Diverse responses of Symbiodinium types to menthol and DCMU treatment
- Published
- Accepted
- Received
- Academic Editor
- James Reimer
- Subject Areas
- Environmental Impacts, Environmental Sciences, Marine Biology
- Keywords
- Symbiodinium, ROS activity, PSII system, Aposymbiotic coral, Symbiodinium cell depletion
- Copyright
- © 2017 Wang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2017. Diverse responses of Symbiodinium types to menthol and DCMU treatment. PeerJ 5:e3843 https://doi.org/10.7717/peerj.3843
Abstract
To understand the mechanism of photosynthetic inhibition and generation of reactive oxygen species (ROS) in Symbiodinium types under stress, chemicals such as dichlorophenyl dimethylurea (DCMU) are widely used. Moreover, DCMU and recently menthol were used to generate aposymbiotic cnidarian hosts. While the effects of DCMU on Symbiodinium cells have been extensively studied, no studies have shown the mechanism behind menthol-induced coral bleaching. Moreover, no study has compared the effects of DCMU and menthol treatments on photosystem II (PSII) activity and generation of ROS in different Symbiodinium types. In this study, we utilized five freshly isolated Symbiodinium types (S. minutum (B1), S. goreaui (C1), C3, C15, and S. trenchii (D1a)) to compare the effects of DCMU and menthol treatments. Symbiodinium cells were exposed to DCMU and menthol at different concentrations for 4 h. Results showed that values of the 50% inhibitory concentration (IC50) for PSII inhibition were 0.72∼1.96 mM for menthol-treated cells compared to 29∼74 pM for DCMU-treated cells. Diverse responses of Symbiodinium types were displayed in terms of PSII tolerance to menthol (S. minutum > S. trenchii = C15 > C3 = S. goreaui), and also in the response curves. In contrast, responses were not so diverse when the different types were treated with DCMU. Three of five menthol-treated Symbiodinium types showed instant and significant ROS generation when PSII activity was inhibited, compared to no ROS being generated in DCMU-treated Symbiodinium types. Both results indicated that menthol inhibited Symbiodinium PSII activity through Symbiodinium type-dependent mechanisms, which were also distinct from those with DCMU treatment. This study further confirmed that photosynthetic functions Symbiodinium have diverse responses to stress even within the same clade.
Introduction
Symbiodinium spp. are associated with marine invertebrate hosts, including Protista, Porifera, Cnidaria, and Mollusca (Coffroth & Santos, 2005), and play important functional roles in providing photosynthesis-derived carbon and conserving or recycling host nitrogen metabolites (Davy, Allemand & Weis, 2012). To date, nine (A∼I) Symbiodinium clades and numerous subcladal types have been identified with distinguishable genetic identities and physiological characteristics (reviewed in Stat & Gates, 2011). Among these, only clades A, B, C, and D are widely associated with scleractinian corals. Different Symbiodinium types are known to show variations in their photosynthesis functions (Rowan, 2004; Tchernov et al., 2004; Robinson & Warner, 2006; Sampayo et al., 2008; Wang et al., 2012b; Suggett et al., 2015), resulting in different contributions to their symbiotic associations (Stat, Morris & Gates, 2008; Yuyama & Higuchi, 2014; Pernice et al., 2015). Various stress tolerabilities among different Symbiodinium types were also revealed by high antioxidant plasticity (Krueger et al., 2014).
The association between corals and Symbiodinium is highly vulnerable to physical and chemical disturbances. For example, only 1∼2 °C above the summer average under moderate to high irradiance is enough to disrupt the symbiotic relationship and end up with loss of symbionts from coral hosts, resulting in ‘coral bleaching’ (Fitt et al., 2001; Lesser & Farrell, 2004). The mechanism of Symbiodinium depletion (reviewed in Weis, 2008), from cnidarian host cells, is generally attributed to damage by reactive oxygen species (ROS) generated from photoinhibition of Symbiodinium during stress (e.g., high temperature, irradiance, or herbicides, e.g., dichlorophenyl dimethylurea (DCMU) treatment) (Jones et al., 1998; Jones, 2004; Jones, 2005) or stress on the coral host’s metabolism (Jones, 2004). Disruption of Symbiodinium-coral symbiosis can also be achieved by some chemicals such as heavy metals (Jones, 1997 and sunscreen contamination (Danovaro et al., 2008). However, there is no direct evidence to indicate coral bleaching caused by ROS generated by DCMU-treated Symbiodinium (Jones, 2004).
