused by the long-term loss of Slc26a1.
At present, study findings indicate that many genes expressed in the testis are dispensable for mouse fertility (e.g., FBXW17 (Chen et al., 2022b), ASB12 (Zhang et al., 2022), USP26 (Felipe-Medina et al., 2019)). Our results exclude SLC26A1 as a contraceptive target and male infertility factor and will help avoid duplication of effort by researchers and save time and money in other laboratories. There is only a remote possibility of a germ-cell-specific dominant mutation. These results can also help reproductive researchers determine target gene research priorities and focus on genes that are essential for fertility.
Supplemental Information
Antibodies.
SLC26A1, solute carrier 26A; Lin28, lin-28 homolog; SOX9, SRY-box 9; SCP3, synaptonemal complex protein 3; γH2AX, H2AX variant histone; 3β-HSD, IgG3 κ mouse monoclonal 3 beta-HSD.