Tall Pinus luzmariae trees with genes from P. herrerae
- Published
- Accepted
- Received
- Academic Editor
- Tatiana Tatarinova
- Subject Areas
- Agricultural Science, Genetics, Plant Science
- Keywords
- AFLP, Tree breeding, STRUCTURE, NewHybrids, Australes, Interspecific hybrids, Tree species, Monitoring, Random Forest, Adaptive silviculture
- Copyright
- © 2020 Wehenkel et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2020. Tall Pinus luzmariae trees with genes from P. herrerae. PeerJ 8:e8648 https://doi.org/10.7717/peerj.8648
Abstract
Context
Pinus herrerae and P. luzmariae are endemic to western Mexico, where they cover an area of more than 1 million hectares. Pinus herrerae is also cultivated in field trials in South Africa and South America, because of its considerable economic importance as a source of timber and resin. Seed quality, afforestation success and desirable traits may all be influenced by the presence of hybrid trees in seed stands.
Aims
We aimed to determine the degree of hybridization between P. herrerae and P. luzmariae in seed stands of each species located in the Sierra Madre Occidental, Durango, Mexico.
Methods
AFLP molecular markers from samples of 171 trees across five populations were analyzed with STRUCTURE and NewHybrids software to determine the degree of introgressive hybridization. The accuracy of STRUCTURE and NewHybrids in detecting hybrids was quantified using the software Hybridlab 1.0. Morphological analysis of 131 samples from two populations of P. herrerae and two populations of P. luzmariae was also conducted by Random Forest classification. The data were compared by Principal Coordinate Analysis (PCoA) in GenAlex 6.501.
Results
Hybridization between Pinus herrerae and P. luzmariae was observed in all seed stands under study and resulted in enhancement of desirable silvicultural traits in the latter species. In P. luzmariae, only about 16% molecularly detected hybrids correspond to those identified on a morphological basis. However, the morphology of P. herrerae is not consistent with the molecularly identified hybrids from one population and is only consistent with 3.3 of those from the other population.
Conclusions
This is the first report of hybrid vigour (heterosis) in Mexican pines. Information about hybridization and introgression is essential for developing effective future breeding programs, successful establishment of plantations and management of natural forest stands. Understanding how natural hybridization may influence the evolution and adaptation of pines to climate change is a cornerstone to sustainable forest management including adaptive silviculture.
Introduction
Hybridization represents an important evolutionary force that can introduce much more new genetic material than is created by mutation events (Anderson, 1949; Wright, 1964). It can also act as an additional, perhaps more abundant, source of adaptive genetic variation than mutation (Grant & Grant, 1994), by allowing gene flow and recombination (Abbott et al., 2013; Hipp, 2018). Furthermore, hybridization is one of the key sources of species formation and diversity, and many species may have originated by this route (Linder & Risenberg, 2004; Blanckaert & Bank, 2018), perhaps even as much as between 30% and 80% of all species (Wendel, McD & Rettig, 1991). On the other hand, increasing rates of hybridization may also lead to the extinction of unique populations or species because of unsuccessful reproductive efforts or introgression with a more common species (Rhymer & Simberloff, 1996; Blanckaert & Bank, 2018). In times of rapid ongoing climate change, hybridization may thus contribute to further extinctions, sometimes weakening reproductive isolation among species (Owens & Samuk, 2019) as well as supporting the development of novel segregating genotypes that will speed up adaptation to changes in climate (Chunco, 2014; Menon et al., 2019). Knowledge of hybridization has therefore deep practical reasons. Besides the adaptation issues, the presence of hybrid trees in seed stands “contaminates” the species’ gene pool and thus may influence seed quality and afforestation success (Arnold & Hodges, 1995; Rieseberg & Carney, 1998).
The process of hybridization incorporates alleles from one species into the gene pool of another (Harrison, 1993). Interactions between the environment and genetic structure can thus lead to segregation of a novel taxon from parental types. Depending on the degree of differentiation, hybrid offspring of two or more plants of different taxa are sometimes identified as species, subspecies or variants (Futuyma, 1998; Tovar-Sánchez & Oyama, 2004).
Hybrids often display post-mating reproductive difficulties relative to their ancestors. These difficulties include hybrid weakness, sterility and fitness breakdown (Rieseberg & Carney, 1998). However, hybrids are not necessarily uniformly unfit. On the contrary, some genotypic classes may be equally fit or even fitter than the parental taxa (Arnold & Hodges, 1995; Mabaso, Ham & Nel, 2019). The first hybrid generation (F 1) tends to exceed the parental generation in vegetative vigour or robustness, in a condition also known as heterosis. However, early hybrid generations such as F 2 and F 3 are often less vigorous and fertile than their parents due to the break-up of adaptive gene arrangements (Rieseberg & Carney, 1998).
Studies involving hybridization are often based on morphological traits. However, the phenotypic expression of characters of one taxon in another does not necessarily indicate hybridization. Similar characters may occur in species because of phenotypic plasticity, convergent evolution or simply because of a common ancestry, as Linder et al. (1998) observed in wild sunflower. Furthermore, morphological characters yield limited information when the parents and their hybrids are affected by environmental factors such as disease or drought stress, generating a wide range of phenotypic variability. This problem is increased by subsequent backcrossing of the hybrids to either parent species, resulting in morphological characters that become more similar to those of the backcrossed parent species (Chen et al., 2004).
Use of molecular markers to detect interspecific hybridization is more effective than verification by morphological, chemical or cytogenetic analysis, especially as access is available to an almost unlimited number of molecular markers (Rieseberg, Ellstrand & Arnold, 1993; Alexandrov & Karlov, 2018; Jasso-Martínez et al., 2018). Introgressive hybridization in many plant species has been identified by molecular data (Rieseberg, Ellstrand & Arnold, 1993; Arnold, 1997; Delgado et al., 2007; Kaplan & Fehrer, 2007; McVay, Hipp & Manos, 2017). These markers have been useful for diagnosing F 1 and derived hybrid generations, evaluating levels of gene flow among species and reconstructing phylogenetic relationships between hybridizing taxa and their close relatives (Rieseberg, Ellstrand & Arnold, 1993).
Amplified Fragment Length Polymorphism (AFLP) markers have been successfully used to detect introgressive hybridization in plants (Guo et al., 2005; Shasany et al., 2005; Koerber, Anderson & Seekamp, 2013), specifically in pines (Xu, Tauer & Nelson, 2008; Stewart et al., 2010; Vasilyeva & Semerikov, 2014; Ávila Flores et al., 2016). AFLP markers include a more or less large number of polymorphic, di-allelic loci and can be developed relatively easily and at a relatively low cost, even for species about which no prior genetic information is available (Mueller & Wolfenbarger, 1999; Hardy et al., 2003; Paun & Schönswetter, 2012). Possible disadvantages of the AFLP technique such as compiling standardized patterns in a database for interlaboratory use and future reference can be avoided by using specific procedures as recommended by Savelkoul et al. (1999). However, AFLP as dominant marker does not allow identification of homologous alleles and thus scoring of homozygote and heterozygote states (Mueller & Wolfenbarger, 1999).
