Whole-genome analysis of CGS, SAHH, SAMS gene families in five Rosaceae species and their expression analysis in Pyrus bretschneideri
- Published
- Accepted
- Received
- Academic Editor
- Pedro Silva
- Subject Areas
- Bioinformatics, Genomics, Molecular Biology, Plant Science
- Keywords
- CGS, SAHH, SAMS, Rosaceae, Expression analysis, Stone cell
- Copyright
- © 2022 Zhang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. Whole-genome analysis of CGS, SAHH, SAMS gene families in five Rosaceae species and their expression analysis in Pyrus bretschneideri. PeerJ 10:e13086 https://doi.org/10.7717/peerj.13086
Abstract
Cystathionine γ-synthase (CGS), S-adenosyl-L-homocysteine hydrolase (SAHH), and S-adenosy-L-methionine synthetase (SAMS) play an important role in the regulation of plant growth, development, and secondary metabolism. In this study, a total of 6 CGS, 6 SAHH, and 28 SAMS genes were identified from five Rosaceae species (Pyrus bretschneideri, Prunus persica, Prunus mume, Fragaria vesca, and Malus domestica). The evolutionary relationship and microsynteny analysis in five Rosaceae species revealed that duplicated regions were conserved between three gene families (CGS, SAHH, SAMS). Moreover, the chromosomal locations, gene structures, conserved motifs, cis-elements, physicochemical properties, and Ka/Ks analysis were performed by using numerous bioinformatics tools. The expression of different organs showed that the CGS, SAHH and SAMS genes of pear have relatively high expression patterns in flowers and stems, except for PbCGS1. RNA-seq and qRT-PCR combined analysis showed that PbSAMS1 may be involved in the regulation of pear stone cell development. In summary, this study provides the basic information of CGS, SAHH and SAMS genes in five Rosaceae species, further revealing the expression patterns in the pear fruit, which provides the theoretical basis for the regulation of pear stone cells.
Introduction
Pear is an important fruit crop that belongs to the Pyrus genus in the Rosaceae family and has been cultivated for more than two thousand years in China (Xue et al., 2019). There are many types of pear fruits, which are rich in nutrients, and people love them. “Dangshan Su” Pear is originally produced in Dangshan County, Anhui Province, China, and is the most widely cultivated variety in China (Konarska, 2013). The fruit of pear is famous all over the world especially in China and become popular due to its high nutritional and medicinal value (Cao et al., 2018a; Wu et al., 2013). The content of stone cells is an important factor that affects the taste of pear. The less content of the stone cells increase the taste and quality of pear fruits, which is closely related to lignin (Jaime et al., 2015). Because stone cells are formed by the deposition of lignin on the secondary wall (Yan et al., 2014), it is important to reduce the synthesis of lignin.
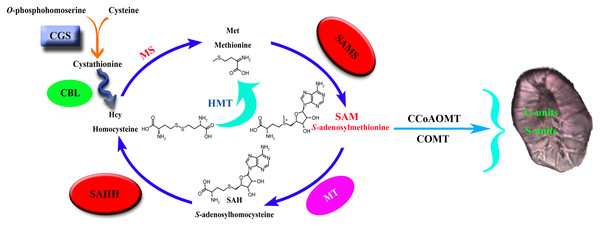
Cystathionine γ-synthase (CGS) is the first step of methionine biosynthesis in higher plants and is a key regulatory step for methionine biosynthesis (Fig. 1). The O-phosphohomoserine (OPH) of aspartic acid (Asp) combines with the sulfhydryl group of cysteine to form cystathionine (Galili, Amir & Fernie, 2016). Threonine synthase (TS) competes with CGS for their common substrate OPH, which is metabolized into threonine (Thr) and isoleucine (Ile) (Thompson et al., 1982; Amir, Hacham & Galili, 2002). CGS and TS jointly control the biosynthesis of methionine. S-adenosyl-L-homocysteine hydrolase (SAHH) is the only eukaryotic enzyme that decomposes S-adenosyl-L-homocysteine (SAH), which is the key to maintaining SAH levels in plants. Sadenosylmethionine (SAM) is the methylation donor in the methylation reaction of all organisms. SAH is a byproduct of the reaction after the methylation donor is transferred to the acceptor, and it binds and competes with SAM, thereby inhibiting the activity of methyltransferase (Nguyen, Yablon & Chen, 2001; Luka, Mudd & Wagner, 2009). Therefore, SAH is a strong product inhibitor of SAM-dependent methyltransferase, which is hydrolyzed into homocysteine and adenylate by SAHH.
Figure 1: Lignin biosynthesis is controlled by carbon metabolism.
CGS, cystathionine γ-synthase; CBL, cystathionine b-lyase; MS, methionine synthase; SAHH, S-adenosylhomocysteine hydrolase; HMT, homocysteine S-methyltransferase; SAMS, S-adenosylmethionine synthase; MT, methionine S-methyltransferase; CCoAOMT, caffeoyl CoA O-methyltransferase; COMT, caffeic acid O-methyltransferase.S-adenosy-L-methionine synthetase (SAMS) is a key enzyme in plant metabolism that catalyzes the synthesis of SAM from ATP and Met (Han et al., 2016). It has the functions of transmethylation, transthiolation and transamination (Belbahri et al., 2000; Finkelstein, 1990; Mato et al., 1997). Transmethylation mainly provides methyl groups for plants (Bottiglieri, Hyland & Reynolds, 1994). In the biosynthetic pathway of lignin, the methylation of G- and S-lignin monomers is mainly catalyzed by the two enzymes COMT and CCoAOMT (Humphreys, Hemm & Chapple, 1999; Osakabe, Tsao & Li, 1999; Ma & Xu, 2008), and the methyl group required for the reaction is provided by the synthesis of SAM (Bottiglieri, Hyland & Reynolds, 1994), so S-adenosylmethionine synthase is the key to lignin synthesis.
In plants, animals, and microorganisms, the transmethylation reaction is usually involved in the modification of almost all metabolites. In most methylation reactions, SAM is the methyl donor used by all organisms, while SAH is a by-product of the reaction after the transfer of the methyl donor to receptor (Luka, Mudd & Wagner, 2009; Moffat & Weretilnyk, 2010). SAH is a strong product inhibitor of SAM-dependent methyltransferase, which is hydrolyzed to homocysteine and adenosine by SAHH. SAHH is a key enzyme that maintains the methylation potential of cells (Tanaka et al., 1997; Miller et al., 1994). Inhibition of this enzyme will lead to increased accumulation of SAH, thereby inhibiting the methylation pathway through a feedback inhibition mechanism. CGS controls the synthesis of met. Overexpression of CGS can increase the level of met by 15 times and significantly increase the level of s-methylmethionine (SMM). SMM is considered to correspond to met (Baddiley & Jamieson, 1955; Eckert et al., 2009; Rao & Rossmann, 1973). SAM is also an effector of CGS post-transcriptional autoregulation, and the increase of SAM level down-regulates the level of CGS (Chiba et al., 2003). SAMS catalyzes the synthesis of SAM from ATP and Met and provides the necessary methyl groups for the lignin biosynthesis process. In summary, it can be seen that the three families of CGS, SAHH and SAMS are closely related.
