Seed morphometric characteristics of European species of Elatine (Elatinaceae)
- Published
- Accepted
- Received
- Academic Editor
- Marion Röder
- Subject Areas
- Plant Science, Taxonomy
- Keywords
- Determination key, Ephemerals, Amphibious species, Seed coat, Morphology, Malphigiales, SEM, Micromorphology, Seeds traits, Population
- Copyright
- © 2017 Popiela et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2017. Seed morphometric characteristics of European species of Elatine (Elatinaceae) PeerJ 5:e3399 https://doi.org/10.7717/peerj.3399
Abstract
Elatine L. contains ca. 25 small, herbaceous, annual species distributed in ephemeral waters in both hemispheres. All species are amphibious and characterized by a high degree of morphological variability. The importance of seed morphology in Elatine taxonomy has been emphasized by many authors. The degree of seed curvature and seed coat reticulation have been traditionally considered very important in recognizing individual species of this genus. Seed morphometric characteristics of 10 Elatine species, including all European native taxa, are provided on the basis of material from two or three populations of each species. A total of 24–50 seeds were studied from each population, altogether 1,260 images were used for the morphometric study. In total, six parameters were measured from SEM pictures: object surface area, profile specific perimeter (object circuit), rectangle of the object (a) length, rectangle of the object (b) width, angle of the seed curvature, and number of pits in the seed coat counted in the middle row. Our study shows that the range of morphological variation of seeds in European species of Elatine is great, both between the species and the populations. Discrimination analysis showed that all six traits significantly differentiate the populations studied (λ = 0.001, p < 0.001), and the greatest contributions were “number of pits”, “rectangle_a”, and “the angle curvature”. Multidimensional scaling based on a correlation matrix of Mahalanobis distance of the six features studied revealed the greatest similarity between the three populations of E. alsinastrum, E. macropoda, and E. hexandra. Regarding interspecific differences, a Kruskal–Wallis tests showed that, in many cases, lack of statistically significant differences between species relative to the studied seed traits. If distinction of species is only based on seeds, especially if only a few seeds are evaluated, the following species pairs can be easily confused: E. alsinastrum and E. orthosperma, E. hexandra and E. macropoda, E. campylosperma and E. hydropiper, as well and E. gussonei and E. hungarica. We found no diversity in seed coat micromorphology within pits that could have potential taxonomic importance. An identification key and descriptions of species are provided on the basis of seeds traits.
Introduction
Elatine L. is one of the two genera in the Elatinaceae, a family in Malpighiales (Tucker, 1986; Davis & Chase, 2004), and contains ca. 15–25 ephemeral amphibious species (Heywood et al., 2007). To the present knowledge, ten native taxa occur in Europe; however, Flora Europea lists seven (Cook, 1968) and Euro+Med Plantbase nine native species (Uotila, 2009b). One taxon belongs to the subgenus Potamopithys (Adanson) Seub(E. alsinastrum L.), and the other taxa are classified into subgenus Elatine Seub. (=Hydropiper Moesz): E. triandra Schkuhr (sect. Triandra Seub. (=Crypta (Nutt.) Seub.); E. brochonii Clavaud, E. campylosperma Seub., E. gussonei (Sommier) Brullo, Lanfr., Pavone & Ronsisv., E. hexandra (Lapierre) DC., E. hydropiper L., E. hungarica Moesz, E. macropoda Guss., and E. orthosperma Düben (section: Elatinella Seub.). Two more taxa of sect. Elatinella occur in the New World (in North America E. californica A. Gray and in South American E. ecuadoriensis Molau). Other taxa classified to sect. Triandra mainly occur in temperate regions of the Old and New World, with the probable center of diversity in North and South America. E. ambigua is another taxon from Europe (Uotila, 2009b). It shows no substantial genetic differences in relation to E. triandra (Sramkó et al., 2016) an Asian species also occurring in Europe.
Elatine alsinastrum is characterized by whorled leaves; all other species have opposite leaves, and are mainly distinguished by number of stamens (three, six or eight) and number of perianth lobes (three or four). The shape of leaves is variable, oblong or roundish, petiolate or almost sessile, and depends on environmental conditions. Flowers are sessile or pedunculated, while tiny seeds are oblong, curved or horseshoe-shaped (Cook, 1968; Tucker, 1986).
Recently, Elatine species have been of interest to researchers because of their rarity throughout their range, relatively poorly known distribution and taxonomy, ecology, karyology and phylogenetic relationships (e.g., Popiela, 2005; Misfud, 2006; Uotila, 2009a; Uotila, 2010; Popiela & Łysko, 2010; Popiela & Łysko, 2011; Popiela et al., 2011; Popiela et al., 2012; Popiela, Łysko & Molnár, 2013; Popiela et al., 2015; Takács, 2013; Molnár et al., 2013; Molnár, Popiela & Lukács, 2013; Šumberova & Hrivnak, 2013; Kalinka et al., 2014; Kalinka et al., 2015; Cai et al., 2016; Sramkó et al., 2016). The above-mentioned authors emphasized that the erratic temporal appearance of Elatine species depends mainly on environmental factors; for example, plants develop as aquatic or terrestrial forms, and, moreover, they are morphologically variable depending on the phase of drying on the ground. This variability and the very small size of plants and short-lasting, tiny flowers often make proper identifications difficult. Earlier leaf length and shape, pedicel length and seed shape were widely used for identification of Elatine taxa (Seubert, 1845; Niedenzu, 1925). The importance of seed morphology in Elatine taxonomy has been emphasized by many authors: the degree of seed curvature (i.e., seed shape) and seed coat reticulation have been considered very importan tfor recognizing individual species (Cook, 1968; Uotila, 1974; Uotila, 2010; Tucker, 1986; Misfud, 2006; Molnár et al., 2013).
There have been only a few studies addressing morphological variability of Elatine taxa (Mason, 1956; Molnár et al., 2013; Molnár, Popiela & Lukács, 2013). Recently, (Molnár et al., 2015) examined the level of phenotypic plasticity in Elatine. Analysis of morphological differences between aquatic and terrestrial forms of individual species clearly showed that vegetative traits are highly influenced by environmental factors and only seed traits are stable within species. According to Molnár et al. (2015), only seed morphology (aside from generative characteristics) is valuable for taxonomic purposes.
Consequently, we studied seed morphometric characteristics of 10 Elatine species, including all native European taxa, as a part of comprehensive surveys on taxonomy and phytogeography of this genus that have been conducted by a Hungarian-Polish research team since 2010. We assumed that advanced and methodically uniform seed characteristics are taxonomically important in this genus. Our aims were to (i) find statistical differences between Elatine species relative to seed morphological features, (ii) evaluate intra- and interspeciesseed variability, and then (iii) construct a guide to identifying species based onseed morphological features. Due to the small size of seeds the study was made by using SEM micrographs.