Interspecific hybridization is very common in natural stands of the genus Pinus (Critchfield, 1967; Quijada et al., 1997; Conkle & Critchfield, 1988; López-Upton et al., 2001; Delgado et al., 2007; Ávila Flores et al., 2016; Stacy et al., 2017; Vasilyeva & Goroshkevich, 2018; Mo et al., 2019), because of very weak reproductive barriers between pine species (Little & Righter, 1965; Garrett, 1979; Dungey, 2001); this could be generalized across conifers with similar divergence history (Menon et al., 2018). Interspecific F 1 hybrids in this genus are highly viable and fertile (Critchfield, 1975), which complicates taxonomic classification (Martínez, 1948; Lanner, 1974). Genetic diversity is often high in Pinus because of the usually large populations, cross-fertilization, high mutation rates and long-distance dispersion of pollen and sometimes seeds (Gernandt et al., 2011), as well as interspecific hybridization and introgression (Critchfield, 1967; Critchfield, 1975; Quijada et al., 1997; Conkle & Critchfield, 1988; Ledig, 1998; López-Upton et al., 2001; Ávila Flores et al., 2016). In addition, understanding the phylogenetic relationships between closely related species of pines is also difficult due to retention of ancestral alleles (Delgado et al., 2007; Hernández-León et al., 2013; Ortiz-Martínez & Gernandt, 2016). Moreover, North American hard pines in the subsection Australes share plastid DNA lineages due to introgressive hybridization or incomplete lineage sorting (Ortiz-Martínez & Gernandt, 2016).
Pinus herrerae Martínez and Pinus luzmariae Pérez de la Rosa belong to the subsection Australes, a monophyletic group including 29 pine species (Gernandt et al., 2005; Hernández-León et al., 2013). Herrera’s pine (P. herrerae), previously known as Pinus teocote var. herrerae (Martínez) Silba, is endemic to western Mexico, where it covers an area of about 1 million hectares (1 M ha) (Comisión Nacional Forestal, 2009) in mountain ranges between 16° and 28°N, at elevations ranging from 1,100 m to 2,800 m (Dvorak et al., 2007; Wehenkel et al., 2015). The species is used to produce construction timber and resin (Martínez, 1948). It is also cultivated as an exotic in field trials in South Africa and South America because of its typically very tall, straight trunk (Dvorak et al., 2007). Pinus luzmariae (three-needled egg-cone pine), previously known as Pinus oocarpa var. trifoliata Martínez, was first recognized as a separate species by Pérez de la Rosa (1998). This small to medium-sized tree species is endemic to Mexico and it has been reported as covering an area of about 200,000 ha (Comisión Nacional Forestal, 2009). However, its distribution is not clear because it has been included in the very wide range of P. oocarpa Schiede ex Schltdl. The two largest populations are documented in the southern Sierra Madre Occidental covering about 1,000 hectares in south Durango and about 600 ha in northern Jalisco, respectively. Although no uses have been documented for Pinus luzmariae, it may be used as a source of timber, in the same way as P. oocarpa. The number of mature individuals of this species in its natural habitat is decreasing (Pérez de la Rosa & Farjon, 2013).
Both pine species grow in the Madrean-tropical subregion of the Sierra Madre Occidental, at lower elevations (< 2,400 m). Pinus herrerae often is dominating in subhumid areas whereas P. luzmariae occupies sites with poor soils, although sometimes they grow together (González-Elizondo et al., 2012; González-Elizondo et al., 2013). The ecological niches of these two species are clearly defined by soil pH and climate in the State of Durango (Mexico) (Wehenkel et al., 2015).
Population genetics studies of P. herrerae are scarce (see Wehenkel et al., 2015) and of P. luzmariae non-existent. Hybrids between these two species have not yet been reported so far. The aim of the present study was therefore to use AFLP molecular markers and morphological traits to determine the degree of hybridization between P. herrerae and P. luzmariae in seed stands of each species located in the Sierra Madre Occidental mountain system, Durango, Mexico. Although P. herrerae and P. luzmariae are morphologically very different (Perry, 1991; Farjon & Styles, 1997; García-Arévalo & González-Elizondo, 2003; Pérez de la Rosa & Vargas Amado, 2009), they are genetically closely related and can thus, theoretically, easily hybridize with each other (Dvorak et al., 2000; Ortiz-Martínez & Gernandt, 2016; Gernandt et al., 2018). In addition, we tested the possible P. luzmariae hybrid individuals for clues of possible hybrid vigour (heterosis). We aimed to unravel introgressive hybridization between P. herrerae and P. luzmariae, under the assumption that effective pollen flow has occurred between the two species.
Material and methods
Study sites
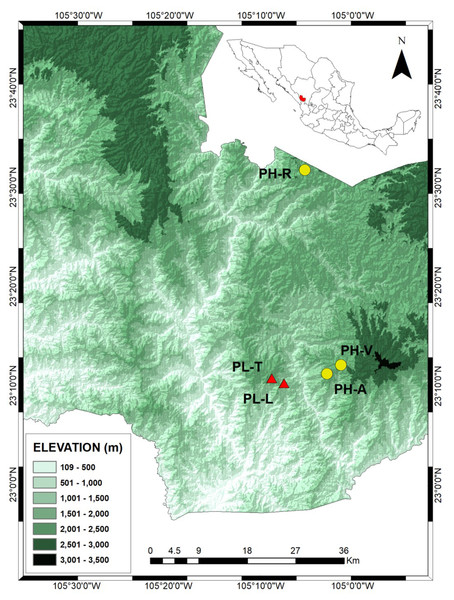
Samples were obtained from trees grown in three Pinus herrerae (PH) and two P. luzmariae (PL) seed stands located in the Sierra Madre Occidental, state of Durango (NW Mexico) (collection permit SEMARNAT SGPA/DGVS/003644/18). The three P. herrerae seed stands were (1) Ranchito (PH-R), (2) Manchón del Abies (PH-A) and (3) Ventana (PH-V). The P. luzmariae stands were (4) Laguna (PL-L) and (5) Tacuache (PL-T). All seed stands are uneven-aged and located in natural populations (Table 1).