Methyltetrahydrofolate reductase (MTHFR) and folic polyglutamate synthase (FPGS) can significantly affect the biosynthesis of lignin, which may help reveal a new regulatory mechanism of lignin biosynthesis (Li et al., 2015; Tang et al., 2014). MTHFR and FPGS participate in plant one-carbon (C1) metabolism, which is essential for the synthesis of Met, SAM, SAH, homocysteine (Hcy), and other methylated compounds in all organisms (Hanson & Roje, 2001). C1 metabolism generally includes tetrahydrofolate (THF) and the Met cycle. The former is responsible for the transfer of the C1 unit, and the latter is responsible for the synthesis and recovery of SAM (the activated form of Met). In the process of lignin biosynthesis, CCoAOMT and COMT need to use SAM as a cofactor to methylate the 3-OH and 5-OH on the aromatic ring, respectively, which leads to the biosynthesis of G and S lignin (Boerjan, Ralph & Baucher, 2003; Roje, 2006). In addition, many C1 metabolism genes show high expression levels in tissues with good lignification of plants, which means that there is potential coordination between C1 metabolism and lignin biosynthesis (Scully et al., 2018; Srivastava et al., 2015; Villalobos et al., 2012).
At present, the CGS, SAHH and SAMS genes of some species have been studied, such as CGS1 in Arabidopsis (Chiba et al., 2003); PvCGS and PvSAHH1 in switchgrass (Bai et al., 2018); PtoSAHHA, PtoSAHHB in poplar (Du et al., 2014); SAHH1-SAHH2, SAMS1-SAMS4 (Yang et al., 2019) in Arabidopsis; FvSAMS (Kovacs et al., 2020) in strawberry and so on. Previous studies have been shown that overexpression of AtCGS leads to a significant increase in Met and SMM (Gakière et al., 2002); down-regulation of PvSAHH1 will increase the level of SAH and reduce the ratio of SAM/SAH, thereby reducing the accumulation of lignin (Bai et al., 2018); the decrease of AtSAMS3 can reduce the content of plant lignin (Shen, Li & Tarczynski, 2002). However, there are few reports on the bioinformatics and expression analysis related to the CGS, SAHH and SAMS gene families of pear (Pyrus bretschneideri). This study was conducted in the five Rosaceae species of pear, peach (Prunus persica), plum (Prunus mume), strawberry (Fragaria vesca), and apple (Malus domestica). Classification, conserved domains, chromosome location, microcollinearity, etc. were analyzed. Meanwhile, RNA-seq and qRT-PCR at different developmental stages of the CGS, SAHH and SAMS gene families of pear were analyzed to understand their expression patterns. The genes related to lignification in pear were screened out, and the study of five Rosaceae (CGS, SAHH, SAMS families) also laid the foundation for improving the quality of pear fruits.
Materials and Methods
Plant materials
The plant material collected from Pyrus bretschneideri Rehd. ‘Dangshan Su’, which is grown on horticulture orchards (Dangshan County, Anhui Province). The 40-year-old Dangshan crisp pear tree has been artificially pollinated. Uniform sized fruits were collected at eight-different development stages: 15 DAF (days after flowering), 39 DAF, 47 DAF, 55 DAF, 63 DAF, 79 DAF, 102 DAF, and 145 DAF. In addition, flowers, inflorescence stems, leaves, and buds samples were also collected in the same year. All of the samples were stored at −80 °C for further use.
Identification and database search of CGS, SAHH, SAMS family members
The Pyrus bretschneideri and plum (Prunus mume) genomes were downloaded from the Genome Database For Rosaceae (https://www.rosaceae.org/), and the sequence information of strawberry (Fragaria vesca), peach (Prunus persica), and apple (Malus domestica) was downloaded from the Phytozome database (phytozome.jgi.doe.gov/pz/portal.html). The already known Arabidopsis thaliana CGS, SAHH and SAMS gene sequences were used as query (Chiba et al., 2003; Du et al., 2014; Shen, Li & Tarczynski, 2002). Secondly, the HMM (hidden Markov model) was searched through Pfam (http://pfam.xfam.org/) website to obtain the specific characteristic domain of CGS, SAHH, and SAMS families. Then the sequence was compared with BLASTP (E = <1.0E−10) in pear, strawberry, peach, apple, and plum genome database by using BioEdit software, and three gene family members were screened out among five Rosaceae species. Subsequently, SMART (http://smart.embl-heidelberg.de/) and the Pfam database were used to analyze the conserved domains of these genes, and the sequences without conserved domains and redundant sequences were removed (Cheng et al., 2018). Finally, the family members of the five Rosaceae species were identified.
Comparative phylogeny analysis of CGS, SAHH, SAMS gene family
The amino acid and cds sequences of CGS, SAHH and SAMS genes were aligned by using clustalw tool in MEGA5.1 software, and the phylogenetic tree was constructed by Neighbor-Joining (N-J) method (bootstrap = 1,000; substitution model = p-distance) and Maximum Likelihood (ML) method (bootstrap = 1,000; substitution model = WAG). The evolutionary tree of these gene families was constructed between pear, strawberry, peach, apple, and plum.
Analysis of genetic parameters in CGS, SAHH, SAMS gene family
The EXPASY (https://web.expasy.org/protparam/) online tool was used to predict isoelectric point (pI) and molecular weight (Mw). WoLF PSORT (https://www.genscript.com/wolf-psort.html) was used to predict subcellular localization.
Conserved motifs and gene structure analysis of CGS, SAHH, SAMS
The online tool MEME (http://meme-suite.org/tools/meme), set the number of motifs to 20, the Minimum width, and Maximum width to 6 and 200. Finally use the TBTools (Chen et al., 2020) software to draw a conservative motif diagram. The gene structure was analyzed by GSDS (http://gsds.gao-lab.org/).
Chromosomal locations and mode of duplication events
The genomic annotation data of five species were collected for chromosomal locations between three gene families. Finally, chromosomal locations were mapped with MapInspect software. Furthermore, the duplicate_gene_classifier tool of MCScanX was used to identify the duplication events in each species (Wang et al., 2012). In addition, the DnaSP 5.0 software was used to analyze the non-synonymous substitution rate (Ka), synonymous substitution rate (Ks) of duplicated genes.
Promoter cis-acting elements and intraspecific microcollinearity analysis
The 2,000 bp promoter sequences upstream of the three gene family members were obtained from the pear genome database, and PlantCARE online webtool (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) was used to analyze the cis-acting elements on the promoter region. Then, TBtools (Chen et al., 2020) software was used to draw the intraspecies microcollinearity map.