Material & Methods
Plant material and cultivation
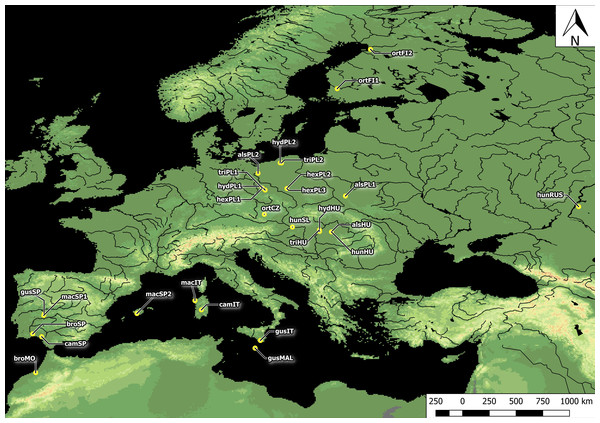
Plants studied were collected across Europe. In total, seeds were collected from all 10 Elatine species and from three populations each, with an exception of very rare E. brochonii and E. campylosperma, two populations, so altogether 28 populations were used for the study. The distance between the populations of each species ranged from approximately 10–2,000 km. For the localities of the original material, and the voucher specimens, see Table 1 and Fig. 1. The studied seeds were gathered directly from the field, or from cultivated plants grown from the original material; in some cases seeds from herbarium specimens were used. Culture was conducted at the Center for Molecular Biology at the University of Szczecin, Poland and/or a the Department of Botany at the University of Debrecen, Hungary. Plants were grown in climate-controlled culture chambers with 12 h/day light and 30,000 lux light intensity, temperatures: under light, 22 ± 2 °C, and under dark, 18 ± 2 °C.
| Nr | Acronym | Name | Origin*,** | Latitude | Longitude | Collector, voucher | No. of seeds | Approx. distance between two/three populations (km) |
|---|---|---|---|---|---|---|---|---|
| 1. | alsHU | E. alsinastrum L. | Hungary: Konyár** | 47.31 | 21.67 | Molnár V. A. DE- 22226 | 50 | 620 |
| 2. | alsPL1 | E. alsinastrum L. | Poland: Staw Noakowski* | 50.80 | 23.03 | Popiela A. SZUB- 008756 | 50 | |
| 3. | alsPL2 | E. alsinastrum L. | Poland: Strzelczyn | 53.01 | 14.54 | Popiela A. SZUB- 015968 | 50 | |
| 4. | broMO | E. brochonii Clavaud | Morocco: Ben Slimane** | 33.62 | −7.07 | Lukács B. A. DE-43230 | 44 | 420 |
| 5. | broSP | E. brochonii Clavaud | Spain: San Silvestre de Guzmán** | 37.4 | −7.36 | Molnár V. A. DE-37684 | 49 | |
| 6. | camIT | E. campylosperma Seub. | Italy: Sardegna, Gesturi** | 39.73 | 9.03 | Molnár V. A. DE-37423 | 47 | 1,380 |
| 7. | camSP | E. campylosperma Seub. | Spain: El Rocio, Donana** | 37.12 | −6.49 | Molnár V. A. DE-37681 | 50 | |
| 8. | gusMAL | E. gussonei (Sommier) Brullo, Lanfr., Pavone & Ronsisv. | Malta: Gózó: Ta’ Sannat** | 36.01 | 14.25 | Molnár V. A. & Lukács B. A. DE-43229 | 50 | 1,265 |
| 9. | gusSP | E. gussonei (Sommier) Brullo, Lanfr. Pavone & Ronsisv. | Spain: Casar de Cáceres** | 39.33 | −6.25 | Molnár V. A. DE-43231 | 50 | |
| 10. | gusIT | E. gussonei | Italy: Sicily, Modica** | 36.76 | 14.77 | Molnár V. A. DE-38750 | 50 | |
| 11. | hexPL1 | E. hexandra (Lapierre)DC. | Poland: Janików (Janikowo) | 51.57 | 14.96 | Popiela A. SZUB- 015964 | 33 | 115 |
| 12. | hexPL2 | E. hexandra (Lapierre) DC. | Poland: Milicz* | 51.55 | 17.35 | Popiela A. SZUB- 010851 | 50 | |
| 13. | hexPL3 | E. hexandra (Lapierre) DC. | Poland: Ruda Milicka* | 51.53 | 17.34 | Dajdok Z. SZUB- 011097 | 50 | |
| 14. | hunRUS | E. hungarica Moesz | Russia: Volgograd** | 49.76 | 45.7 | Mesterházy A. DE-37484 | 42 | 1,375 |
| 15. | hunSLO | E. hungarica Moesz | Slovakia: Okánikowo | 47.78 | 17.88 | Eliáš P. SZUB- 010523 | 24 | |
| 16. | hunHU | E. hungarica Moesz | Hungary: Konyár** | 47.31 | 21.67 | Molnár V. A. DE-22266 | 50 | |
| 17. | hydHU | E. hydropiper L. | Hungary: Tiszagyenda** | 47.36 | 20.52 | Molnár V. A. DE-22273 | 39 | 550 |
| 18. | hydPL1 | E. hydropiper L. | Poland: Parowa | 51.38 | 15.23 | Popiela A. | 45 | |
| 19. | hydPL2 | E. hydropiper L. | Poland: Kwecko Lake | 54.02 | 16.69 | Popiela A.,Prajs B. SZUB- 015705 | 43 | |
| 20. | macIT | E. macropoda Guss. | Italy: Sardegna: Olmedo** | 40.63 | 8.41 | Molnár V. A. DE-37424 | 50 | 900 |
| 21. | macSP1 | E. macropoda Guss. | Spain: Casar de Cáceres** | 39.19 | −6.29 | Molnár V. A. DE-37692 | 46 | |
| 22. | macSP2 | E. macropoda Guss. | Spain: Mallorca: Cap Blanc/SaTore* | 39.38 | 2.77 | Popiela A., SZUB”-- 015969 | 42 | |
| 23. | ortCZ | E. orthosperma Düben | Czech Republic: Klášter* | 49.02 | 15.15 | Šumberova K. | 45 | 1,260 |
| 24. | ortFI1 | E. orthosperma Düben | Finland: Kokemäki | 61.23 | 22.23 | Suominen J, H 439800 | 25 | |
| 25. | ortFI2 | E. orthosperma Düben | Finland: Oulu* | 65.06 | 25.47 | Mesterházy A. DE-43232 | 50 | |
| 26. | triHU | E. triandra Schkuhr | Hungary: Kisköre* | 47.50 | 20.50 | Molnár V. A. DE-22282 | 41 | 570 |
| 27. | triPL1 | E. triandra Schkuhr | Poland: Janików | 51.57 | 14.96 | Popiela A., SZUB- 010520 | 47 | |
| 28. | triPL2 | E. triandra Schkuhr | Poland: Bobięcińskie Małe Lake | 54.01 | 16.82 | Dambska I., SZUB- 010862 | 50 |
Figure 1: Distribution of Elatine populations studied.
For acronyms, see Table 1.Elatine hungarica, E. hydropiper and E. triandra are protected species in Hungary and were sampled with the permission of the Hortobágy National Park Directorate (Permission id.: 45-2/2000, 250-2/2001).