| Abbreviatedstand name | Property | Seed stand | Municipality | Latitude | Longitude | Elevation |
|---|---|---|---|---|---|---|
| (N) | (W) | (m) | ||||
| PH-R | Comunidad Milpillas | Ranchito | Pueblo Nuevo | 23° 31′46.8″ | 105° 05′11.3′ | 2,511 |
| PH-A | Comunidad Lajas | Manchon del Abies | Pueblo Nuevo | 23° 11′15.1″ | 105° 02′45.5′ | 2,318 |
| PH-V | Comunidad Lajas | Ventana | Pueblo Nuevo | 23° 12′08.3″ | 105° 01′13.7″ | 2,396 |
| PL-L | Comunidad Lajas | Laguna | Pueblo Nuevo | 23° 10′18.4″ | 105° 07′25.4′ | 1,960 |
| PL-T | Comunidad Lajas | Tacuache | Pueblo Nuevo | 23° 10′47.8″ | 105° 08′46.0″ | 2,140 |
The three PH seed stands grow on slightly acidic soil (pH 5.2 ± 0.4 (standard deviation [SD]), with H+representing 27.4 ± 6.2 SD of total exchangeable cations) (Wehenkel et al., 2015). The Julian date of the last frost date in spring (Sday) was 118 (equivalent to April 28) ±13 days SD. The elevation ranges between 2,318 and 2,511 m above sea level in the study area, with annual rainfall between 1,046 and 1,116 mm. The mean temperature varies from about 11 to 13 °C. The PL stands are also situated on slightly acidic soils with pH 5.0 ± 0.4 SD and H+ representing 29.3 ± 5.6% SD of total exchangeable cations, although at lower elevations with an earlier Sday and higher temperatures. Their elevation varies from 1,960 to 2,140 m above sea level, Sday is 77 (equivalent to March 18 ± 13 days SD, with annual rainfall of between 1,107 and 1,139 mm. The mean temperature ranges between 14 and 16 °C.
The PH-R and PH-A stands are separated from PL-L and PL-T by a deep (1,400 m) canyon and by a distance of 8.1–11 km (Fig. 1). The three PH stands include typical specimens of P. herrerae, i.e., tall trees, of height up to 40 m. However, both populations of P. luzmariae under study showed uncommon increased fitness relative to other populations of the same species (e.g., Pérez de la Rosa, 1998; García-Arévalo & González-Elizondo, 2003), as they are taller (see more in ‘Discussion’).
Figure 1: Locations of the Pinus herrerae (yellow circles) and Pinus luzmariae stands (red triangles) in the State of Durango, Northwest Mexico.
Locations of the Pinus herrerae (Pinus teocote var. herrerae) (yellow circles) and Pinus luzmariae stands (red triangles) in the State of Durango, Northwest Mexico. The P. herrerae seed stands were 1) Ranchito (PH-R), Manchon del Abies (PH-A) and Ventana (PH-V). The P. luzmariae stands were Laguna (PL-L) and Tacuache (PL-T).Fluorescence-based semi-automated AFLP analysis
Needles were collected from a total of 171 adult, dominant and superior putative phenotypes, i.e., plus trees, according to previously described selection criteria (Wehenkel et al., 2015), of both P. herrerae and P. luzmariae (33–35 per stand). Dendrometric variables were also recorded in all seed stands, including coordinates, height (H) and diameter at breast height (DBH) of each sampled tree. The samples were placed in individual tubes with a few drops of ethanol and stored at −10 °C until DNA extraction.
DNA was extracted using the QIAGEN DNeasy96 plant kit, according to the steps described in the product manual. DNA fingerprints were obtained by amplified fragment length polymorphism (AFLP), according to a modification of the protocol of Vos et al. (1995), outlined by Ávila Flores et al. (2016). The restriction enzymes used were EcoRI (selective primer: 5′-GACTGCGTACCAATTCNNN-3′) and MseI (selective primer: 5′-GATGAGTCCTGAGTAANNN-3′). The primer combination E01/M03 (EcoRI-A/MseI-G) was used in the pre-AFLP amplification.
Selective amplification was carried out with the fluorescent-labelled (FAM) primer pair E35 (EcoRI-ACA-3) and M63+C (MseI-GAAC). All PCR reactions were carried out in a Peltier Thermal Cycler (MJ Research, Waltham, Massachusetts, USA). The amplified restriction products were electrophoretically separated in a Genetic Analyzer (ABI 3100 16 capillaries), with a GeneScan 500 ROX internal size standard (Applied Biosystems, Foster City, California, USA). The size of the AFLP fragments was resolved with the GeneScan® 3.7 and Genotyper® 3.7 software packages (Applied Biosystems, Foster City, California, USA).
The amplified restriction products were scored automatically. Only high quality fragments above the signal threshold of 50 (minimum peak height) (according to ABI manual) and with a maximum peak width of 1.0, minimum fragment size of 75 base pairs (bp), maximum fragment size of 450 bp and tolerance +/- bp of 0.4 were considered. Two fragments were only considered when the peak-peak distance between the two signals was at least 0.5 bp. The quality and reproducibility of the analysis were verified by inclusion of reference samples in each plate and independent repetition (replicate PCRs) of analysis at least 16 samples (i.e., a minimum of 16 randomly chosen individuals from each plate). In all replicates, the AFLP pattern was the same as in the first analysis (Simental-Rodríguez et al., 2014; Ávila Flores et al., 2016).
Two binary AFLP matrices were generated from the presence (code 1) or absence (code 0) at probable band positions (Table S1). The bands detected represented the presence of a dominant genetic variant (plus phenotype) with unknown mode of inheritance of this band position (detected fragment length) (Vuylsteke et al., 1999; Krauss, 2000). The absence of a band indicated the presence of only recessive genetic (allelic) variants at the given position (locus). To minimize the rate of size homoplasy (Vekemans & Hardy, 2004; Caballero, Quesada & Rolán-Alvarez, 2008) and technical artefacts (Krauss, 2000), only the polymorphic loci (fragment lengths) with frequencies of occurrence of between 5 and 95% were selected for study (SanCristobal et al., 2006).
Defining pure individuals and molecular identification of hybrids
The trees PH-V4, PH-V49, PH-V52, PH-V64 and PH-V127, and PL-T28, PL-T31, PL-T37, PL-T103, PL-T130 were defined as individuals of “pure” Pinus herrerae (PH) and P. luzmariae (PL), respectively, (hereinafter called pure individuals or pure trees) identified by their genetic affiliation probability and by their morphological traits (see details below).
When PH or PL stands include common hybrid trees, they should possess a genome that is a combination of alleles derived from trees belonging to both species. These hybrids can be detected by genetic data obtained from molecular marker analysis (Xu, Tauer & Nelson, 2008; Ávila Flores et al., 2016).
The resulting AFLP loci from the 171 tree samples were used to determine the degree of introgressive hybridization between PH and PL in the analysis, conducted with STRUCTURE version 2.1 (Pritchard, Stephens & Donnelly, 2000; Falush, Stephens & Pritchard, 2007) and NewHybrids version 1.1 Beta 3 software (Anderson & Thompson, 2002). Both software programs have been used to identify putative hybrids in Pinus with dominant markers such as AFLP (Xu, Tauer & Nelson, 2008; Ávila Flores et al., 2016). The systematic Bayesian clustering approach applying Markov Chain Monte Carlo (MCMC) estimation, as implemented in STRUCTURE was used to test the affiliation of individuals to each species. The MCMC process started by randomly assigning individuals to a pre-determined population (group or species) number (K) (here K = 2, Fig. 2). Repeated many times in the burn-in process (burn-in period of 10,000 cycles), comprising 100,000 iterations, variant (allele) frequencies were estimated in each population and individuals re-assigned using those frequency estimates. In the course of the process, the convergence progressed toward reliable membership probabilities of individuals to a population (or species) (Porras-Hurtado et al., 2013).