Expression patterns and transcriptomic analysis of CGS, SAHH, SAMS gene family
Download the transcriptome data of eight different developmental stages (15 DAF, 39 DAF, 47 DAF, 55 DAF, 63 DAF, 79 DAF, 102 DAF, and 145 DAF) of four pear varieties: Yali (P. bretschneideri), Starkrimson (P. communis), Nanguoli (P. ussuriensis) and Kuerlexiangli (P. sinkiangensis) fragrant pear from the NCBI database (Accession no. SRP070620). The expression profiles of CGS, SAHH and SAMS in the development of pear fruit were analyzed.
According to the RNA extraction kit of Tiangen Biotechnology Effective Company, the total RNA of Dangshansu pear fruit is extracted, and then cDNA is synthesized by the PrimeScriptRT reagent Kit with gDNA Eraser (Perfect Real Time) kit produced by the company. The Primer Premier 5.0 software was used to design specific primers. Detailed information about the primers is shown in Table S1. The relative expression levels of CGS, SAHH and SAMS genes in pear fruits were analyzed by qRT-PCR. The experiment uses the CFX96 fluorescent quantitative PCR instrument (Bio-Rad, Hercules, CA, USA), and the reaction system is 10 μL of SYBR® Premix Ex TaqTM II (2×) (TaKaRa, Kusatsu, Shiga, Japan), 2 μL of cDNA, 6.4 μL of water, and 0.8 μL of the upstream and downstream primers. This experiment was conducted with three biological and technical replication for each sample, and the relative expression of genes was calculated by 2−ΔΔCT method (Livak & Schmittgen, 2001).
Results
Identification and amino acid characteristics of CGS, SAHH, SAMS family members
The members of CGS (6), SAHH (6), and SAMS (28) were identified from five Rosaceae species, which renamed in pear: PbCGS1, PbSAHH1-PbSAHH2, PbSAMS1-PbSAMS6; peach: PpCGS1, PpSAHH, PpSAMS1-PpSAMS4; plum: PmCGS1, PmSAHH, PmSAMS1-PmSAMS4; strawberry: FvCGS1, FvSAHH, FvSAMS1-FvSAMS6; and apple: MdCGS1-MdCGS2, MdSAHH, MdSAMS1-MdSAMS8 (Table 1). The amino acid length of CGS members is between 412–618 aa, most of SAHH is 485 aa; only PbSAHH2 is 502 aa; and the length of SAMS is between 253–448 aa, which has the biggest difference in length among apples. The molecular weights of CGS and SAHH are relatively high, mainly between 53.3–66.4 kDa, PmCGS1 is relatively low, 45.9 kDa; SAMS is mainly in the range of 41.1–44.6 kDa, only FvSAMS3, MdSAMS4, and MdSAMS6 individual members are different. Except for the isoelectric points of PbCGS1, FvSAMS2, and MdCGS2 greater than 7, the other members are all less than 7, indicating that the amino acids are generally acidic. Prediction of subcellular location shows that four members of CGS gene family are found in the chloroplast, PbCGS1 is in the chloroplast or endoplasmic reticulum, and PmCGS1 may be in various positions of cells; 31 members of SAHH and SAMS gene family are in cytoplasmic, MdSAMS4 is shown in the chloroplast or extracellular, while FvSAMS2 and MdSAMS6 are displayed in the chloroplast.
| Species | Sequence ID | Name | Protein | ||||
|---|---|---|---|---|---|---|---|
| Length (aa) | Mw (kDa) | pI | Subcellular localization | Formula | |||
| Pear | Pbr038433.1 | PbCGS1 | 618 | 66.4 | 9.09 | chlo/E.R | C2885H4628N804O849S20 |
| Pbr005412.1 | PbSAHH1 | 485 | 53.3 | 5.72 | cyto | C2358H3751N639O716S26 | |
| Pbr016485.1 | PbSAHH2 | 502 | 55.1 | 6.22 | cyto | C2434H3874N666O732S28 | |
| Pbr008602.1 | PbSAMS1 | 393 | 43.0 | 5.59 | cyto | C1906H2998N522O583S14 | |
| Pbr037756.1 | PbSAMS2 | 393 | 43.0 | 5.59 | cyto | C1902H2999N521O584S15 | |
| Pbr026061.1 | PbSAMS3 | 391 | 43.1 | 5.49 | cyto | C1909H3006N522O584S14 | |
| Pbr006707.1 | PbSAMS4 | 394 | 43.2 | 5.56 | cyto | C1907H3005N525O587S15 | |
| Pbr020754.1 | PbSAMS5 | 390 | 42.7 | 5.97 | cyto | C1897H3009N519O575S14 | |
| Pbr018549.1 | PbSAMS6 | 379 | 41.2 | 5.53 | cyto | C1810H2860N502O565S15 | |
| Peach | Prupe.3G264300.1 | PpCGS1 | 522 | 56.0 | 6.51 | chlo | C2490H3981N677O744S21 |
| Prupe.1G165200.1 | PpSAHH | 485 | 53.4 | 5.81 | cyto | C2363H3760N640O712S26 | |
| Prupe.1G107000.1 | PpSAMS1 | 393 | 42.9 | 5.68 | cyto | C1899H3000N520O582S15 | |
| Prupe.3G004000.1 | PpSAMS2 | 393 | 43.0 | 5.77 | cyto | C1899H2995N529O581S15 | |
| Prupe.6G306200.1 | PpSAMS3 | 390 | 42.9 | 5.71 | cyto | C1904H3001N521O581S14 | |
| Prupe.7G128500.1 | PpSAMS4 | 390 | 42.8 | 6.08 | cyto | C1895H3005N523O577S14 | |
| Plum | Pm015918 | PmCGS1 | 412 | 45.9 | 6.24 | chlo/nucl/cyto/plas/extr/E.R | C2052H3257N545O595S25 |