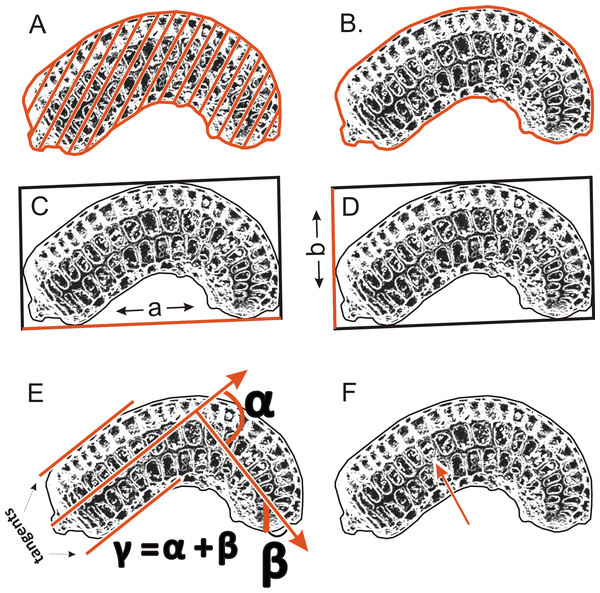
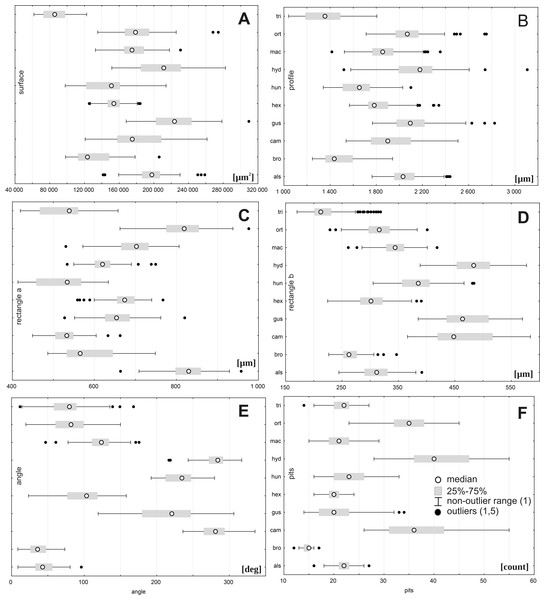
To determinate the variability and diagnostic features of seeds, 24–50 seeds obtained from several individuals from each population (Table 1) were used. A total of more than 1,500 scanning electron microscope (SEM) images of the seeds were obtained at ×200 magnification using an SEM (Zeiss Evo, Molecular Biology and Biotechnology Center, University of Szczecin, Szczecin, Poland); however, 1,260 images were used for the morphometric study, because all cracked seeds were excluded. In total, six parameters were measured (Fig. 2): (A) object surface area; (B) profile specific perimeter (object circuit); (C) object rectangle a (length); (D) object rectangle b (width); (E) the angle of curvature; (F) number of pits on the seed coat counted in the middle row. Moreover, membrane presence and pit shape were evaluated.
Figure 2: The method of measuring of seed.
(A) surface; (B) profile; (C) rectangle a; (D) rectangle b; (E) the angle of curvature (γ = α + β); (F) number of pits in the middle row.To examine micromorphology of seed coat, 48 pictures at ×500, ×2,000, ×4,000, and ×7,000 were taken (Zeiss Evo SEM; Laboratory of Confocal and Electron Microscopy, Faculty of Biology, Adam Mickiewicz University, Poznań, Poland)
Data analysis
To distinguish the characteristics that have the greatest impact on population and species discrimination, multiple discriminant analysis was used. Wilks λ was used to measure the discriminatory power of the model (0—perfect discrimination; 1—no discrimination). Interpretation of discriminant functions was performed using canonical analysis.
For visualization of the relationship between species and populations, Mahalanobis distance-based unweighted pair-group method using arithmetic averages to construct (UPGMA) trees was applied. Canonical values were shown using categorized scatterplots. The most discriminative traits were also independently tested by the non-parametric Kruskal–Wallis tests. All calculations were made in Statistica v. 12.5 software.
Results
Variability of populations within species
Discrimination analysis showed that all six traits significantly differentiate the populations studied (λ = 0.001, p < 0.001). Of these, the greatest contributions were as follows: number of pits, rectangle a (length), and the angle of curvature (Table 2).
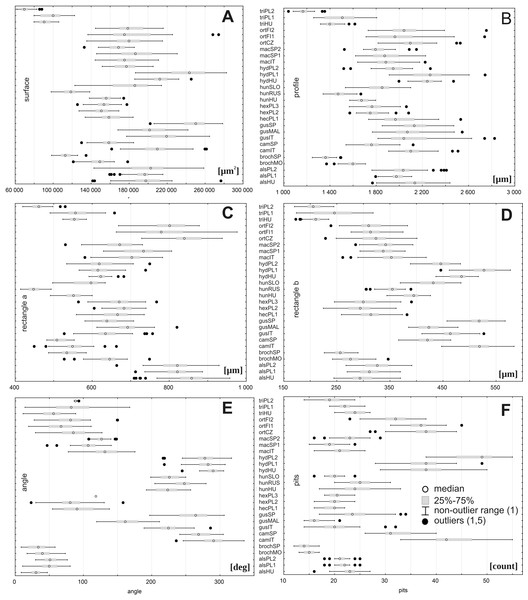
Based on a Kruskal–Wallis tests, we found no statistically significant differences (p = 0.05) between populations studied of each European Elatine species regarding the following traits: (A) surface (all species except E. campylosperma, E. hungarica, E. hydropiper), (B) profile (all species except E. campylosperma, E. hexandra, E. hungarica, E. hydropiper), (C) rectangle a (all species except E. brochonii), (D) rectangle b (all species), (E) the angle of curvature (all species), (F) number of pits (all species except E. gussonei, E. hungarica, E. triandra) (Table 3). Accordingly, the traits studied did not show statistically significant variation between populationsof the following species: E. alsinastrum, E. macropoda and E. orthosperma.
| N = 1,260 | λ = .00016F(162, 7217) = 153.07p < 0.0001 | |||||
|---|---|---|---|---|---|---|
| λ (Wilks) | Fragm. (Wilks) | F (27.1227) | p | Toler. | R2 | |
| The angle of curvature | 0.000461 | 0.342896 | 87.0870 | 0.0001 | 0.751829 | 0.248171 |
| Rectangle a | 0.000492 | 0.320751 | 96.2370 | 0.0001 | 0.254119 | 0.745881 |
| Number of pits | 0.000839 | 0.188320 | 195.8703 | 0.0001 | 0.989553 | 0.010447 |
| Surface | 0.000298 | 0.530541 | 40.2124 | 0.0001 | 0.169730 | 0.830270 |
| Rectangle b | 0.000229 | 0.690923 | 20.3291 | 0.0001 | 0.262387 | 0.737613 |
| Profile | 0.000199 | 0.793047 | 11.8592 | 0.0001 | 0.632832 | 0.367169 |
However, there were large ranges of variation for some traits, especially within the following populations: E. orthosperma from Finland, Fin1 (for acronyms see Table 1) (surface: SD 36131.9 and rectangle a: SD 78.9), E. hungarica from Slovakia (profile: SD 498.1), E. triandra from Poland, PL1 (rectangle b:SD 39.9 and the angle of curvature: SD 33.7), and E. hydropiper from Hungary (number of pits: SD 5.3) (Fig. 3, Table 4). Conversely, the smallest ranges of variation were observed in the following populations: E. triandra from Poland, PL2 (surface: SD 5897.8), E. triandra from Hungary (rectangle a: SD 15.3), E. brochonii from Spain (profile: SD 51.7 and rectangle b: SD 13.6), E. hydropiper from Hungary and E. macropoda from Spain, SP2 (angle of curvature, SD 10.5; SD 10.3, respectively), and E. brochonii from Morocco (number of pits: SD 0.9) (Fig. 3, Table 4).