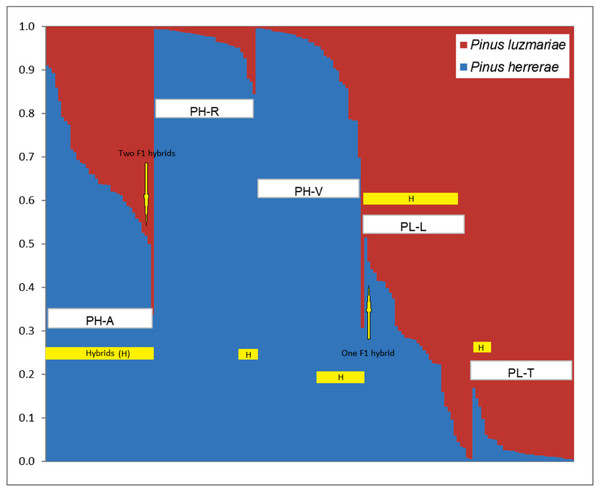
Figure 2: Identification of two populations (K = 2) based on 348 AFLP from three Pinus herrerae seed stands (PH) (Pop 1 = blue) and two Pinus luzmariae seed stands (PL) (Pop 2 = orange) using STRUCTURE.
Identification of two populations (K = 2) based on AFLP data from three Pinus herrerae seed stands (PH) (Pop 1 = blue) and two Pinus luzmariae seed stands (PL) (Pop 2 = red) (171 individuals in total), with Structure, version 2.1 software. PH-A with 35 hybrids, PH-R with six hybrids, PH-V with 14 hybrids, PL-L with 30 hybrids and PL-T with seven hybrids; PH-A, Manchon del Abies; PH-R, Ranchito; PH-V, Ventana; PL-L, Laguna and PL-T, Tacuache.If the probability of PH (or PL) affiliation of a putative PH (or PL) tree was less than 95% according to STRUCTURE, then this individual was recorded as a candidate hybrid. The affiliation probability was measured by the proportion of the dominant STRUCTURE populations in the studied stands (Table S2). Individuals were identified as first-generation (F 1) hybrids when the probability of PH affiliation with a PL tree was in the range 48–52% (Xu, Tauer & Nelson, 2008; Ávila Flores et al., 2016).
Use of the Markov chain Monte Carlo (MCMC) methodology and 100,000 sweeps after BurnIn (10,000 cycles), the NewHybrids 1.1 software (Anderson & Thompson, 2002) is suitable for the situation studied here, where only two diploid species appear to be hybridizing. By applying this software, Anderson (2008) showed that just ten AFLP were adequate to accurately separate parental and F 1 genotypes from later generation hybrid classes. A sample of M individuals, putative pure individuals as well as hybrid individuals, is obtained and genotyped at the L loci of codominant and dominant genetic markers. This software contemplates six genotype classifications (pure species 1, pure species 2, F 1 hybrids, F 2 hybrids, and the first backcross generation to pure species 1 or pure species 2) and estimates the probability that each individual belongs to the different classes (Anderson & Thompson, 2002; Anderson, 2008; Xu, Tauer & Nelson, 2008) (Table S3). A tree was assigned to one of the hybrid classes with a posterior probability of at least 95%.
To visualize individual and species differences, Principal Coordinate Analysis (PCoA) was also performed using the binary AFLP data matrix produced, Nei‘s Genetic Distance (Nei, 1972; Nei, 1978) and GenAlex 6.501 software (Peakall & Smouse, 2012). The PCoA diagrams were elaborated with the first, second and third coordinate.
The accuracy of the software STRUCTURE and NewHybrids (burn-in period of 10,000 cycles, 100,000 iterations) in detecting hybrids was quantified using the computer program Hybridlab 1.0 (Nielsen, Bach & Kotlicki, 2006). However, this software simulates intraspecific hybrids from population samples of co-dominant nuclear genetic markers, whereas the AFLP-technique can detect only dominant genetic markers. Here, the accuracy corresponds with the number of correctly identified individuals for a hybrid generation over the actual number of individuals assigned to that generation (Marie, Bernatchez & Garant, 2011).
Assuming that the fixed band differences between PH and PL were homozygous (expected for fixed polymorphisms), a subset of 11 diagnostic AFLP loci of the five pure PH and PL trees distinguishing the two parental species (100% of one reference parental species had the band whereas 0% of the other parental species did not) were used to simulate three intraspecific hybrid generations (F 1 (N = 50) and F 2, F 1PL, F 1PH, F 2PL, F 2PH, F 1PL-PL and F 1PH-PH backcrosses; N = 125 for each). The majority of the used AFLP loci did not show fixed band differences between PH and PL. Consequently, it was not possible to reliably identify the heterozygote or homozygote state by means of the AFLP bands, as found also by LaRue, Grimm & Thum (2013). Nevertheless, these three simulated intraspecific hybrid generations were also created when using all polymorphic AFLP loci assuming that the AFLP band was always the dominant homozygote and the recessive variant the recessive homozygote. Finally, we performed STRUCTURE and NewHybrids analyses to estimate their accuracy using the two simulation datasets.
Morphological detection of hybrids
To test the results of the AFLP analysis, morphological analysis was conducted on samples from the same trees in populations PH-A, PH-V, PL-L and PL-T sampled for the AFLP analysis. At least 31 individual trees were analysed for cone traits and 11 for needle traits per species. Samples of branchlets, needles and cones were collected for taxonomic determination and morphometric examination, and voucher specimens were deposited in the CIIDIR herbarium (acronym according to Thiers, 2019), the collection of the Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional of the Instituto Politécnico Nacional (Tables S4 and S5). Morphological characters were selected from those used by Martínez (1948) and Pérez de la Rosa (1998) in their descriptions of P. herrerae and P. luzmariae, respectively, as well as those used by García-Arévalo & González-Elizondo (2003) to distinguish these two species in the study area, considering sheath, needle and cone characters (Table 2, Tables S6 and S7, Fig. S1). Characters that do not possess discrete or different states between P. herrerae and P. luzmariae according to these authors were excluded from the analysis as they have no informative value for this study, e.g., persistence of fascicle sheaths (persistent in both), and cone peduncle (peduncle present, oblique and about the same diameter in both species). Some of the characters were measured at ×40 with the aid of a Carl Zeiss Dicovery.V8 stereo microscope. Individual and species differences were pictured by PCoA using the distance of Huff, Peakall & Smouse (1993) and GenAlex 6.501.