While use of DCMU to generate aposymbiotic cnidarian hosts and the effects of DCMU on Symbiodinium cells have extensively been studied (Murata et al., 2007; Takahashi et al., 2013; Fransolet, Roverty & Plumier, 2014; Aihara, Takahashi & Minagawa, 2016; Parrin & Blackstone, 2017), use of menthol as an efficient bleaching agent has only recently been established. In recent years, menthol was found to successfully induce bleaching in coral and sea anemone hosts (Wang et al., 2012a; Dani et al., 2016; Matthews et al., 2016).
Menthol is a cyclic terpene alcohol which can cause local anesthetic effects in neuronal and skeletal muscles by blocking voltage-operated sodium channels (Haeseler et al., 2002). This effect has led to its use as a marine anesthetic (Moore, 1989; Lauretta et al., 2014). A variety of different membrane receptors are known to respond to menthol stimulation, including transient receptor potential (TRP)M8 that results in an increase in intracellular Ca2+ concentrations and causes a cold sensation in vertebrates (McKemy, Neuhausser & Julius, 2002; Okazawa et al., 2000; Peier et al., 2002; Hans, Wilhelm & Swandulla, 2012). Menthol is also a cytotoxic compound to plant tissues (Muller & Hauge, 1967; Brown, Hegarty & Charlwood, 1987), causing a drastic reduction in the number of intact mitochondria and Golgi bodies in seedling roots (Lorber & Muller, 1976), inhibiting respiration and photosynthesis (Pauly, Douce & Carde, 1981), and decreasing cell membrane permeability (Muller et al., 1969).
Recently, Wang et al. (2012a) demonstrated that menthol can be used to effectively to induce bleaching in the corals Isopora palifera and Stylophora pistillata. Dani et al. (2016) further hypothesized that menthol-induced activation of a host TRP receptor might increase the intracellular calcium concentration. Modulation of calcium homeostasis, which is known to upregulate the autophagic pathway (Smaili et al., 2013), could therefore trigger Symbiodinium depletion through a phagolysosomal process. Whether the PSII breakdown in the endosymbiotic Symbiodinium in corals is directly or indirectly caused by menthol needs to be clarified. Moreover, no studies have compared responses of different Symbiodinium types to both menthol and DCMU, which would investigate the important but under-explored topic of functional diversity among Symbiodinium species.
In this study, we tested the responses of different Symbiodinium types to DCMU and menthol exposure by evaluating the concentration for 50% inhibition of PSII activity and the generation of ROS. Results of this study will provide information on diverse responses among different Symbiodinium types to menthol treatment and is a first step towards understanding mechanisms of Symbiodinium cell depletion caused by menthol treatment.
Materials and Methods
Symbiodinium were isolated from five coral species. Parts of colonies of Acropora humilis, Galaxea fasicularis, Isopora palifera, and Porities lutea corals were collected from reefs within Kenting National Park, Taiwan (21°55′54″N, 120°44′45″E). A sample collection permit (no. 488-100-01) was obtained from the Kenting National Park Authority as part of the long-term environmental monitoring project. Coral colonies were transferred to the laboratory within 3 h in an aerated plastic box, and maintained in an aquarium tank under conditions described in Wang et al. (2011). Corals were acclimatized to laboratory aquarium (Wang et al., 2012a; Wang et al., 2012b) settings (25 °C and 70 µmol photons m−2 s−1 light) for 1 week before initiating experiments. The glass sea anemone, Exaiptasia pulchella (Grajales & Rodríguez, 2014), originally collected from a discharge trench of the Tongkun Fishery Research Institute, Taiwan, had been maintained in a tank (45 × 30 × 30 cm) equipped with illumination and temperature control for more than 1 year.