| Pm007821 | PmSAHH | 485 | 53.5 | 5.81 | cyto | C2365H3764N640O712S29 | |
| Pm008469 | PmSAMS1 | 393 | 42.9 | 5.68 | cyto | C1899H3000N520O582S15 | |
| Pm012890 | PmSAMS2 | 393 | 43.0 | 5.60 | cyto | C1906H2999N521O583S14 | |
| Pm003235 | PmSAMS3 | 390 | 42.9 | 5.79 | cyto | C1904H3003N521O579S15 | |
| Pm026349 | PmSAMS4 | 390 | 42.9 | 6.08 | cyto | C1897H3009N523O577S15 | |
| Strawberry | mrna04420 | FvCGS1 | 545 | 58.4 | 6.57 | chlo | C2605H4148N706O775S21 |
| mrna06564 | FvSAHH | 485 | 53.4 | 5.90 | cyto | C2367H3769N641O711S26 | |
| mrna09668 | FvSAMS1 | 390 | 42.7 | 6.02 | cyto | C1890H2986N522O576S15 | |
| mrna13614 | FvSAMS2 | 371 | 41.1 | 8.53 | chlo | C1856H2870N508O516S18 | |
| mrna13617 | FvSAMS3 | 253 | 27.6 | 5.76 | cyto | C1228H1931N335O366S10 | |
| mrna22927 | FvSAMS4 | 386 | 42.4 | 5.82 | cyto | C1881H2968N516O571S15 | |
| mrna22974 | FvSAMS5 | 394 | 43.0 | 5.50 | cyto | C1901H3055N521O583S15 | |
| mrna24556 | FvSAMS6 | 393 | 43.1 | 5.66 | cyto | C1909H3007N525O584S14 | |
| Apple | MD17G1051900 | MdCGS1 | 523 | 56.1 | 6.68 | chlo | C2501H3992N678O743S20 |
| MD09G1054200 | MdCGS2 | 547 | 58.9 | 7.62 | chlo | C2622H4188N714O776S23 | |
| MD13G1281900 | MdSAHH | 485 | 53.3 | 5.72 | cyto | C2355H3751N639O714S26 | |
| MD04G1091900 | MdSAMS1 | 390 | 42.7 | 5.97 | cyto | C1896H2999N519O577S14 | |
| MD04G1187700 | MdSAMS2 | 398 | 43.7 | 5.64 | cyto | C1930H3046N532O592S15 | |
| MD09G1292700 | MdSAMS3 | 396 | 43.5 | 5.77 | cyto | C1922H3029N533O586S15 | |
| MD12G1109900 | MdSAMS4 | 423 | 46.3 | 6.07 | chlo/extr | C2059H3269N559O622S15 | |
| MD12G1201100 | MdSAMS5 | 395 | 43.6 | 5.66 | cyto | C1931H3048N530O587S15 | |
| MD13G1141700 | MdSAMS6 | 448 | 49.1 | 6.20 | chlo | C2183H3449N597O658S17 | |
| MD16G1138300 | MdSAMS7 | 393 | 43.0 | 5.59 | cyto | C1904H3004N518O583S15 | |
| MD17G1283400 | MdSAMS8 | 406 | 44.6 | 5.74 | cyto | C1975H3103N541O600S17 | |
Note:
Chlo, Chloroplast; Cyto, Cytoplasmic; Nucl, Nucleus; plas, Plastosome; Extr, Extracellular; E.R., Endoplasmic reticulum.
Phylogeny, classification, conservative motifs, and gene structure analysis of CGS, SAHH, SAMS genes
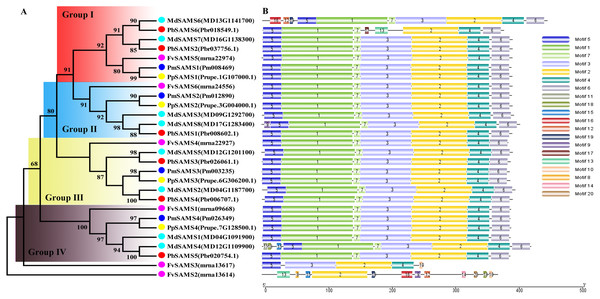
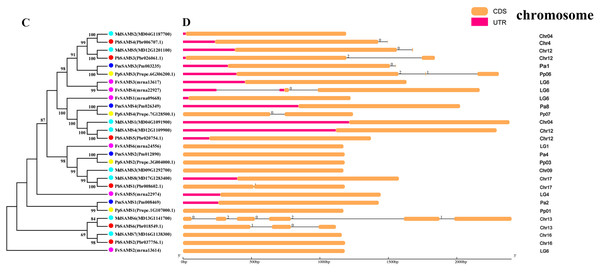
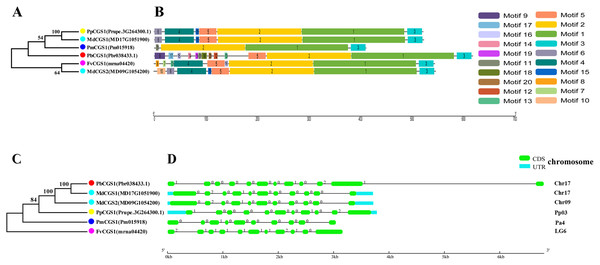
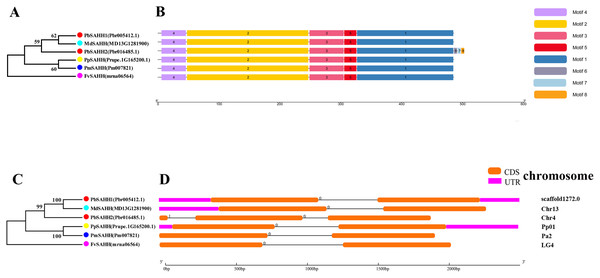
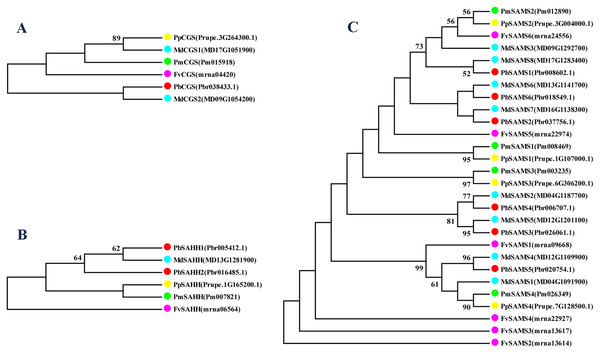
In this study, NJ method was used to construct the phylogenetic tree (Figs. 2A–5A). The conserved motif analysis and gene structure analysis corresponds to the phylogenetic tree constructed by amino acid sequence and cds sequence. The analysis of the SAMS gene family phylogenetic tree showed that the SAMS genes of the five species were mainly divided into four subfamilies, namely Group I, II, III, and IV (Fig. 2A). In addition, FvSAMS2 and FvSAMS3 form independent branches. The SAMS family members formed 10 gene pairs, and only three of them had high bootstrap values (≥99). Twenty motifs of the SAMS gene family were identified by using the MEME tool (Fig. 2B). Except for the two independent branches of FvSAMS2 and FvSAMS3, the C-terminal conserved motifs of the four subfamilies include motif2, motif4, and motif6. Only PbSAMS6 does not include motif3; except for the conservative motifs of MdSAMS4, MdSAMS6, and MdSAMS8 at the N-terminal, most types and distributions are similar. The only PbSAMS6 exists in motif17, and the only FvSAMS2 exists in motif8 and motif20 indicating that they may have specific functions. The SAMS gene family is not all composed of CDS and UTR. Each subfamily and independent branch has UTR-free regions, and 16 of them have UTR regions (Fig. 3D). The C-terminal conserved motifs of the CGS gene family members are all consistent, including motif2, motif1, and motif3. Each member of the N-terminal contains a unique motif, which may contain some other functions; the UTR region and the non-UTR region each account for half (Fig. 4). Among the six members of the SAHH gene family, except for the PbSAHH2 conserved motif, which contains motif6, motif7, and motif8, the remaining conserved motifs are the same. These motifs may give the member unique functions. UTR region and the non-UTR region also account for half (Fig. 5). Taken together, the conserved motif composition and gene structure characteristics of each family are very similar, which supports their close evolutionary relationship and the reliability of constructing evolutionary trees.