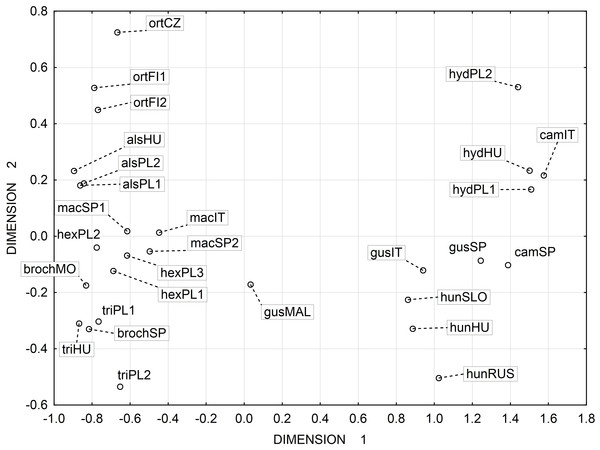
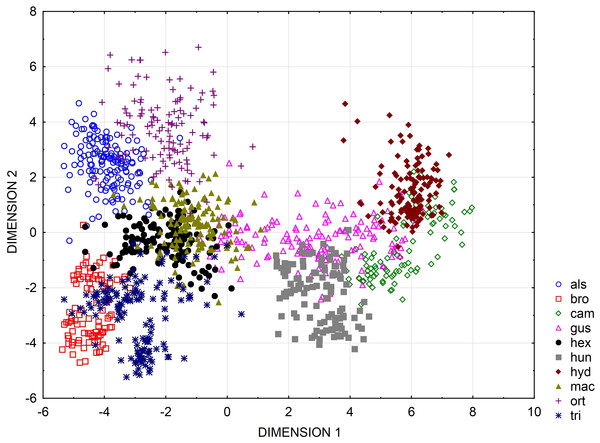
Multidimensional scaling based on a correlation matrix of Mahalanobis distance of the six features studied revealed the greatest similarity between the three populations of the following species: E. alsinastrum, E. macropoda, E. hexandra (Fig. 4).
Variability between species
Discriminant analysis showed that all variables could discriminate species (λ < 0.01). The greatest impact was from the following features: number of pits, the angle of curvature and rectangle a (Table 5).
| alsHU | alsPL1 | alsPL2 | brochMO | brochSP | camIT | camSP | gusIT | gusMAL | gusSP | hexPL1 | hexPL2 | hexPL3 | hunHU | hunRUS | hunSLO | hydHU | hydPL1 | hydPL2 | macIT | macSP1 | macSP2 | ortCZ | ortFI1 | ortFI2 | triHU | triPL1 | triPL2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| alsHU | abcf | abcf | cdef | abcdef | cde | cdef | acde | acef | abce | abce | abcde | abcde | bcde | cdef | cdef | cdef | bcde | bef | abce | ef | f | ef | abcd | abce | abcdef | |||
| alsPL1 | abcf | abcdf | cdef | abcdef | cde | cdef | acde | ac | abc | abce | abcde | abce | cde | bcdef | abcdef | cdef | ce | e | ce | f | f | f | abcd | abc | abcd | |||
| alsPL2 | abcf | abcdf | cdef | abcdef | cde | cdef | acde | ac | abc | abce | abcde | abce | cde | cdef | cdef | cdef | ce | e | abce | f | f | f | abcd | abcd | abcd | |||
| brochMO | abcf | abcf | abcf | c | abcdef | cdef | abdef | abde | abdef | be | f | ef | cdef | cdef | de | abdef | abdef | abdef | abdef | abcde | def | abcf | abcf | abcf | cf | f | ace | |
| brochSP | abcf | abcdf | abcdf | c | abdef | abdef | abcdef | abcde | abcdef | abce | bcef | bcef | def | def | abde | abcdef | abcdef | abcdef | abcdef | abcdef | abcdef | abcdef | abcf | abcdef | f | ef | ef | |
| camIT | cdef | cdef | cdef | abcdef | abdef | ab | cf | cef | cf | acdef | abcdef | abcdef | abdf | abdf | bf | a | abcdef | bcdef | abcdef | cde | cde | cde | abdef | abdef | abdef | |||
| camSP | abcdef | abcdef | abcdef | cdef | abdef | ab | abcf | abcef | abcf | bcdef | cdef | cdef | f | f | abc | abc | c | cef | acdef | cdef | bcde | cde | bcde | abde | adef | abdef | ||
| gusIT | cde | cde | cde | abdef | abcdef | cf | abcf | f | ade | abde | abd | abcf | abcdf | f | f | af | ad | cde | abd | acdef | cdef | acdef | abcdef | abcde | abcde | |||
| gusMAL | cdef | cdef | cdef | abde | abcde | cef | abcef | f | ad | abde | abdf | abcf | abcf | c | ef | ef | ef | f | d | abdf | cdef | def | def | abcdef | abcdef | abcde | ||
| gusSP | acde | acde | acde | abdef | abcdef | cf | abcf | f | f | adef | abdef | abde | abcd | abcd | ab | f | f | af | abde | abcdef | abde | acdef | acdef | acdef | abcde | abcde | abcdef | |
| hexPL1 | acef | ac | ac | be | abce | acdef | bcdef | ade | ad | adef | b | b | bcdef | bcef | de | adef | adef | def | cf | f | cf | abcdf | abc | abcd | ||||
| hexPL2 | abce | abc | abc | f | bcef | abcdef | cdef | abde | abde | abdef | b | cdef | cdef | cde | abdef | abdef | adef | ade | a | abcf | f | abcf | bcf | c | abcd | |||
| hexPL3 | abce | abce | abce | ef | bcef | abcdef | cdef | abd | abdf | abde | b | cd | bcef | cd | abdef | abdef | def | a | abcf | f | abcf | abcdef | abc | abcd | ||||
| hunHU | abcde | abcde | abcde | cdef | def | abdf | f | abcf | abcf | abcd | bcdef | cdef | cd | abf | abdf | bf | bc | abcef | c | bcdef | bcdef | bcdef | ade | ade | abdef | |||
| hunRUS | abcde | abce | abce | cdef | def | abdf | abcdf | abcf | abcd | bcef | cdef | bcef | abf | abcdf | abcdf | abcf | abc | abcef | abc | abcef | abce | abce | de | de | def | |||
| hunSLO | bcde | cde | cde | de | abde | bf | f | c | ab | de | cde | cd | abf | bf | abf | f | c | c | bcdef | cdef | cdef | abde | abde | abde | ||||
| hydHU | cdef | bcdef | cdef | abdef | abcdef | abc | f | ef | f | adef | abdef | abdef | abf | abcdf | bf | ab | abdef | bcdef | abdef | acde | cde | acde | abdef | abdef | abcdef | |||
| hydPL1 | cdef | abcdef | cdef | abdef | abcdef | abc | f | ef | f | adef | abdef | abdef | abdf | abcdf | abf | ab | abcdef | abcdef | abdef | acde | acde | acde | abdef | abdef | abcdef | |||
| hydPL2 | cdef | cdef | cdef | abdef | abcdef | a | c | af | ef | af | def | adef | def | bf | abcf | f | ab | ab | def | cdef | def | cde | cde | cde | abdef | abdef | abcdef | |
| macIT | bcde | ce | ce | abdef | abcdef | abcdef | cef | ad | f | abde | ade | bc | abc | c | abdef | abcdef | def | bcf | ef | ef | abcde | abcd | abcd | |||||
| macSP1 | bef | e | e | abcde | abcdef | bcdef | acdef | cde | d | abcdef | a | a | abcef | abcef | c | bcdef | abcdef | cdef | bf | f | f | abcdef | abcd | abcd | ||||
| macSP2 | abce | ce | abce | def | abcdef | abcdef | cdef | abd | abdf | abde | c | abc | abdef | abdef | def | bcf | ef | bcf | abcde | abcd | abcdf | |||||||
| ortCZ | ef | f | f | abcf | abcdef | cde | bcde | acdef | cdef | acdef | cf | abcf | abcf | bcdef | abcef | bcdef | acde | acde | cde | bcf | bf | bcf | abcdf | abcdf | abcdf | |||
| ortFI1 | f | f | f | abcf | abcf | cde | cde | cdef | def | acdef | f | f | f | bcdef | abce | cdef | cde | acde | cde | ef | f | ef | abcd | abcf | abcdf | |||
| ortFI2 | ef | f | f | abcf | abcdef | cde | bcde | acdef | def | acdef | cf | abcf | abcf | bcdef | abce | cdef | acde | acde | cde | ef | f | bcf | abcd | abcdf | abcdf | |||
| triHU | abcd | abcd | abcd | cf | f | abdef | abde | abcdef | abcdef | abcde | abcdf | bcf | abcdef | ade | de | abde | abdef | abdef | abdef | abcde | abcdef | abcde | abcdf | abcd | abcd | f | ||
| triPL1 | abce | abc | abcd | f | ef | abdef | adef | abcde | abcdef | abcde | abc | c | abc | ade | de | abde | abdef | abdef | abdef | abcd | abcd | abcd | abcdf | abcf | abcdf | |||
| triPL2 | abcdef | abcd | abcd | ace | ef | abdef | abdef | abcde | abcde | abcdef | abcd | abcd | abcd | abdef | def | abde | abcdef | abcdef | abcdef | abcd | abcd | abcdf | abcdf | abcdf | abcdf | f |
Notes:
- a
-
surface
- b
-
profile
- c
-
rectangle a
- d
-
rectangle b
- e
-
angle of curvature
- f
-
number of pits
Figure 3: Boxplots of the most discriminative seed traits among 28 studied populations of Elatine.