To prepare freshly isolated Symbiodinium (FIS), coral fragments with 5∼10 cm2 of live tissue and excised tentacles from sea anemones were homogenized and washed with artificial seawater (ASW) prepared from Instant Ocean (Aquarium Systems, France) as described by Wang et al. (2011). The dominant FIS types used in this study were identified as Symbiodinium ITS2 types C1 (S. goreaui), C3, C15, D1a (S. trenchii), and B1 (S. minutum) from S. pistillata, A. humilis, P. lutea, G. fasicularis and E. pulchella, respectively, based on denaturation gradient gel electrophoresis (DGGE) according to Wang et al. (2011).
PSII activities of FIS were determined by the maximum quantum yield (Fv∕Fm), and the minimum (F0) and maximum (Fm) fluorescence levels were measured to calculate the variable fluorescence [Fv, (Fv = Fm − F0)] using a DIVING-PAM fluorometer (Walz, Germany) at a setting of 8 for the measuring light and saturating flash of actinic light.
When determining values of the half-maximal inhibitory concentration (IC50), triplicate samples with 5 ml of FIS (around 106 cells ml−1) of each type showing Fv∕Fm values of >0.6 (a value generally considered normal/healthy, Fitt et al., 2001) were transferred to a 50-ml Falcon tube and centrifuged at 417 × g for 3 min to collect Symbiodinium. The algal pellet was then re-suspended in 5 ml of different concentrations of menthol- or DCMU-ASW and maintained at 25 °C under illumination of around 70 µmol photons m−2 s−1 of photosynthetically active radiation (PAR). Wang et al. (2011) suggested that the PSII activity of FIS begins to fluctuate after incubation for 4 h, so the effects of menthol and DCMU were determined as the 4-h IC50. Fv∕Fm values of FIS in menthol or DCMU were directly determined with the DIVING-PAM fluorometer without dark adaptation at 4 h of incubation, and were converted to PSII inhibition by comparing to values in fresh isolates as below: where (Fv∕Fm)0h and (Fv∕Fm)4h values were respectively measured at 0 and after 4 h of incubation. After plotting PSII inhibition against the logarithm-transformed concentration of menthol (or DCMU), the equation for calculating the IC50 was obtained from the best curve-fit-model provided in SigmaPlot 10.0 software.
ROS generated in menthol- or DCMU-treated FIS were determined with a 2′, 7′-dichlorofluorescin diacetate (H2DCFDA) probe. FIS (around 106 cells ml−1, cells counted as in Wang et al., 2012b) of each Symbiodinium type was incubated in ASW containing 1.73 mM menthol (for S. minutum, this was 2.43 mM) or 130 pM DCMU as the positive control, for 4 h in which the PSII activity of FIS would be completely inhibited. The mentioned concentration used to examine ROS generation refers to the highest concentration causing maximum inhibition of PSII activity. Also, during the trials of menthol-induced bleaching of the coral hosts, the effective concentration varied between different hosts associated with different Symbiodinium types, but all displayed significant photosystem II (PSII) breakdown in the endosymbiotic Symbiodinium (Fig. S1). ROS in Symbiodinium were detected by incubating 1 ml of an algal suspension with 5 µl H2DCFDA (10 mM in dimethyl sulfoxide) for 30 min in the dark, followed by fluorescence microscopic examination (Mydlarz & Jacobs, 2004) and fluorescence determination (Wang et al., 2011). The fluorescence data of ROS signals are expressed in arbitrary fluorescence units per cell.