Figure 2: Systematic evolution and conserved motif analysis of SAMS genes family.
(A) The phylogenetic tree of SAMS genes was conserved by the N-J method (It is constructed from amino acid sequences). (B) Conserved motif in SAMS proteins.Figure 3: Systematic evolution and gene structures analysis of SAMS genes.
(C) The phylogenetic tree of SAMS genes was conserved by the N-J method (It is constructed from cDNA sequences). (D) Exon-intron organization of SAMS genes.Figure 4: Systematic evolution, conserved motif and gene structures analysis of CGS genes family.
(A) The phylogenetic tree of CGS genes was conserved by the N-J method (It is constructed from amino acid sequences). (B) Conserved motif in CGS proteins. (C) The phylogenetic tree of CGS genes was conserved by the N-J method (It is constructed from cDNA sequences). (D) Exon-intron organization of CGS genes.Figure 5: Systematic evolution, conserved motif and gene structures analysis of SAHH genes family.
(A) The phylogenetic tree of SAHH genes was conserved by the N-J method (It is constructed from amino acid sequences). (B) Conserved motif in SAHH proteins. (C) The phylogenetic tree of SAHH genes was conserved by the N-J method (It is constructed from cDNA sequences). (D) Exon-intron organization of SAHH genes.Meanwhile, we also constructed the ML phylogenetic tree (Fig. 6), and it is clear from the comparison between the two trees that the gene pairs formed due to high homology are basically consistent, indicating that the construction of the phylogenetic trees are accurate. Only in the CGS gene family, the homology of PbCGS1, FvCGS1 and MdCGS2 were not quite consistent in the two phylogenetic trees. Since the NJ phylogenetic tree is a distance method, which indicates the degree of similarity between sequences. While the amino acid sequences of FvCGS1 and MdCGS2 are closer, so they form a gene pair. ML phylogenetic tree indicates a series of features that change over time, and from an evolutionary perspective, it is possible that PbCGS1 and MdCGS2 are more closely related. Thus leading to a less consistent situation of these three genes.
Figure 6: The maximum likelihood phylogenetic tree of CGS, SAHH, SAMS gene families.
(A) The maximum likelihood phylogenetic tree of CGS gene family. (B) The maximum likelihood phylogenetic tree of SAHH gene family. (C) The maximum likelihood phylogenetic tree of SAMS gene family.Characteristic domain analysis
Further analysis of the characteristic domains of five Rosaceae species from three families (Fig. S1) (Cheng et al., 2019). The result shows that the characteristic domain of CGS family members is Cys_Met_Meta_PP (PF01053). As can be seen from Fig. S1, according to Pfam’s annotation information (http://pfam.xfam.org/family/Cys_Met_Meta_PP), this domain uses pyridoxal phosphate (PLP) as a cofactor to participate in cysteine and methionine amino acid metabolism (Ferla & Patrick, 2014). PLP is the active form of vitamin B6. PLP is a versatile catalyst, as a coenzyme in many reactions, including decarboxylation, deamination, and transamination reactions (Hayashi, 1995; John, 1995; Kirsch & Eliot, 2004). The characteristic domain of SAHH family members is AdoHcyase_NAD (SM00997), according to Pfam’s annotation results (http://pfam.xfam.org/family/PF00670). S-adenosine-L-homocysteine acid hydrolase (AdoHcyase) is an activated methyl cycle enzyme responsible for the reversible hydration of S-adenosyl-L-homocysteine to adenosine and homocysteine. AdoHcyase is a ubiquitous enzyme that binds to and requires NAD+ as a cofactor (Sganga et al., 1992). The characteristic domains of SAMS family members mainly include S-AdoMet_synt_N (PF00438), S-AdoMet_synt_M (PF02772), and S-AdoMet_synt_C (PF02773). The three domains of S-adenosylmethionine synthetase have the same alpha + beta folding. S-adenosylmethionine synthase is an enzyme that catalyzes the formation of S-adenosylmethionine (AdoMet) from methionine and ATP (Horikawa et al., 1990). AdoMet is an important methyl donor for demethylation and a propylamine donor in polyamine biosynthesis. These characteristic domains indicate their important functions and further prove the accuracy of the identified members. However, the three members of FvSAMS2, FvSAMS3, and PbSAMS6 are missing one domain, which may change their functions.
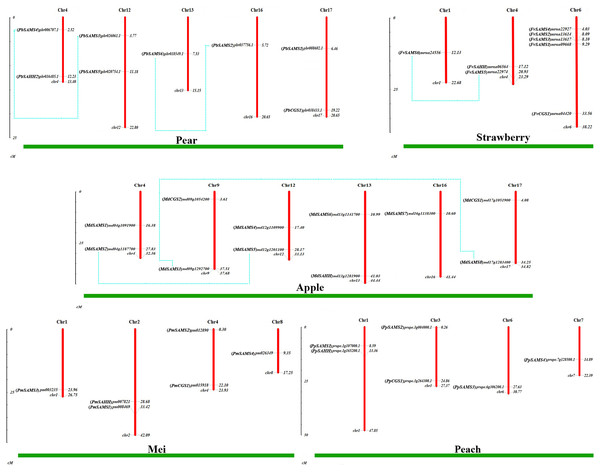
Chromosome location and gene duplication analysis of CGS, SAHH, SAMS family members
To understand the distribution of CGS, SAHH and SAMS families on chromosomes, the chromosome locations of five species were analyzed (Fig. 7). Except for PbSAHH1, all members of CGS, SAHH and SAMS gene family members were located on chromosomes. In Pyrus bretschneideri, eight members are distributed on chromosomes 4, 12, 13, 16, and 17. In Fragaria vesca, eight members were mainly distributed on chromosome 6, and the rest on chromosomes 1 and 4. The 11 members of Malus domestica were evenly distributed on chromosomes 4, 9, 12, 13, 16, and 17. In Prunus mume, six members are distributed on chromosomes 1, 2, 4, and 8. In Prunus persica, six members are distributed on chromosomes 1, 3, 6, and 7.
Figure 7: Chromosomal locations and gene duplication of five Rosaceae species.