Notations: boxes indicate 25–75 percentiles, white point indicate medians, whiskers exclude outliers, black points indicate outliers. For acronyms, see Table 1. (A) surface; (B) profile; (C) rectangle a; (D) rectangle b; (E) angle; (F) pits.Figure 4: Multidimensional scaling based on a correlation matrix of Mahalanobis distance for seed traits among 28 populations of Elatine.
For acronyms, see Table 1| Acronym | Surface (µm2) | Profile (µm) | Rectangle_a (µm) | Rectangle_b (µm) | The angle of curvature (°) | Numer of pits |
|---|---|---|---|---|---|---|
| alsHU | 22108.8 | 152.4 | 55.0 | 26.1 | 12.5 | 2.1 |
| alsPL1 | 12280.7 | 69.8 | 34.2 | 22.7 | 16.8 | 1.3 |
| alsPL2 | 23478.5 | 139.6 | 51.6 | 28.0 | 11.7 | 1.5 |
| brochMO | 14826.5 | 86.4 | 37.3 | 21.0 | 14.2 | 0.9 |
| brochSP | 7424.4 | 51.7 | 22.7 | 13.6 | 14.2 | 1.3 |
| camIT | 23754.8 | 154.9 | 37.4 | 34.7 | 21.4 | 5.0 |
| camSP | 12567.2 | 106.3 | 19.7 | 23.1 | 16.1 | 3.6 |
| gusIT | 23082.4 | 237.0 | 44.7 | 27.4 | 17.7 | 3.4 |
| gusMAL | 18526.5 | 172.1 | 39.8 | 24.8 | 23.0 | 1.6 |
| gusSP | 17567.0 | 121.0 | 29.1 | 26.0 | 26.9 | 4.1 |
| hexPL1 | 12783.1 | 194.6 | 41.8 | 27.8 | 26.1 | 1.6 |
| hexPL2 | 9186.5 | 87.2 | 27.3 | 26.0 | 23.6 | 1.7 |
| hexPL3 | 10467.0 | 110.1 | 39.3 | 32.9 | 10.3 | 1.4 |
| hunHU | 7222.9 | 59.2 | 24.9 | 19.4 | 17.4 | 4.0 |
| hunRUS | 8318.2 | 84.2 | 17.5 | 17.8 | 17.4 | 2.7 |
| hunSLO | 24936.3 | 498.1 | 40.1 | 30.3 | 15.9 | 1.9 |
| hydHU | 11886.9 | 101.5 | 18.3 | 19.2 | 10.5 | 5.3 |
| hydPL1 | 22799.7 | 252.3 | 35.7 | 30.5 | 16.7 | 4.2 |
| hydPL2 | 12877.0 | 221.3 | 41.1 | 22.2 | 20.6 | 4.3 |
| macIT | 14752.8 | 121.9 | 45.0 | 30.6 | 21.4 | 2.1 |
| macSP1 | 21061.9 | 153.9 | 45.6 | 22.4 | 18.3 | 1.8 |
| macSP2 | 16799.6 | 160.0 | 41.1 | 35.2 | 10.3 | 2.5 |
| ortCZ | 18174.3 | 271.2 | 51.0 | 34.8 | 25.7 | 3.8 |
| ortFI1 | 36131.9 | 241.2 | 78.9 | 24.5 | 24.3 | 3.0 |
| ortFI2 | 17093.8 | 177.3 | 45.3 | 30.7 | 25.4 | 3.0 |
| triHU | 6365.6 | 67.3 | 15.3 | 13.9 | 16.4 | 2.0 |
| triPL1 | 11403.4 | 140.7 | 36.4 | 39.9 | 33.7 | 1.8 |
| triPL2 | 5897.8 | 55.6 | 22.1 | 18.5 | 11.7 | 2.0 |
| N = 1,260 | λWilks: .00252 F (54,6352) = 262.68 p < 0.0001 | |||||
|---|---|---|---|---|---|---|
| λ (Wilks) | Fragm. (Wilks) | F (9.1245) | p | Toler. | R2 | |
| The angle of curvature | 0.005051 | 0.499212 | 138.7702 | 0.0001 | 0.632234 | 0.367766 |
| Rectangle a | 0.004048 | 0.622838 | 83.7685 | 0.0001 | 0.298593 | 0.701407 |
| Number of pits | 0.007668 | 0.328805 | 282.3820 | 0.0001 | 0.916368 | 0.083632 |
| Surface | 0.002735 | 0.921836 | 11.7295 | 0.0001 | 0.153021 | 0.846979 |
| Profile | 0.002759 | 0.913793 | 13.0504 | 0.0001 | 0.186761 | 0.813239 |
| Rectangle b | 0.002717 | 0.927840 | 10.7585 | 0.0001 | 0.509627 | 0.490373 |
The Kruskal–Wallis tests showed, in many cases, lack of statistical significance between species relative to the studied seed traits (Table 6). Regarding the trait surface, only E. triandra seeds showed statistical significance compared with all species tested. Analysis of all characteristics showed the least amount of statistically significant differences between the following species pairs: E. alsinastrum and E. orthosperma, E. hexandra and E. macropoda, as well as E. gussonei and E. hydropiper (Table 6).
| E. alsinastrum | E. brochonii | E. campylosperma | E. gussonei | E. hexandra | E. hungarica | E. hydropiper | E. macropoda | E. orthosperma | E. triandra | |
|---|---|---|---|---|---|---|---|---|---|---|
| E. alsinastrum | abcdf | abcdef | acdef | abcef | abcde | cdef | abcde | aef | abcde | |
| E. brochonii | abcdf | abcdef | abcdef | bcdef | cdef | abdef | abcdef | abcdef | acef | |
| E. campylosperma | abcdef | abcdef | abcef | acdef | abdef | abc | cdef | bcde | abdef | |
| E. gussonei | acdef | abcdef | abcef | abde | abcdf | ef | abcde | acdef | abcde | |
| E. hexandra | abcef | bcdef | acdef | abde | bcdef | abcdef | ad | abcf | abcdf | |
| E. hungarica | abcde | cdef | abdef | abcdf | bcdef | abcdef | abcdef | abcdef | abde | |
| E. hydropiper | cdef | abdef | abc | ef | abcdef | abcdef | abcdef | acde | abcdef | |
| E. macropoda | abcde | abcdef | cdef | abcde | ad | abcdef | abcdef | bcdef | abcde | |
| E. orthosperma | aef | abcdef | bcde | acdef | abcf | abcdef | acde | bcdef | abcdf | |
| E. triandra | abcde | acef | abdef | abcde | abcdf | abde | abcdef | abcde | abcdf |
Notes:
- a
-
surface
- b
-
profile
- c
-
rectangle a
- d
-
rectangle b
- e
-
angle of curvature
- f
-
number of pits
There was a large range of variation for the taxa studied regarding the following traits: seed size (traits: surface, profile, and rectangle a), especially within E. hungarica (SD 27183.7, SD 285.9, and SD 62.0, respectively); the angle of curvature, E. gussonei (SD 44.7); and number of pits, E. campylosperma (SD 7.2). The smallest variation was present in E. triandra (surface SD 14587.3, profile: SD 171.8, rectangle a: SD 54.6) and E. brochonii (rectangle b SD 20.6, the angle of curvature SD 14.7, pits: SD 1.3). The characteristics associated with size (surface, profile, rectangle a, rectangle b) revealed that the following species had the smallest seeds: E. brochonii and E. triandra, while the largest seeds in the studied species belonged to E. gussonei and E. hydropiper (Fig. 5, Table 7).