| Morphological traits | Pinus herrerae | Pinus luzmariae | ||||
|---|---|---|---|---|---|---|
| max | mean | min | max | mean | min | |
| Cone shape (ovoid (1) vs. widely ovoid (2)) | 1 | 1 | 1 | 2 | 2 | 2 |
| Cone width (cm) | 3.5 | 3.1 | 2.8 | 5.2 | 4.8 | 4.1 |
| Cone scale position (ascendant (1) vs. divergent (3)) | 1 | 1 | 1 | 2 | 2 | 2 |
| Cone scale length (cm) | 1.5 | 1.3 | 1.1 | 1.9 | 1.7 | 1.7 |
| Cone scale width (cm) | 0.8 | 0.7 | 0.6 | 1.1 | 0.9 | 0.8 |
| Apophysis width (mm) | 5 | 4.6 | 4 | 8 | 6.8 | 6 |
| Keel (inconspicuos (0) vs. prominent (1)) | 1 | 0.4 | 0 | 0 | 0 | 0 |
| Leaf sheath length (cm) | 1.3 | 1.1 | 0.9 | 2.0 | 1.8 | 1.5 |
| Leaf sheath diameter (mm) | 1.4 | 1.2 | 1.0 | 1.9 | 1.8 | 1.7 |
| Needle number | 3.0 | 3.0 | 3.0 | 3.5 | 3.2 | 3.0 |
| Needle length (cm) | 15.9 | 13.4 | 11.3 | 28.8 | 24.4 | 21.5 |
| Needle width (mm) | 1.0 | 0.8 | 0.7 | 1.3 | 1.2 | 1.1 |
| Needle thickness (mm) | 0.3 | 0.2 | 0.1 | 0.6 | 0.5 | 0.4 |
| Stomata rows (dorsal face) | 9.0 | 7.1 | 5.3 | 10.0 | 9.7 | 9.0 |
| Stomata rows (ventral faces) | 3.3 | 3.1 | 3.0 | 6.0 | 5.0 | 4.0 |
In order to detect PH and PL hybrids identified by morphological traits, we first identified five pure PH (PH-V 4, 49, 52, 64, 127) and five pure PL (PL-T 28, 31, 37, 103, 130) trees, applying a genetic affiliation probability larger than 0.99 according to STRUCTURE and NewHybids and clearly assignable by morphological traits. Since not every independent morphological trait was normally distributed and continuous, the species assignation of each tree and “morphological” hybrids was established by Random Forest (Breiman, 2001) using the caret package and function “train” (Kuhn, 2008; Williams et al., 2018) available in the free statistical application R 3.5.2 (R Development Core Team, 2018). For this purpose, the PH trees were labeled with the value “1” (corresponding to the presence of PH) and the PL trees were labelled with “0” (corresponding to the absence of PH) in this presence–absence classification model. The model for both the cone traits and needle traits, respectively, was fit using a 5-fold cross-validation repeated 10 times (i.e., using 80% of the dataset as training set and the remaining 20% as testing set). Random Forest is a nonparametric tree-based classifier and hence does not require variable scaling and can successfully handle non-normality (Strobl, Malley & Tutz, 2009) as well as categorical and confounding variables (Dormann et al., 2013). Caret package (short for Classification And REgression Training) is a complete framework for building machine learning models (http://caret.r-forge.r-project.org).
Using all morphological traits listed in Table 2, a tree was classified as a possible PH (or PL) tree if it was assigned to each of those species with the highest assignment probability (i.e., > 50%). If the posterior probability of PH (or PL) affiliation of a possible PH (or PL) tree was less than 95% according to Random Forest, then, that tree was considered as a putative PH (or PL) hybrid.
The predictive ability of the Random Forest model was evaluated using the True Skill Statistic (TSS; Allouche, Tsoar & Kadmon, 2006) using the caret package in R (for more details see Escobar-Flores et al., 2018). TSS (also known as the Hanssen–Kuipers discriminant) is an appropriate alternative to Area Under a Receiver Operating Characteristic (ROC) Curve (AUC; Fawcett, 2006) in cases where model predictions are formulated as presence–absence models and an improvement to the widely used kappa. TSS not only accounts for both omission and commission errors, but is not affected by the sample size of each class. The TSS is defined as sensitivity + specificity –1, and ranges from −1 to +1, where +1 indicates a perfect classification model and values of zero or less indicate performance no better than random (Allouche, Tsoar & Kadmon, 2006; Tatler, Cassey & Prowse, 2018).
Results
Molecular detection of hybrids
The AFLP primer combination yielded 348 polymorphic bands of 75-450 base pairs across all individual specimens of Pinus herrerae (PH) and Pinus luzmariae (PL). PH yielded 338 and PL 316 polymorphic bands. Both species shared 304 AFLP fragments (87.4% of polymorphic bands detected).
Figure 2 shows the percentage of hybridization obtained with the STRUCTURE software, for K = 2, the three PH seed stands have a dominant genetic variant (blue) and the two PL seed stands contain another dominant genetic variant (red). Based on a 5% probability of introgression of gene content, 92 (53.8%) putative hybrids between PH and PL were found in all the seed stands analysed. Thus, 18% of the individuals in the Ranchito PH stand (PH-R) were putative hybrids; all PH individuals in the Manchón del Abies PH stand (PH-A) displayed genetic introgression from PL, and 14 of the 35 individuals in the Ventana PH stand (PH-V) were putative hybrids (40.0%). Regarding P. luzmariae, 30 (85.7%) putative hybrids were detected in the Laguna stand (PL-L), whereas seven individuals (21.2%) in the Tacuache P. luzmariae stand (PL-T) displayed introgression with P. herrerae. Five trees were first-generation hybrids (F 1), as indicated by introgression of between 48 and 52%: three trees in the PH-A stand and another two in the PL-L stand (Fig. 2, Table 3).
| STRUCTURE 2.1 | NewHybrids 1.1 | |||||
|---|---|---|---|---|---|---|
| Seed stand | Sample number | Hybrid number | F1 hybrid number | Hybrid number | F1 hybrid number | Backcrossingnumber |
| PH-A | 35 | 35 | 2 | 9 | 0 | 0 |
| PH-R | 33 | 6 | 0 | 0 | 0 | 0 |
| PH-V | 35 | 14 | 0 | 2 | 0 | 1 |
| PL-L | 35 | 30 | 1 | 33 | 0 | 14 |
| PL-T | 33 | 7 | 0 | 21 | 0 | 4 |
| Total | 92 | 3 | 65 | 0 | 19 | |
Notes:
- PH
-
Pinus herrerae
- PL
-
Pinus luzmariae seed stands
- PH-A
-
Manchon del Abies
- PH-R
-
Ranchito
- PH-V
-
Ventana
- PL-L
-
Laguna
- PL-T
-
Tacuache
NewHybrids software clearly identified 65 (38%) putative hybrids between PH and PL (Table 3). No putative hybrids were found in the Ranchito PH stand (PH-R). In total, 25.7% of the individuals in the PH-A were putative hybrids, and two of the 35 individuals in the Ventana PH stand (PH-V) were putative hybrids (5.7%). A large majority (94.2%) of the individuals in the Laguna P. luzmariae stand (PL-L) were identified as putative hybrids, whereas 64% of the individuals in the Tacuache P. luzmariae seed stand (PL-T) displayed genetic introgression with P. herrerae. Only one tree, located in the PL-L stand, was detected as a first-generation hybrid (F 1) (Table 3).