Dose effects (4-h IC50) of menthol and DCMU on PSII inhibition of FIS were calculated from samples of each treatment. The curve equations for IC50 calculation were determined by the best-fit regression curve of PSII inhibition (%) against the logarithm-transformed reagent concentration, which are described in Eq. (1) for menthol and (2) for DCMU treatment: (1) and (2)
Parameters and regression coefficients of the equations are listed in Table 1.
| Phylotype | Menthol | DCMU | |||||||
|---|---|---|---|---|---|---|---|---|---|
| a | b | x0 | y0 | r2 | a | b | x0 | r2 | |
| B1 (S. minutum) | 53.71 | 0.045 | 0.23 | 6.33 | 0.992 | 86.57 | 0.18 | 2.06 | 0.997 |
| C1 (S. goreaui) | 98.35 | 0.115 | −0.11 | 8.13 | 0.995 | 72.86 | 0.21 | 1.84 | 0.997 |
| C3 | 94.45 | 0.050 | −0.06 | 6.44 | 0.996 | 74.39 | 0.23 | 1.59 | 0.999 |
| C15 | 96.63 | 0.033 | 0.01 | 2.66 | 0.999 | 75.73 | 0.16 | 1.86 | 0.995 |
| D1a (S. trenchii) | 111.22 | 0.105 | 0.07 | 10.15 | 0.989 | 73.53 | 0.22 | 1.57 | 0.998 |
Comparisons of 4-h IC50 values between Symbiodinium types were made using a one-way analysis of variance (ANOVA) followed by Fisher’s least significance difference (LSD) test, with a significance level of 0.05. The coefficient of variance (CV) was used to evaluate the variation in IC50 measurements between replicates.
Results
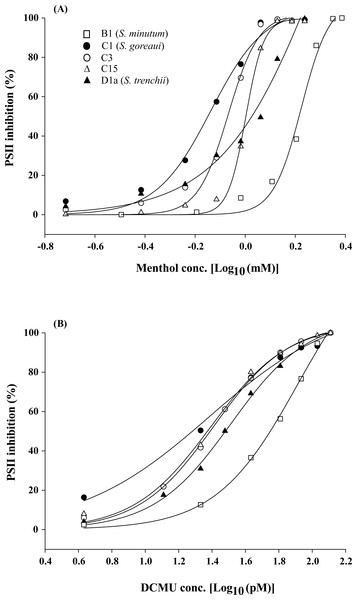
When Symbiodinium algae were incubated in menthol-supplemented ASW for 4 h, all five Symbiodinium types (S. minutum, S. goreaui, C3, C15, and S. trenchii) displayed typical dose–response curves under menthol concentrations of 0.19∼2.43 mM (Fig. 1A). In contrast to menthol inhibition at millimolar levels, DCMU-treated samples displayed PSII inhibition of FIS at picomolar levels (4∼129 pM) (Fig. 1B).
Figure 1: Dose effects of menthol and dichlorophenyl dimethylurea (DCMU) on inactivation of photosystem II (PSII) function in freshly isolated Symbiodinium.
The means of PSII inhibition (n = 3) after 4 h of incubation in menthol (A) or DCMU (B) are plotted in the presence of various reagent concentrations.Regression coefficients for curve fitting ranged 0.989∼0.999 for menthol treatments and 0.995∼0.999 for DCMU treatments. When converting parameter “b” in Table 1 to a slope factor (curve steepness) as described by Motulsky & Christopoulos (2003), values for menthol treatments were divided into two groups which included high (20.0∼30.3 for S. minutum, types C3, and C15) and low slope factors (8.7∼9.5 for S. goreaui and S. trenchii). But those for DCMU-treated FIS displayed only one group of slope factors that ranged 4.3∼6.3. The 4-h IC50 values for menthol and DCMU treatments on five Symbiodinium types were calculated, and results are listed in Table 2. Mean 4-h IC50 values for menthol ranged 0.72∼1.96 mM with CV values ranging 1.1%∼12.3%, and those for DCMU ranged 29∼74 pM with CV values of 2.3%∼14.1% (Table 2). Two sets of data significantly varied among Symbiodinium types (menthol, F4,11 = 17.54, p < 0.001; DCMU, F4,10 = 12.00, p < 0.01), and S. minutum was found to be the most tolerant type to both menthol and DCMU irritation (p < 0.05). In contrast to the comparable tolerability to DCMU among different Symbiodinium types, S. trenchii and type C15 were significantly more tolerant to menthol than were S. goreaui and C3 (p < 0.05).