Figure 5: Boxplots of the most discriminative seed traits among Elatine species studied.
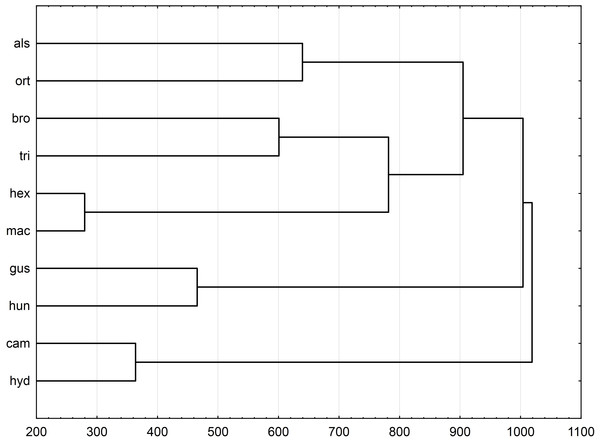
Notations: boxes indicate 25–75 percentiles, white points indicate medians, whiskers exclude outliers, black points indicate outliers. For acronyms, see Table 1. (A) surface; (B) profile; (C) rectangle a; (D) rectangle b; (E) angle; (F) pits.Figure 6: Morphological relationships of seeds among surveyed Elatine species displayed by Mahalanobis distance-based UPGMA cluster based on the following features: rectangle a, angle of curvature, and number of pits.
For acronyms, see Table 1.| Surface (µm2) | Profile (µm) | Rectangle_a (µm) | Rectangle_b (µm) | Angle of curvature (°) | Number of pits | |
|---|---|---|---|---|---|---|
| E. alsinastrum | 20213.4 | 142.3 | 50.1 | 27.8 | 16.7 | 1.8 |
| E. brochonii | 20881.3 | 133.8 | 58.9 | 20.6 | 14.7 | 1.3 |
| E. campylosperma | 30981.9 | 219.0 | 35.1 | 56.7 | 20.8 | 7.2 |
| E. gussonei | 26873.7 | 184.4 | 44.7 | 46.1 | 44.7 | 4.5 |
| E. hexandra | 11356.3 | 165.5 | 37.1 | 30.6 | 26.0 | 1.6 |
| E. hungarica | 27183.7 | 285.9 | 62.0 | 35.7 | 20.3 | 3.7 |
| E. hydropiper | 31653.4 | 250.3 | 33.6 | 41.5 | 17.3 | 6.6 |
| E. macropoda | 19610.4 | 148.5 | 50.3 | 30.6 | 20.9 | 2.5 |
| E. orthosperma | 22819.4 | 239.2 | 60.9 | 31.3 | 26.2 | 4.2 |
| E. triandra | 14587.3 | 171.8 | 54.6 | 32.2 | 27.5 | 2.7 |
The classification matrix of the discriminant analysis showed that the level of classification varied from 86% (rectangle a, the angle of curvature, number of pits) to 84% (surface, profile, rectangle a, rectangle b, the angle of curvature, number of pits). The highest values of classification were found for E. alsinastrum, E, brochonii, E. hydropiper, and E. orthosperma (all greater than 90%). The lowest values were found for E. campylosperma (57%, 55%) (Table 8).
| E. alsinastrum | E. brochonii | E. campylosperma | E. gussonei | E. hexandra | E. hungarica | E. hydropiperer | E. macropoda | E. orthosperma | E. triandra | Correct n | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Correct classification (%) | A | 96 | 94 | 57 | 85 | 79 | 85 | 94 | 83 | 95 | 88 | 86 |
| B | 97 | 92 | 55 | 78 | 71 | 82 | 95 | 78 | 93 | 91 | 84 | |
| E. alsinastrum | A | 144 | 1 | 0 | 0 | 3 | 0 | 0 | 2 | 0 | 0 | 150 |
| B | 145 | 1 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 150 | |
| E.brochonii | A | 1 | 87 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 93 |
| B | 1 | 86 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 1 | 93 | |
| E. campylosperma | A | 0 | 0 | 55 | 0 | 0 | 17 | 25 | 0 | 0 | 0 | 97 |
| B | 0 | 0 | 53 | 0 | 0 | 16 | 28 | 0 | 0 | 0 | 97 | |
| E. gussonei | A | 0 | 0 | 1 | 126 | 0 | 1 | 4 | 16 | 0 | 0 | 148 |
| B | 0 | 0 | 1 | 115 | 0 | 3 | 6 | 23 | 0 | 0 | 148 | |
| E. hexandra | A | 1 | 1 | 0 | 0 | 105 | 0 | 0 | 26 | 0 | 0 | 133 |
| B | 1 | 2 | 0 | 0 | 95 | 0 | 0 | 35 | 0 | 0 | 133 | |
| E. hungarica | A | 0 | 0 | 1 | 16 | 0 | 99 | 0 | 0 | 0 | 0 | 116 |
| B | 0 | 0 | 1 | 20 | 0 | 95 | 0 | 0 | 0 | 0 | 116 | |
| E. hydropier | A | 0 | 0 | 6 | 2 | 0 | 0 | 119 | 0 | 0 | 0 | 127 |
| B | 0 | 0 | 5 | 1 | 0 | 0 | 121 | 0 | 0 | 0 | 127 | |
| E. macropoda | A | 5 | 0 | 0 | 0 | 17 | 0 | 0 | 115 | 0 | 1 | 138 |
| B | 5 | 0 | 0 | 1 | 24 | 0 | 0 | 107 | 0 | 1 | 138 | |
| E. orthosperma | A | 4 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 114 | 0 | 120 |
| B | 6 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 112 | 0 | 120 | |
| E. triandra | A | 0 | 1 | 0 | 0 | 15 | 1 | 0 | 0 | 0 | 121 | 138 |
| B | 0 | 1 | 0 | 0 | 9 | 1 | 0 | 2 | 0 | 125 | 138 | |
| Total classified | A | 155 | 90 | 63 | 144 | 146 | 118 | 148 | 160 | 114 | 122 | 1,260 |
| B | 158 | 90 | 60 | 137 | 138 | 115 | 155 | 168 | 112 | 127 | 1,260 | |
| (Correct n) − (Tot. class.) | A | −5 | 3 | 34 | 4 | −13 | −2 | −21 | −22 | 6 | 16 | |
| B | −8 | 3 | 37 | 11 | −5 | 1 | −28 | −30 | 8 | 11 |
Notes:
- A
-
rectangle a, angle of curvature, pits
- B
-
surface, profile, rectangle a, rectangle b, angle of curvature, number of pits
UPGMA clusters of Mahalanobis distance based on rectangle a, the angle of curvature, and number of pits yielded two groups: species with straight or nearly straight seeds, and species with curved and U-shaped seeds (Fig. 6). The greatest similarity was found between seeds of E. hexandra and E. macropoda, and E. campylosperma and E. hydropiper. The spatial distribution of observed characteristics of the analyzed species is depicted as a categorized scatterplot based on canonical analysis values (Fig. 7).