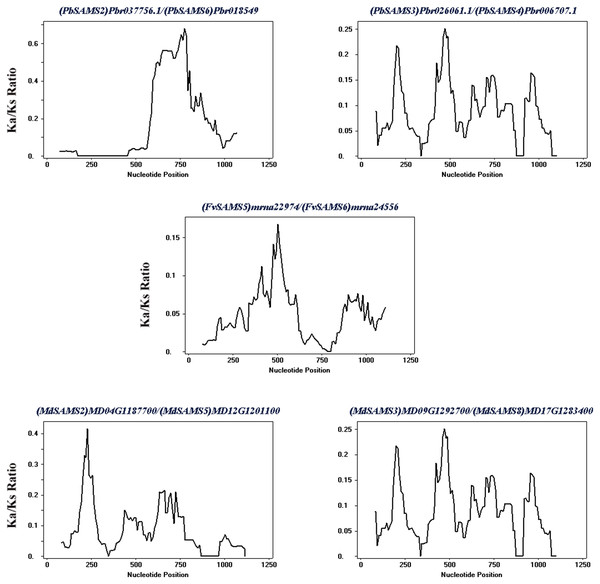
The species name is marked above the green line. duplicated gene pairs are connected with dotted lines.Subsequently, the evolutionary relationship analysis of these three gene families and the chromosome locations, it is found that five gene pairs have different modes of duplication events. They are all shown in Fig. 7 and each pair is connected together with a dotted line. The analysis showed that they have a high percentage of segmental duplication, indicating that the amplification of SAMS gene family through a segmental duplication event. According to Table 2 and Fig. 8, Ka is less than Ks and the peak value is not 1. Also we used functional motifs for analysis and comparison (Table S2), and found that the calculated Ka and Ks values of the two results are very close, and the results are consistent. It indicates that the gene has purification and selection effects, and the structure and function are not seriously affected.
| Duplicated genes | Ka | Ks | Ka/Ks | Duplicated type |
|---|---|---|---|---|
| (PbSAMS2) Pbr037756.1/(PbSAMS6) Pbr018549.1 | 0.0389 | 0.2679 | 0.1452 | Segmental duplication |
| (PbSAMS3) Pbr026061.1/(PbSAMS4) Pbr006707.1 | 0.0170 | 0.2044 | 0.0832 | Segmental duplication |
| (FvSAMS5) mrna22974/(FvSAMS6) mrna24556 | 0.0452 | 1.0571 | 0.04276 | Segmental duplication |
| (MdSAMS2) MD04G1187700/(MdSAMS5) MD12G1201100 | 0.0180 | 0.2512 | 0.0717 | Segmental duplication |
| (MdSAMS3) MD09G1292700/(MdSAMS8) MD17G1283400 | 0.0078 | 0.2149 | 0.0363 | Segmental duplication |
Figure 8: Sliding window analysis of duplicated genes.
At the top of each sliding window is a duplicate gene pair.Intraspecies microcollinearity
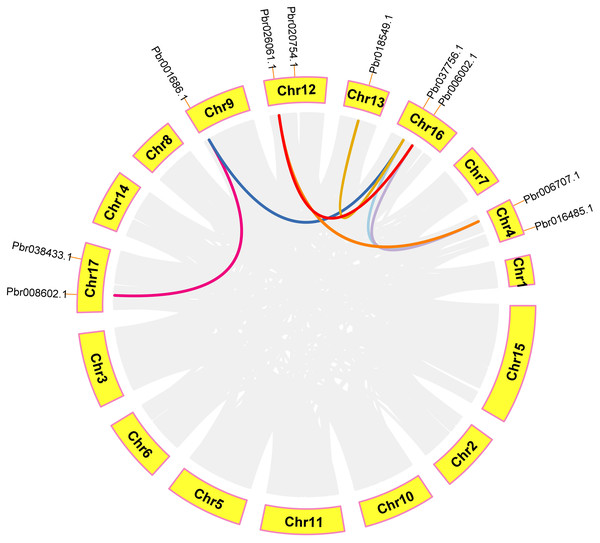
In order to further analyze the collinearity relationship of CGS, SAHH and SAMS gene families in pear, and we identified seven pairs of collinearity genes in a pear that have high homology, indicating that may be due to similar functions (Fig. 9). Respectively: Pbr008602.1/Pbr001686.1, Pbr018549.1/Pbr037756.1, Pbr026061.1/Pbr006002.1, Pbr026061.1/Pbr006707.1, Pbr018549.1/Pbr037756.1, Pbr037756.1/Pbr006707.1, Pbr006002.1/Pbr006707.1.
Figure 9: Analysis of microcollinearity within pear species.
Each collinear gene pair is connected with colored lines.Identification of cis-acting elements analysis
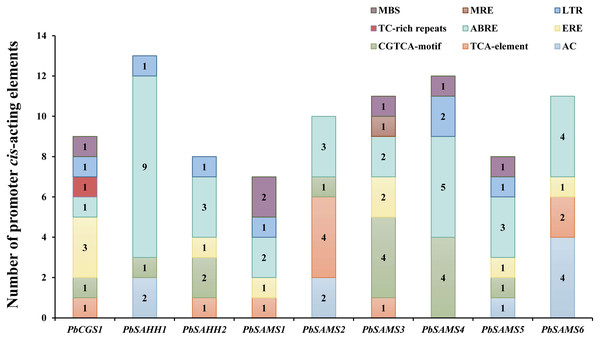
To better understand the promoter characteristics of members of the CGS, SAHH and SAMS gene families in pear, we analyzed their cis-acting elements (Fig. 10). Among these nine genes, only PbSAMS3 contains MRE (light response element), indicating that only the expression of this member may be regulated by light. These members also contain elements related to defense and abiotic stress, such as TC-rich repeats (defense and stress response), MBS (drought induction), LTR (low-temperature response). Except for PbSAMS2 and PbSAMS6, all other members contained at least one element of MBS or LTR, indicating that PbCGS, PbSAHH, PbSAMS are closely related to pear drought and low-temperature stress. We also found many CGTCA-motif, TCA-element, ABRE elements and ERE elements related to MeJA, SA, ABA and ethylene hormone response. All of the nine members contained ABRE elements, a total of 32, which are the most abundant cis-acting elements, indicating that they have the most extensive response to abscisic acid. The cis-acting elements TCA-element and ERE related to SA and ethylene were identified in six different members. CGTCA-motif, a cis-acting element related to MeJA, was identified among the seven members, a total of 14, which is the second most abundant cis-acting element. In addition to these elements, we also found AC elements that bind to MYB, which are related to lignin synthesis (Cao et al., 2016). These results indicate that PbCGS, PbSAHH and PbSAMS not only play a role in the synthesis of lignin, but also participate in the light response process, abiotic stress, and hormone response.
Figure 10: Analysis of cis-acting elements of PbCGS, PbSAHH and PbSAMS.