| Phylotype | Cnidarian host | Menthol | DCMU | ||
|---|---|---|---|---|---|
| mM | CV (%) | pM | CV (%) | ||
| B1 (S. minutum) | Exaiptasia pulchella | 1.96 ± 0.13a | 6.4 | 74 ± 8a | 10.9 |
| C1 (S. goreaui) | Stylophora pistillata | 0.72 ± 0.09b | 12.3 | 29 ± 3b | 9.8 |
| C3 | Acropora humilis | 0.86 ± 0.02b | 2.7 | 37 ± 1b | 2.3 |
| C15 | Porities lutea | 1.01 ± 0.01c | 1.1 | 33 ± 3b | 10.1 |
| D1a (S. trenchii) | Galaxea fasicularis | 1.07 ± 0.07c | 6.2 | 52 ± 7b | 14.1 |
Notes:
a, b and c indicate the significance between data after statistical analysis at P = 0.05.
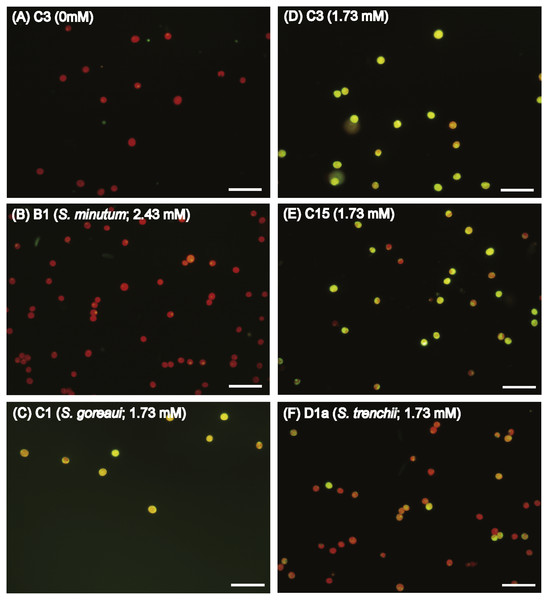
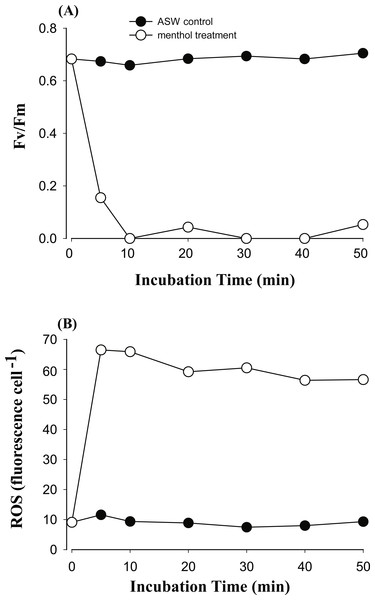
In order to explore if ROS are the cause of menthol inhibition of PSII activity of FIS, the generation of algal ROS under a condition of complete PSII activity inhibition by menthol or DCMU treatment was further examined. According to a fluorescence microscopic examination, no DCMU-treated type displayed green fluorescence, indicating no ROS signal, as found in the ASW control (Fig. S2). However, ROS generation in menthol-treated Symbiodinium varied among types. As shown in Fig. 2B, S. minutum, consistent with the ASW control in Fig. 2A, showed almost no ROS signal. For S. trenchii, there were only mild ROS signals detected (Fig. 2C). In contrast to S, minutum and S. trenchii, as shown in Figs. 2D–2F, types C15, C3, and S. goreaui displayed intense green fluorescence, representing considerable ROS generation. Direct measurements of the fluorescence intensity derived from an ROS probe also indicated that the five Symbiodinium types had varied responses to menthol irritation (Table 3). Menthol-treated S. goreaui, C3, and C15 types displayed almost double the extent of ROS fluorescence (with mean values of 58∼68 fluorescence units cell−1) of that of S. trenchii (35 ± 3 fluorescence units cell−1), and more than three times that of S. minutum (18 ± 2 fluorescence units cell−1) (Table 3). ROS fluorescence intensities obtained from menthol-treated S. minutum and all DCMU-treated types were comparable to background levels (with mean values of 18∼28 fluorescence units cell−1), even though Fv∕Fm values were reduced to <0.1. In order to monitor the times needed for Symbiodinium types to respond to menthol irritation, Fv∕Fm values and ROS levels of the C3 type were examined every 5 or 10 min after suspending the alga in menthol-supplemented ASW. As shown in Fig. 3A, the Fv∕Fm value was significantly reduced from 0.685 to <0.2 within 5 min of incubation, and to nearly 0 after 10 min of incubation. As with the decrease in Fv∕Fm value, ROS generation in the menthol treated Symbiodinium reached a maximum within 5 min of incubation (Fig. 3B).