The accuracy test showed that the method NewHybrids (NH) correctly assigned at least 88% of naturally occurring “pure” PH and PL individuals using 11 diagnostic AFLP and all 348 AFLP. STRUCTURE (STR) presented much more errors, especially with the 11 diagnostic AFLP. Using the 11 diagnostic loci, for both methods detections of 1st and 2nd (F 2, F 1PL and F 1PH backcrosses) generation hybrids were 100% and nearly 100%, for 3rd generation hybrids (F 2PL, F 2PH, F 1PL-PL and F 1PH-PH backcrosses) this decreased further to 0.59% in STR and 0.49% in NH (posterior probability (PP) of at least 95%). Using the all 348 AFLP, simulations demonstrated lower rates of inaccurately than the test with 11 diagnostic loci. STR and NH correctly assigned 100% of simulated F 1 hybrids and nearly 100% of 2nd generation hybrids. Moreover, NH correctly assigned 100% of simulated 3rd generation hybrids, too. Using STR, a lower percentage of 3rd generation hybrids were correctly assigned (50%) (PP of at least 95%) (Table 4).
| Class | 11 AFLP loci | 348 AFLP loci | ||
|---|---|---|---|---|
| STR | NH | STR | NH | |
| 1st gen | 1 | 1 | 1 | 1 |
| 2nd gen | 1 | 0.99 | 1 | 0.99 |
| 3rd gen | 0.59 | 0.49 | 0.50 | 1.00 |
| Hybrid Avg. | 0.86 | 0.83 | 0.83 | 1.00 |
| PH | 0.13 | 0.88 | 0.80 | 0.88 |
| PL | 0.13 | 0.88 | 0.78 | 0.95 |
The results of the Principal Coordinates Analysis (PCoA) comparing genetic differences between individual specimens of PH and PL are shown in Fig. S2. At the individual level, the first three coordinates in PCoA explained 13.4% of the variability.
Morphological detection of hybrids
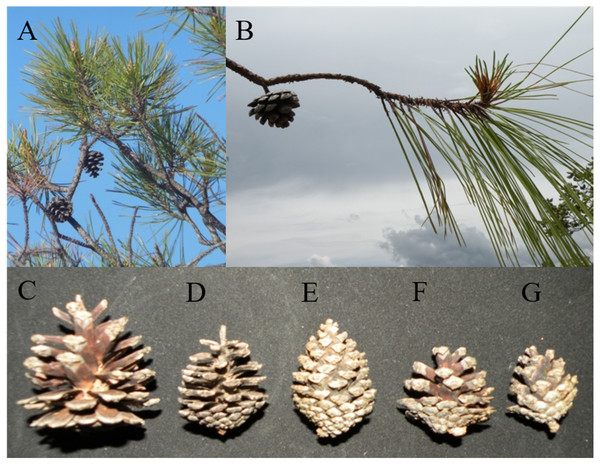
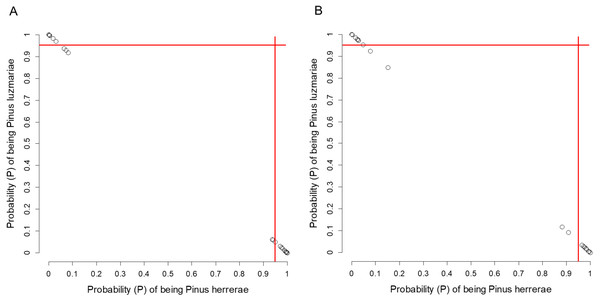
Pinus herrerae and P. luzmariae are morphologically distinct and easily recognized by several needle traits as well as by the width, scale position and scale length of the cone. However, various morphological intermediates between the two species were found (Figs. 3 and 4), including hybrids confirmed by Random Forest (Table 5, Fig. 5) considering seven cone (hybrid proportion of 4.6%) and eight needle traits (4.5%). Every observation was correctly classified (TSS = +1). At the individual level, the first three coordinates in PCoA only explained 36.2% of the variability in seven cone traits, but 61.7% of the variability in eight needle traits (Figs. S3 and S4). Hybrids identified by 15 morphological traits matched only 13.4% of the molecularly detected hybrids, and 5.7% of hybrids were only found by morphological traits.
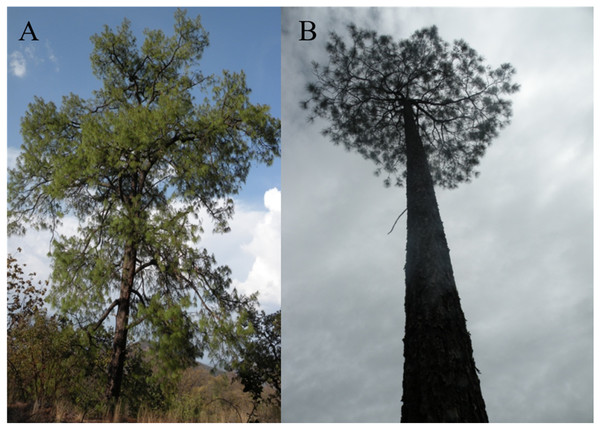
Figure 3: Images from a typical Pinus luzmariae (A) and a Pinus luzmariae hybrid (B).
Images from a typical Pinus luzmariae (Bolaños in Jalisco, Mexico, 2013) (A) and a Pinus luzmariae hybrid (seed stand “Laguna” (PL-L), tree 102, 26 m stem height) (B).Figure 4: Typical branches, needles and cones of Pinus herrerae and Pinus luzmariae and variation of P. luzmariae cones.
Typical branches, needles and cones of Pinus herrerae (A) and Pinus luzmariae (B) and variation of P. luzmariae cones: typical (C), different hybrid forms (D–G).| Cone traits | Needle traits | |||
|---|---|---|---|---|
| Seed stand | Sample number | Hybrid number | Sample number | Hybridnumber |
| PH-A | 35 | 3(7) | 34 | 0(2) |
| PH-V | 34 | 0(10) | 31 | 2(3) |
| PL-L | 31 | 1(3) | 13 | 0(4) |
| PL-T | 31 | 2(9) | 11 | 2(3) |
| Total | 131 | 6(29) | 89 | 4(12) |
Notes:
- PH
-
Pinus herrerae
- PL
-
Pinus luzmariae seed stands
- PH-A
-
Manchon del Abies
- PH-R
-
Ranchito
- PH-V
-
Ventana
- PL-L
-
Laguna
- PL-T
-
Tacuache
Number in brackets, hybrid number at four of seven cone traits detected and at four of eight needle traits detected.