Figure 7: Categorized scatterplot based on canonical analysis value for seeds of the European species of Elatine.
For acronyms, see Table 1.Discussion
Our study shows that in Elatine tested seed variability is mainly associated with size-connected traits, especially surface, profile, rectangle b, and, to a lesser extent, rectangle a. This allowed us to draw the conclusion that to distinguish seeds of these species the most useful traits are the angle of curvature and number of pits, and to a lesser extent rectangle a (length). These findings confirm previous knowledge about the usefulness of these features in Elatine taxonomy (Misfud, 2006; Uotila, 2009a; Uotila, 2010; Molnár et al., 2013; Molnár et al., 2015). Nevertheless, our study revealed that the range of variation of European Elatine morphological features is large, both between species and the populations of each species.
Regarding intraspecific variability, the traits studied were not statistically significantly different between studied populations of the following taxa: E. alsinastrum, E. macropoda, E. hexandra. Conversely, E. gussonei, E. campylosperma E. hungarica and E. hydropiper seeds showed statistically significant intrapopulation variability. The taxonomic status of the first three species is still being elucidated. Elatine gussonei, an enigmatic plant of the Mediterranean, was first described as a variety of E. hydropiper and was later classified as a separate species (Brullo et al., 1988; Misfud, 2006; Molnár et al., 2013; Molnár, Popiela & Lukács, 2013). Elatine campylosperma was described by Seubert (1845) from Sardinia, and later greatly neglected by most researchers by synonymizing this species under E. macropoda; at present, it is considered a separate species (Kalinka et al., 2015). Elatine hungarica was last collected in 1960 and rediscovered in Hungary in 1998 (Molnár et al., 1999); for years its taxonomic status was under discussion (Molnár et al., 2013).
Our present study showed that regarding shape statistically only E. alsinastrum and E. orthosperma seeds are nearly straight and seeds of all other species are curved to varying degrees; the range of variation in some species is large in this respect, especially in E. gussonei, E. triandra, and E. hexandra.
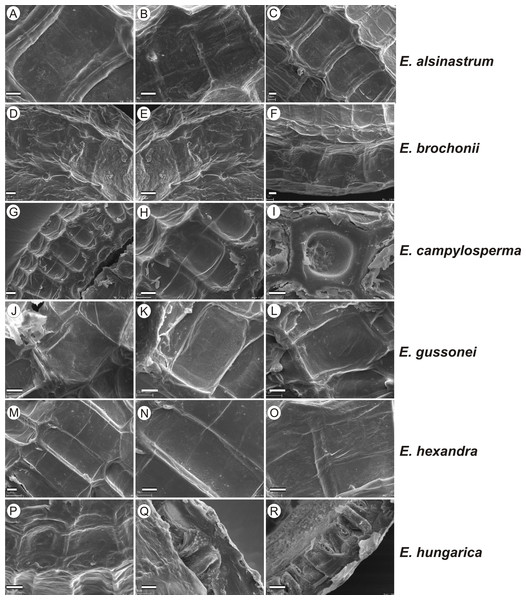
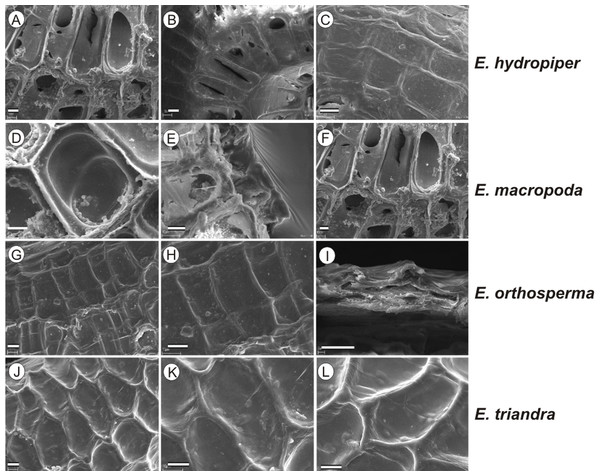
If distinction of species is only based on seeds, it would be easy to confuse the following species pairs: E. alsinastrum and E. orthosperma, E. hexandra and E. macropoda, E. campylosperma and E. hydropiper, andE. gussonei and E. hungarica, especially if only a few seeds are evaluated. Previously, Misfud (2006), who worked on Malta and Mallorca populations, pointed out the importance of distinction based on greater seed curvature in E. gussonei compared with E. macropoda; although this is true if averages are used, there is a substantial amount of overlap in curvature and this could lead to confusion if the curvature of only few seeds are analyzed. This was also confirmed by our results. Misfud (2006) also drew attention to the distinctive seed testa reticulation, and claimed that the wide hexagonal shape of pits in E. gussonei and smaller number of pits/row (15 ± 3) are very difficult to confuse with E. macropoda’s 21 ± 3 narrow pits/row. Our study yielded different results: seeds of E. macropoda populations had similar number of pits [(13–)19–23–(29)] compared to E. gussonei [(17–)23(–32)]. However, because Misfud (2006) did not precisely describe the method of counting pits (especially in which row pits were counted), it is difficult to compare our results. Molnár et al. (2013) pointed out that seeds of E. hungarica are much more curved than those of E. gussonei, and especially of E. macropoda and E. orthosperma, but somewhat less curved than those of E. hydropiper. Our current study revealed that the range of variation for the feature the angle of curvature of E. hungarica seeds is similar to that of E. gussonei, and more curved seeds are found in E. hydropiper and E. campylosperma. These results are basically consistent with observations of Molnár et al. (2013), especially considering that more varied material was used in the current study. Our research confirms observations of Misfud (2006) and Molnár et al. (2013) concerning the evident semilunar membrane on the concave side of seeds (Figs. 8–11). The membrane was present and clearly visible in all highly curved fresh seeds of the following species: E. gussonei, E. hydropiper, E. hungarica, and E. campylosperma. Regarding the seed testa, a very distinctive network-shape ornamentation pattern of E. triandra as visible (Figs. 9J– 9L, 11). Elatine campylosperma seeds showed the most distinctive reticulation, and were characterized by a large number of narrow, rectangular pits (Fig. 10), and round-shaped pits (Figs. 8G– 8I). Similarly, rectangular-shaped pits were found in seeds of the following species: E. alsinastrum, E. triandra, E. orthosperma, E. hydropiper, and E. macropoda; however, hexagonal pits were also present (Figs. 10 and 11). The pit shapes of E. gussonei, E. hungarica, E. brochonii, and E. hexandra are usually both hexagonal and rectangular, with a predominance of the former (Figs. 10 and 11). Similar observations for some of these species were made by Misfud (2006), Molnár et al. (2013), Molnár, Popiela & Lukács (2013). We believe that the shape of pits may be an additional feature that helps distinguish seeds of individual species (Figs. 8 and 10). However, we found no diversity in seed coat micromorphology within pits (e.g., pores, strophioles) that could have potential taxonomic importance. Seed coats within pits were smooth with the exception of irregular strips, and the porosity of the seed coat is visible only in the inner layer of cracked seeds (Figs. 8Q–8R, 9I). Ornamentation pattern (pit shape) becomes distinct as the seeds dry up. The outer layer of the seed coat is very thin and easily destroyed ( Figs. 9A–9B; 9D–9F).
Figure 8: The diversity in seed coat micromorphology of Elatine alsinastrum (a–alsHu; b, c–alsPL1), E. brochonii (a, b–broMO; c–broSP), E. campylosperma (a, b–camIT; c–camSP), E. gussonei (a, b–gusMAL; c–gusSP), E. hexandra (a, b–hexPL1; c–hexPL2), E. hungarica (a, b–hunR; c–hunSL).