Figure 2: Diverse reactive oxygen species (ROS) generation levels among Symbiodinium types when treated with artificial seawater (ASW) containing menthol.
Freshly isolated Symbiodinium (FIS) was incubated in menthol-supplemented ASW for 4 h, followed by 2′, 7′-dichlorofluorescin diacetate labeling and microscopic examination of fluorescence. Menthol concentrations used in FIS incubation were 1.73 mM for types C1 (S. goreaui), C3, C15, and D1a (S. trenchii), and 2.43 mM for B1 (S. minutum), which would cause complete breakdown of PSII activity in the algae. ROS signals in type C3 treated with the ASW control (A) and menthol-treated Symbiodinium types B1, D1a, C15, C3, and C1 (B∼F) are presented with a representative photo; n = 3, scale bar in the photo represents 50 µm.| Phylotypes | ROS signal | |
|---|---|---|
| Menthol | DCMU | |
| (fluorescence units cell−1) | ||
| B1 (S. minutum) | 18 ± 2 | 15 ± 2 |
| C1 (S. goreaui) | 64 ± 6 | 12 ± 1 |
| C3 | 68 ± 9 | 18 ± 2 |
| C15 | 58 ± 8 | 18 ± 1 |
| D1a (S. trenchii) | 35 ± 3 | 17 ± 2 |
Figure 3: The time course of photosystem II (PSII) activity decline and reactive oxygen species (ROS) generation when treating the Symbiodinium C3 with menthol and artificial seawater (ASW).
Changes in PSII activity (A) and ROS levels (B) in type C3 treated with 1.73 mM menthol were determined and plotted with incubation time. ASW-incubated congeneric Symbiodinium was used as a control.Discussion
This study indicated that millimolar levels of menthol significantly inhibited PSII activities of five freshly isolated Symbiodinium types. Loss of photosynthetic activity in Symbiodinium might explain the coral bleaching phenomenon of menthol-treated corals and sea anemones (Wang et al., 2012a; Dani et al., 2016; Matthews et al., 2016). However, various sensitivities and response modes to menthol irritation indicate that different Symbiodinium types did not react uniformly to the chemicals. Diverse physiological performances among different Symbiodinium types were observed with respect to thermal tolerance (Rowan, 2004; Tchernov et al., 2004; Robinson & Warner, 2006; Sampayo et al., 2008; Wang et al., 2012b; Suggett et al., 2015), thermal stress-induced reactive oxygen release and antioxidant plasticity (Krueger et al., 2014), and expression of photosynthesis-related genes (Parkinson et al., 2016). Therefore, it is reasonable to expect that different Symbiodinium types would exhibit diverse sensitivities to menthol (Fig. 1, Table 1). However, when PSII activities were completely shut down by menthol, responses of the Symbiodinium types could be divided into two groups, ROS generating (S. goreaui, C3, and C15) and non-ROS generating (S. minutum and S. trenchii). The reason for such a distinction needs to be addressed in future studies.