Figure 5: Posterior probability (P) of being Pinus herrerae and Pinus luzmariae using Random Forest classification.
Posterior probability (P) of being Pinus herrerae (PH) and Pinus luzmariae (PL) that a tree belongs to a particular class (PH or PL) using a Random Forest classification and (A) using seven cone traits, (B) using eight needle traits; True Skill Statistic (Allouche, Tsoar & Kadmon, 2006) = +1. If the P of PH (or PL) affiliation of a possible PH (or PL) tree was less than 0.95 (red lines), then, that tree was considered as a putative PH (or PL) hybrid.Clues of possible hybrid vigour (heterosis) in P. luzmariae
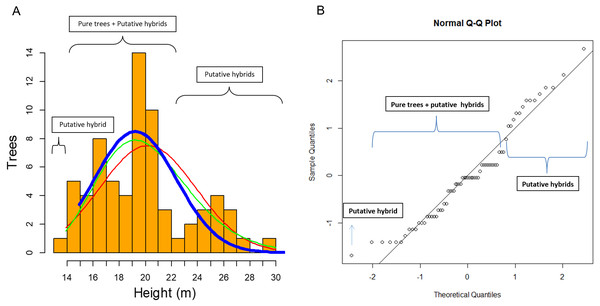
In this study of 69 P. luzmariae (pure and hybrid) trees, the hybrid heights and DBHs were much heterogeneously distributed than the dimensions of the pure trees. The smallest (one tree with 14 m height) and the tallest trees (14 trees with 23–30 m) were hybrids. The pure trees presented a normal distribution (probability) in which the expected proportion of trees higher than 24 m was much lower than the observed frequency of the tallest hybrids (Fig. 6).
Figure 6: Clues of possible hybrid vigour in Pinus luzmariae.
Clues of possible hybrid vigour in Pinus luzmariae: (A) Histogram (tree number) of tree heights (m), red line = normal distribution (probability) of all (69) pure P. luzmariae trees and putative hybrids under study, green line = logarithm normal distribution of all pure P. luzmariae trees and putative hybrids under study, blue bold line = normal distribution of the pure P. luzmariae trees under study, (B) normal Q-Q-plot of all (69) pure P. luzmariae trees and putative hybrids under study.Discussion
Species crossability in pines is of great theoretical and practical interest (Lopez et al., 2018; Vasilyeva & Goroshkevich, 2018). Many pine hybrids, including several Mexican species, have been planted in trials across southern Africa in different conditions and climate regimes (Hongwane et al., 2018). Here, we report for the first time about the occurrence of hybrids in Pinus luzmariae, a little known species, introgressed by P. herrerae, revealing taller trees in comparison to all populations previously known for the species (as compared with those described in Pérez de la Rosa, 1998 and García-Arévalo & González-Elizondo, 2003). Populations of the introgressed P. luzmariae include trees 14 to 30 m (vs. 6-12 m in most other populations) (Fig. 6). This can be interpreted as hybrid vigour or heterosis, being the first report for Mexican pines. Other studies have shown that hybrid pines in the country do not differ from the pure trees in relation to vigour or robustness, e.g., Pinus oocarpa × P. pringei (López-Upton et al., 2001) and P. arizonica × P. engelmannii (Ávila Flores et al., 2016). In comparison with other populations of the same species, populations of the introgressed P. luzmariae (PL) display some important characters that are used to select superior forest trees (Kedharnath, 1984), i.e., good growth vigour, superior height, good self-pruning, and straight cylindrical bole. In the two introgressed populations, P. luzmariae formed almost pure, relatively dense stands with a few specimens of P. herrerae (PH) and Pinus devoniana in association, in contrast to the open, mixed stands in which P. luzmariae usually grows.
The high percentage of AFLP fragments (87%) shared by P. herrerae and P. luzmariae resulted in a large proportion of putative hybrids (54% by STRUCTURE (STR) and 38% by NewHybrids (NH)) using a posterior probability of at least 95%. The accuracy test detecting the different hybrid classes showed comparable results to other studies. However, NH detected more of both, accurate hybrids and individuals of pure species, than STR (Table 4). This explains the notable difference in the putative hybrid number found between STR (92 hybrids) and NH (65 hybrids). Therefore, the results presented by NH are probably more precise.
Previous studies have reported that the accuracy of these software can differ greatly depending on the population context (Vähä & Primmer, 2006). Using AFLP and a PP of at least 90%, LaRue, Grimm & Thum (2013) presented 100% accuracy rate of simulated F 1 hybrids, but lower percentages of F 2 and backcrosses (about 91% and 92%, respectively). Cullingham et al. (2011) found a mean power of 74% to detect hybrids using microsatellites and a PP of at least 90% for F 1 hybrids and PP < 90% for other hybrid classes.
The high degree of introgression can be explained by the relatively recent diversification of species in the subsection Australes and the very weak reproductive barriers between them (Little & Righter, 1965; Garrett, 1979; Dvorak et al., 2000; Vargas-Mendoza et al., 2011; Gernandt et al., 2018). Similar weak reproductive barriers and high recent speciation rates have been recorded for madrones (Arbutus spp.) and oaks (Quercus spp.) (González-Elizondo, González-Elizondo & Sørensen, 2012; González-Elizondo, González-Elizondo & Zamudio, 2012; González-Elizondo et al., 2013; Hipp et al., 2019), the other two tree genera that, along with pines, dominate in the temperate forests of the Sierra Madre Occidental in western Mexico, where this study was carried out. Introgressive hybridization, although usually not obvious, may be more important in evolution than those cases in which hybridization is evident (Anderson, 1949; Hipp et al., 2019).
The interspecific gene transfer between the two pine species studied here is also supported by (i) wind pollination, (ii) longevity of individual trees, (iii) overlapping generations, (iv) large effective population sizes, and (v) weak physical barriers caused by sympatric distribution (Ávila Flores et al., 2016). The relatively high diversity and high levels of gene flow in trees (relative to herbs and shrubs) is favoured by their outcrossed mating system and long distance seed dispersal (Petit & Hampe, 2006).