Potential cis-elements in the 5′ regulatory sequences of the nine genes.Comparative phylogenetic analysis and function prediction
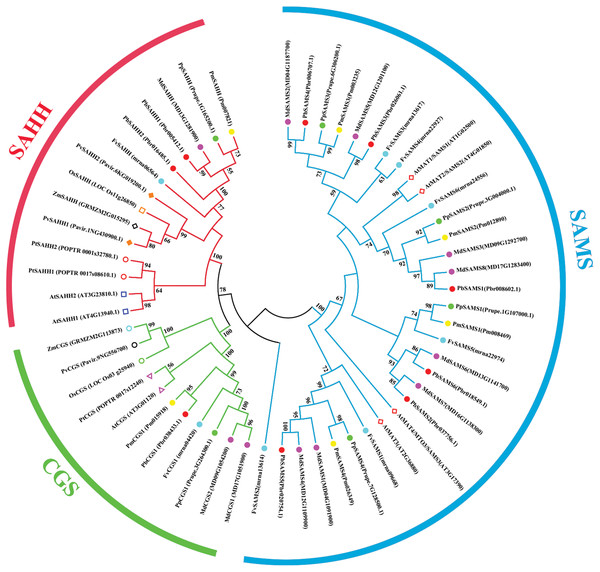
In this study, 10 species of CGS, SAHH, and six species of SAMS were constructed to form an interspecies complex phylogenetic tree (Fig. 11), and the members of the CGS, SAHH and SAMS families in pear were studied. We constructed 10 species of CGS, SAHH, and six species of SAMS to form an interspecific complex phylogenetic tree, and studied the members of the CGS, SAHH and SAMS families in pear. PbCGS1 and AtCGS are very close to the branch of the phylogenetic tree. In previous studies, it was found that overexpression of AtCGS significantly increased Met and SMM. CGS is the rate-limiting enzyme in this metabolic pathway (Gakière et al., 2002). PbCGS1 may also have a similar role.
Figure 11: Phylogenetic tree analysis of CGS, SAHH and SAMS species in different plants.
The interspecific phylogenetic tree is composed of three families: CGS, SAHH, and SAMS. The green, red and blue lines represent the three families, respectively.Numerous studies have found that down-regulating PvSAHH1 will increase SAH levels and reduce the SAM/SAH ratio, thereby reducing the accumulation of lignin (Bai et al., 2018). PvSAHH1 is in the same sub-branch as PbSAHH1 and PbSAHH2, indicating that these two genes may have this function. However, PbSAHH1 and PbSAHH2 are not in the same gene pair, and their functions may be slightly different. Four members of the SAMS family have been identified in Arabidopsis thaliana. Among these four members, AtSAMS3 shows different functions from other members. The reduction of AtSAMS3 results in the decrease of lignin content in plants, indicating that AtSAMS3 plays a role in lignin biosynthesis (Shen, Li & Tarczynski, 2002). The SAMS members in pear are evenly distributed near AtSAMS3, such as PbSAMS1, PbSAMS2, PbSAMS5, etc., indicating that these members may participate in lignin biosynthesis.
Expression profile analysis of PbCGS, PbSAHH and PbSAMS genes
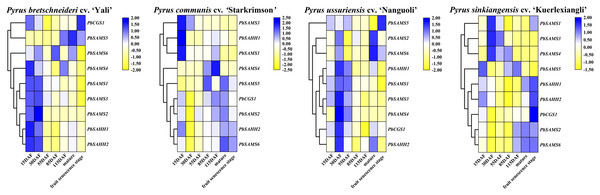
PbCGS1 expressed higher in the first three periods (15 DAF, 30 DAF, and 55 DAF) in Nanguoli, but lower in the other three pear. PbSAMS2 was also only expressed higher in the first three periods in Yali (Fig. 12). The expression levels of PbSAHH1 and PbSAHH2 were lower in the first three periods of Kuerlexiangli, but higher in the other three pear. The expression of PbSAMS4 was only low in the first three stages of Starkrimson. The expression levels of PbSAMS5 and PbSAMS6 in the corresponding period are not consistent with the changing trend of lignin synthesis and stone cell development, indicating that there is no connection with it. These identical genes have different expression in different varieties of pear fruit. By promoter analysis predicts that these members are closely related to drought stress. It may be different due to the differences between geographical, cultivation and environmental conditions, and it is inconsistent in pear fruit (Arzani et al., 2008). The expression levels of PbSAMS1 and PbSAMS3 in Yali, Starkrimson, Nanguoli and Kuerlexiangli were higher in the first three stages, but lower in the four stages after flowering. This is consistent with the trend of lignin synthesis and stone cell development (Cai et al., 2010; Cao et al., 2016; Cheng et al., 2016). These two members may be involved in lignin synthesis and stone cell development.
Figure 12: Expression profile analysis of PbCGS, PbSAHH and PbSAMS in the development of four pear fruits.
The heat map is generated by the TBtools software based on the RNA-seq database. Gene transcription levels are indicated by different colors on the heat map.Analysis of PbCGS, PbSAHH and PbSAMS genes expression patterns in Dangshan Su Pear
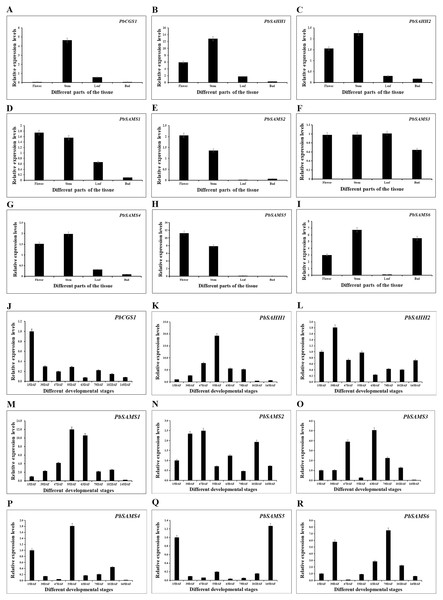
In order to further understand the different functions of the three gene families in pear, we studied the expression patterns of their different tissues (Fig. 13). All members have shown higher expression levels in stems, and three members of PbSAMS1, PbSAMS2 and PbSAMS5 have the highest level in flowers. PbCGS1 is only highly expressed in stems. PbSAHH1, PbSAHH2, PbSAMS2, PbSAMS4 and PbSAMS5 are mainly expressed in flowers and stems. PbSAMS1 is mainly expressed in flowers, stems and leaves. PbSAMS3 has high expression levels in flowers, stems, leaves and buds. PbSAMS6 is abundantly expressed in flowers, stems and buds, but hardly expressed in leaves. The content of stone cells is a key factor affecting the quality of pear fruit. The change of lignin content is closely related to the changes in stone cell content. Therefore, we studied the expression patterns of these nine genes in Dangshan Su pear at eight different development stages by qRT-PCR (Fig. 13). The expression levels of PbCGS1 was the highest in 15 DAF, and the expression levels were low in the remaining seven periods, indicating that they may play an important role in this period. The expression level of PbSAMS5 was the highest in 15 DAF and 145 DAF, and the expression level was lower in the middle stages of development. PbSAHH2 and PbSAMS2 have high expression levels in the early and later stages of development, but moderate expression levels in the middle stages of development. The expression levels of PbSAMS3, PbSAMS4, and PbSAMS6 were only highest in the first and middle stages of development but were not high in the other stages. The expression of PbSAHH1 and PbSAMS1 is the highest at 55 DAF, which is consistent with the changing trend of lignin content in pear fruits (Cai et al., 2010; Jin et al., 2013), indicating that these two genes may be involved in the synthesis and regulation of lignin in pear fruits.