Menthol and other monoterpenes, because of their lipophilic nature, are known to inhibit growth of plant tissues by inducing lipid oxidation, which affects the membrane structure and function (Zunino & Zygadlo, 2004; Singh et al., 2006; Kaur et al., 2011). As proposed by Wang et al. (2012a), the mechanism of menthol-induced bleaching might be attributable to Ca2+ stimulated exocytosis. This has been demonstrated in previous results from transcriptomic studies suggesting the disruption of calcium homeostasis in both corals and sea anemones during stress-induced bleaching (Desalvo et al., 2008; Moya et al., 2012). Also, Dani et al. (2016) have proposed that menthol treatment could be responsible in inducing symbiophagy through Ca2+-triggered mechanisms. Preliminary experiments conducted by Wang et al. (2012a) also suggest that menthol inhibition of Symbiodinium PSII activity may play a role in the expulsion of the algal cells or the digestion of the Symbiodinium cells by the host.
Generation of ROS in the menthol-treated clade C Symbiodinium might be derived from a lipid-oxidation process or a reaction of a TRPM8-like channel receptor. In Symbiodinium C3, ROS generation was initiated and reached a maximum within 5 min of incubation (Fig. 3B), indicating a typical ROS burst reaction as found in infected plants (Wojtaszek, 1997). The production of large amounts of ROS indicates a quick defensive response to stress. As to S. minutum, the lack of ROS detected in the menthol-induced PSII shutdown might be attributed to cells containing higher antioxidant activities and/or a lack of a TRPM8-like channel receptor. Comparisons of antioxidant networks revealed that S. minutum produced more superoxide dismutase and ascorbate peroxidase than S. goreaui when confronting heat stress (Krueger et al., 2014). No ROS generation in menthol-treated S. trenchii might be attributed to the “stress-tolerant” nature of members of Symbiodinium clade D (Toller, Rowan & Knowlton, 2001; LaJeunesse et al., 2008), in which ROS levels were found to remain unaffected by heat stress (McGinty, Pieczonka & Mydlarz, 2012).
In conclusion, activity of (TRP)M8 might not be a general phenomenon. We observed diverse responses based on Symbiodinium types to menthol treatment. In addition to (TRP)M8, menthol might also be inducing symbiophagy (Dani et al., 2016) in the hosts of certain Symbiodinium types. Although, studies (including this one) have tried to understand the mechanisms involved in loss of Symbiodinium cells when treated with menthol, the clarity as to what really happens is still lacking and needs to be explored in more detail in future studies.
Supplemental Information
Maximum quantum yield measurements of Symbiodinium in Isopora palifera
An example study on maximum quantum yield measurements of Symbiodinium in Isopora palifera during menthol bleaching. Bleaching treatment was conducted with a repeat incubation of 8 h in 0.58 mM menthol/artificial seawater (ASW) following with 16 h in ASW. Measurement was stopped at the third day of incubation due to low Symbiodinium population. Data are presented with mean ± S.D (n = 8).
Diverse reactive oxygen species (ROS) generation levels
Diverse reactive oxygen species (ROS) generation levels among Symbiodinium types when treated with artificial seawater (ASW) containing DCMU. Freshly isolated Symbiodinium (FIS) was incubated in DCMU-supplemented ASW for 4 h, followed by 2′, 7′-dichlorofluorescin diacetate labeling and microscopic examination of fluorescence. DCMU concentrations used in FIS incubation were 0.13 mM for all Symbiodinium tested, which would cause complete breakdown of PSII activity in the algae. ROS signals in type C3 treated with the ASW control (A) and DCMU-treated Symbiodinium types B1, D1a, C15, C3, and C1 (B F) are presented with a representative photo. Scale bar in the photo represents 50 mm.