Of the five seed stands studied, PL-L displayed the highest degree of hybridization (94%), confirmed by AFLP as well as cone and needle traits (Tables 3 and 4). According to the PCoA results (Figs. S2, S3 and S4), many PL-L individuals were genetically closely related to P. herrerae individuals. The high phenotypic plasticity and more luxuriant growth found in both populations of P. luzmariae under study (Tables 3 and 5) are a consequence of the hybrid origin. Crossbreeding or heterozygosity promotes variability, as found by Strauss (1987) for heterozygous trees of Pinus attenuata Lemm. derived from crossbreds. Resistance to disease, pathogens or environmental stresses has been a target in tree breeding towards interspecific hybrids. For example, Pinus patula Schltdl. & Cham. has been crossed with P. tecunumanii F.Schwerdtf. ex Eguiluz et J.P.Perry and with P. oocarpa in plantations in South Africa to increase tolerance to a fungal pathogen. The resulting hybrids of these three Mexican pines have a low frost tolerance, so new crosses were made until the finding that P. patula × P. tecunumanii from high elevations has a higher frost tolerance than P. patula × P. tecunumanii from lower elevations (Mabaso, Ham & Nel, 2019).
The high degrees of hybridization have several possible consequences: (i) extinction of one of the PH or PL parental species due to wasted mating effort or genetic swamping; (ii) reinforcement of species boundaries; (iii) creation of a third, hybrid species; (iv) formation of a stable hybrid zone; and (v) partial introgression between the two hybridizing lineages (Chunco, 2014).
The stands of P. luzmariae we studied appear to represent a stable hybrid zone, like the Pinus engelmannii stand reported by Ávila Flores et al. (2016). This conclusion is supported by the fact that the P. luzmariae population displays higher fitness than other populations of the species. Hybrid speciation does not occur in the studied populations as the hybrids are not spatially or ecologically isolated from the parental species, and no novel variants of morphological traits were found (Ungerer et al., 1998). The PH-A displayed the highest degree of hybridization in three PH studied (at least 37.1% consisting of at least 25.4% trees detected by NewHybrids and four extra trees detected by the cone and needle traits) (Tables 3 and 5). PH-A was located next to the two P. luzmariae seed stands (PL-L and PL-T), and it is expected that it intercepts large amounts of P. luzmariae pollen. We can, therefore, conclude that gene flow has occurred in both directions, from PL to PH and vice versa. However, gene flow from PH to PL seems to be more effective as more hybrids were found in the PL stands, both of which are located at lower elevations than the PH stands (Table 1).
Despite the large number of hybrids detected in the studied stands, the frequency of first-generation (F 1) hybrids and backcrossing was low (Table 3), indicating that hybrid crossing was usual in the seed stands. Similar results have recently been reported in species of Salix and for pines in the subsection Ponderosae and (Fogelqvist et al., 2015; Ávila Flores et al., 2016).
Natural hybridization has also been observed in other Mexican pine species (Gernandt et al., 2018). Previous studies of the subsection Australes identified natural hybridization between P. oocarpa × Pinus caribaea and P. oocarpa × Pinus pringlei only by morphological traits (Styles & Stead, 1982; López-Upton et al., 2001). In a study of Mexican pine species of the subsection Ponderosae, Ávila Flores et al. (2016) observed a high degree of introgressive hybridization between P. engelmannii, Pinus arizonica, Pinus cooperi and Pinus durangensis. AFLP markers detected most of the putative hybrids (58%), and only a few were detected by morphological features (15%). Hybridization was not detected by morphological traits in 74% of all hybrids detected by AFLP. Hybrids and backcrossing were also found in Mexican Arbutus species that are common in disturbed areas (González-Elizondo, González-Elizondo & Sørensen, 2012; González-Elizondo, González-Elizondo & Zamudio, 2012). Natural pairwise and triple hybrids have also been detected in numerous Mexican Quercus stands (e.g., Peñaloza Ramírez et al., 2010; Hipp et al., 2019). Natural hybridization between different Populus species and gene flow between cultivated poplars and native poplar populations have been described for European riparian forests and stands (e.g., Van den Broeck et al., 2004; Smulders et al., 2008; Lexer et al., 2005).
Conclusions
Hybridization between Pinus herrerae and P. luzmariae in seed stands in the Sierra Madre Occidental of Mexico has occurred in both directions to different degrees. Estimates of the success of hybrid individuals may be biased in this study by the fact that sampling was conducted in seed stands (in which plus trees predominate). Further research is necessary to increase our understanding of how hybridization may influence silvicultural traits in Mexican pines, as well as their evolution and adaptation to climate change. The successful survival and reproduction of these hybrids over generations will depend on their attributes, their fitness and the environmental factors influencing them (Strauss, 1987), given that hybridization leads to individuals which widely vary depending of the context, location and involved species (Gompert & Buerkle, 2016).
We conclude that both morphological and molecular approaches are essential to confirm the genetic identity of forest reproductive material as PH and PL frequently hybridize in all seed stands under study. Such information is very important for developing effective future breeding programs and successful establishment of plantations (Ávila Flores et al., 2016; Pérez-Luna et al., 2020) as well as for improving planning of the management of natural stands.
Introgressive hybridization in seed stands of Pinus herrerae and Pinus luzmariae generated outstanding plus trees. Because of their tall, straight trunks, hybrids of the largely unknown Pinus luzmariae represent a promising, valuable source of timber for wood industries as well as for reforestation in poor sites. The hybrid trees may be able to be cultivated after evaluation germplasm and vegetative propagation potential and may be suitable for commercial exploitation. However, further research is needed to examine the performance of hybrids and to assess their fertility and growth relative to those of pure species. Finally, monitoring natural hybridization is important in relation to sustainable forest management in Mexico.
Supplemental Information
Raw data of 348 AFLP, STRUCTURE version 2.1 and NewHybrids 1.1 results obtained from 171 trees in three Pinus herrerae (PH) seed stands and two P. luzmariae (PL) , located in the state of Durango (NW Mexico)
Voucher numbers of the Pinus herrarea individuals in the study
Voucher numbers of the Pinus luzmariae individuals in the study
Raw data of seven cone traits obtained from 131 trees in two Pinus herrerae (PH-V, PH-A) seed stands and two P. luzmariae (PL-T, PL-L)
Raw data of eight needle traits obtained from 89 trees in two Pinus herrerae (PH-V, PH-A) seed stands and two P. luzmariae (PL-T, PL-L)
Separation of Pinus herrerae (PH) and Pinus luzmariae seed stands (PL) by the first and second (A) and by the first and third (B) coordinates in Principal Coordinates Analysis using AFLP
PH-A=Manchon del Abies, PH-R=Ranchito, PH-V=Ventana, PL-L=Laguna and PL-T=Tacuache
Separation of Pinus herrerae (PH) and Pinus luzmariae seed stands (PL) by (A) the first and second and by (B) the first and third coordinates in Principal Coordinates Analysis considering seven cone traits
PH-A=Manchon del Abies, PH-V=Ventana, PL-L=Laguna and PL-T=Tacuache
Separation of Pinus herrerae (PH) and Pinus luzmariae (PL) stands by the first and second and by the first and third coordinates in Principal Coordinates Analysis using eight needle traits
PH-A=Manchon del Abies, PH-V=Ventana, PL-L=Laguna and PL-T=Tacuache.