Scale bar = 10 µm. For acronyms, see Table 1. (A–C) E. alsinastrum; (D–F) E. brochonii; (G–I) E. campylosperma; (J–L) E. gussonei; (M–O) E. hexandra; (P–R) E. hungarica.Figure 9: The diversity in seed coat micromorphology of Elatine hydropiper (a - hydHu, b, c - hydPL1); E. macropoda (a, b -macIT; c–macSP), E. orthosperma (a, b -ortCZ; c - ortFI1), E. triandra (a–triHU; b, c–triPL1).
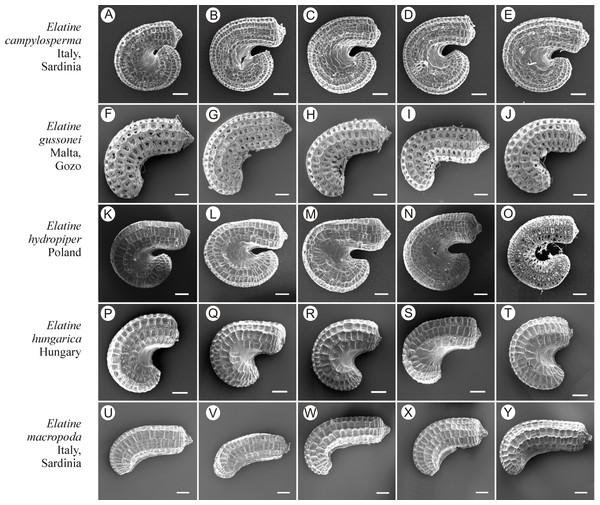
Scale bar = 10 µm. For acronyms, see Table 1. (A–B) E. hydropiper; (D–F) E. macropoda; (G–I) E. orthosperma; (J–L) E. triandra.Figure 10: The diversity of seeds of Elatine campylosperma, E. gussonei, E. hydropiper, E. hungarica, E. macropoda.
Scale bar =200 µm. (A–E) E. campylosperma; (F–J) E. gussonei; (K–O) E. hydropiper; (P–T) E. hungarica; (U–Y) E. macropoda.Figure 11: The diversity of seeds of Elatine orthosperma, E. alsinastrum, E. brochonii, E. hexandra, E. triandra.
Scale bar =200 µm. (A–E) E. orthosperma; (F–J) E. alsinastrum; (K–O) E. brochonii; (P–T) E. hexandra; (U–Y) E. triandra.Our research allowed us to construct a guide that can be useful to identify the studied taxa based on seed traits. We believe that this guide is important for better recognition of these rare and endangered species, and can be useful for elucidating the history of range formation of these taxa in the Holocene and their origin. Elatine subfossil finds were discovered in late-glacial and pre-boreal sediments in the last few centuries (Latałowa, 1992; Brinkkemper et al., 2008; Kowalewski et al., 2013). The ecological amplitude of this species provides robust clues for environmental reconstruction, which must have been a temporarily flooded fresh water area. “...since this type of environment is strongly threatened on a worldwide scale, the presence of these species in the past may also provide interesting information for present nature development projects...” (Brinkkemper et al., 2008).
Identification guide and descriptions for European species of Elatine based on seed morphology presented in Figs. 10 and 11. Note: the guide does not include exceptional values given in parentheses in the descriptions (min. outliers 1.5) 25%–75% (max. outliers 1.5).
| 1 Seeds straight –almost straight –slightly curved, the angle of curvature < 150° | 2 |
| 1* Seeds curved or U-shaped, the angle of curvature ≥ 150 | 8 |
| 2 Number of pits in the seed coat in the middle row ≥ 30 | E. orthosperma |
Seed length (658–)776–854(–971) µm, width (242–)297–334(–389) µm, angle of curvature (55–)61–99(–156)° number of pits in the middle row (23–)32–38(–47), prevailing pit shape rectangular, semilunar membrane absent on the concave side of seeds.
| 2* Number of pits in the seed coat in the middle row <30 | 3 |
| 3 Length of seeds < 600 µm | 4 |
| 3* Length of seeds ≥ 600 µm | 5 |
| 4 Number of pits in the seed coat in the middle row < 17 | E. brochonii |
Seed length (365–)533–645(–813) µm, width (217–)252–276(–312) µm, angle of curvature: 26–47(–79)°, number of pits in the middle row (12–)14–15(–17), prevailing pit shape hexangular, semilunar membrane absent on the concave side of seeds.
| 4* Number of pits in the seed coat in the middle row ≥ 18 | E. triandra |
Seed length (328)467–560(700) µm, width: (158)201–231(274) µm; angle of curvature: 58–89 (136)°, number of pits in the middle row (16)20–23(28), prevailing network-shape of pits in the seed coat, semilunar membrane absent on the concave side of seeds.
| 5 Number of pits in the seed coat in the middle row ≤17 | E. brochonii (for description see above, after line 4) |
| 5* Number of pits in the seed coat in the middle row >17 | 6 |
| 6 Angle of curvature of seeds ≤60° | E. alsinastrum |
Seed length (708–)799–859(–950) µm, width: (230)290–330(391) µm, angle of curvature: 33–56(91)°, number of pits in the middle row: (18)21–23(26), prevailing rectangular shape of pits in the seed coat, semilunar membrane absent on the concave side of seeds.
| 6*Angle of curvature of seeds > 60° | 7 |
| 7 Width of seeds ≥ 320 µm | E. macropoda |
Seed length: (568–)666–732(–830) µm, width: (282–)329–360(–407) µm, angle of curvature (78–)111–134(–167)°, number of pits in the middle row: (13–)19–23(–29); prevailing rectangular shape of pits in the seed coat, usually semilunar membrane absent on the concave side of seeds.
| 7* Width of seeds < 320 µm | E. hexandra |
Seed length: (593–)656–697(–760) µm, width: (223–)283–322(–381) µm; angle of curvation (15–) 77–118(–180)°, number of pits in the middle row: (16–)19–21(–24), the shape of pits in the seed coat hexagonal and rectangular, semilunar membrane absent on the concave side of seeds.
| 8 Number of pits in the seed coat in the middle row ≥ 30 | 9 |
| 8* Number of pits in the seed coat in the middle row <30 | 10 |
| 9 Length of seeds < 600 µm | E. campylosperma |
Seed length: (439–)505–549(–615) µm, width: (274–)419–517(–663) µm, angle of curvation: (222–)265–294(–337)°, number of pits in the middle row: (15–)31–42(–59), narrow rectangular or round shape of pits in the seed coat, semilunar membrane present on the concave side of the seeds.
| 9* Length of seeds ≥600 µm | E.hydropiper |
Seed length: (548–)602–638(–693) µm, width: (367–)454–512(–599) µm, angle of curvation: (246–)273–291(–318)°, number of pits in the middle row: (22–)37–48(–62), prevailing rectangular shape of pits in the seed coat, semilunar membrane present on the concave side of the seeds.
| 10 Length of seeds ≤ 600 µm | E. hungarica |
Seed length: (296–)459–567(–730) µm, width: (284–)357–405(–477) µm; angle of curvation: (161–)213–247(–299)°, number of pits in the middle row: (11–)20–26(–35), prevailing hexagonal shape of pits in the seed coat, semilunar membrane present on the concave side of the seeds.
| 10* Length of seeds > 600 µm | E. gussonei |
Seed length: (539–)627–685(–774) µm, width: (325–)436–509(–620) µm; angle of curvation: (80–)180–247(–347)°, number of pits in the middle row: 17–23(–32), prevailing hexagonal shape of pits in the seed coat, semilunar membrane present on the concave side of the seeds.