Figure 13: Expression patterns of PbCGS, PbSAHH and PbSAMS genes in different tissues and different fruit development stages in Dangshan Su pear.
The expression patterns of PbCGS, PbSAHH and PbSAMS genes in different organs (A-I) of Dangshansu pear. Expression patterns of PbCGS, PbSAHH and PbSAMS genes in Dangshan Su pear at different developmental stages (J-R).Discussion
The size and content of stone cells are the key factors affecting the quality of pear fruit (Cai et al., 2010; Jin et al., 2013). The stone cell is a kind of sclerenchyma cell, which is formed by the continuous deposition of lignin on the secondary wall of parenchyma cells (Yan et al., 2014). Therefore, the formation of a stone cell has a strong correlation with lignin biosynthesis. Through one-carbon metabolism, the three enzymes CGS, SAHH and SAMS control the supply of methyl groups during the methylation reaction (Fig. 1). The CGS controls the synthesis of Met. SAMS catalyzes the synthesis of SAM from ATP and Met. SAHH maintains the SAH level of plants, and SAH competes with SAM binding, thereby inhibiting the activity of methyltransferase. They jointly control the methylation pathway and provide methyl for plant biosynthesis. In the process of G and S lignin biosynthesis, a large number of methyl groups are required. So the screening and identification of these three families are of great significance for an in-depth understanding of pear lignin synthesis and stone cell development regulation.
In this study, we identified 6 CGS, 6 SAHH, and 28 SAMS genes in five Rosaceae species (Table 1). Their isoelectric points are all greater than 7, except for individual ones that are less than 7, indicating that most of the amino acids are acidic. Moreover, most of the CGS family members are located in the chloroplast, and most of the SAHH and SAMS family members are located in the cytoplasm. Phenylalanine ammonia-lyase (PAL) is a key enzyme of lignin biosynthesis in the metabolic pathway of phenylpropane. It is located in the chloroplast and endoplasmic reticulum (Osakabe et al., 2006). Lignin monomer is an important pathway for the metabolism of phenylpropane. Therefore, their biosynthetic pathway is inseparable from the chloroplast and endoplasmic reticulum. So we speculate that three gene families are related to lignin metabolism. Conserved motifs and gene structure may be closely related to the diversity of gene functions (Cao et al., 2018b). We found that the gene structure of the same family members is very similar to the conserved motifs, indicating that their functions are similar, such as FvSAMS5 and PmSAMS1. Their conserved motifs and gene structure (only containing introns, with the same length) are the same. We also found that there are some differences in individual members in each family. Perhaps they have special functions that require special conservative motifs. For example, PbSAMS6 has motif17, and FvSAMS2 exists in motif8 and motif20.
Promoters coordinate to regulate gene expression through cis-acting elements and trans-acting elements (Soliman & Meyer, 2019). The analysis of this study found that a large number of cis-acting elements related to plant hormones, such as CGTCA-motif, TCA-element, ABRE element, and ERE element, are abundant in nine members of pear. These results indicate that these exogenous hormones are widely involved in pear ripening and stress response (Betz, Mccollum & Mayer, 2001). Moreover, we also found some elements involved in biological defense and abiotic stress. For example, TC rich repeats (defense and stress response), MBS (drought-induced), and LTR (low-temperature response), most contain at least one element of MBS or LTR. These results indicate that these members may play an important role in biological defense and abiotic stress. In addition, we only found the light regulatory element (MRE) in PbSAMS3 and speculated that its expression is also related to light response. We also found that the promoter sequences of PbSAHH1, PbSAMS2, PbSAMS5, and PbSAMS6 contained AC elements. Studies have shown that this element can bind to MYB to regulate lignin synthesis (Cao et al., 2016), which suggests that they may be involved in lignin synthesis.
Gene expression patterns can provide important clues for exploring gene functions (Budak & Zhang, 2017; Thomas et al., 2018). Previous studies have shown that CGS affects the expression of methionine (Hacham, Matityahu & Amir, 2013); SAHH is a key enzyme that maintains the methylation potential of cells (Tanaka et al., 1997; Miller et al., 1994); SAMS catalyzes ATP and Met to synthesize SAM, which is the methylated donor for all organisms in the process of methylation. So far, the role of these genes in pear fruit development is still unclear. Stone cells are an important factor affecting the quality of pear fruit, and lignin is the key to the formation of stone cells. Using RNA-seq (No. SRP070620) and qRT-PCR, the expression patterns of nine genes PbCGS/SAHH/SAMS at different developmental stages of pear fruit were compared. Through RNA-seq data analysis, these nine genes are actively involved in the development of pear fruit. Subsequently, the expression levels of PbSAMS1 and PbSAMS3 are similar to the development trend of stone cells in pear fruits. qRT-PCR analysis showed that PbCGS1 was highly expressed in stem, which indicated that it had a great influence on stem development. All genes have different degrees of expression in flowers, stems, leaves, and buds, and they have different degrees of development in these four parts. We also found that PbSAHH1 and PbSAMS1 increased first and then decreased, which is consistent with the development trend of stone cells in pear fruits (Cai et al., 2010; Jin et al., 2013). In Arabidopsis thaliana, it has been reported that the reduction of AtSAMS3 (Shen, Li & Tarczynski, 2002) will lead to the decrease of lignin content in plants, and AtSAMS3 is homologous with PbSAMS1. Combining transcriptome data and qRT-PCR analysis, we speculate PbSAMS1 may be involved in pear fruit lignin synthesis and stone cell development.
Conclusions
This research was conducted in five Rosaceae species (Pyrus bretschneideri, Prunus persica, Prunus mume, Fragaria vesca, and Malus domestica) by using different bioinformatics tools and in vitro experiments. However, six CGS, six SAHH, and 28 SAMS genes were identified. The gene structure and conserved motifs of each family are very similar. Promoter analysis found that they contained a large number of cis-acting elements related to plant hormones, indicating that hormones play an important role in the regulation of expression patterns during plant growth and development. qRT-PCR analysis showed that among the nine PbCGS/SAHH/SAMS genes were showed abundantly significant expression in flowers and stems except for PbCGS1. RNA-seq data and qRT-PCR analysis indicated that PbSAMS1 may be involved in the regulation of pear stone cell formation and lignin biosynthesis. In conclusion, our observations provide a basis for our understanding of the PbCGS/SAHH/SAMS genes in five Rosaceae plants. In addition, this study revealed their role in pear lignin synthesis and provided basic data for the use of molecular biology techniques to regulate pear lignin synthesis and stone cell development.