Melanoleuca subgriseoflava and M. substridula—two new Melanoleuca species (Agaricales, Basidiomycota) described from China
- Published
- Accepted
- Received
- Academic Editor
- Héctor Mora-Montes
- Subject Areas
- Biodiversity, Molecular Biology, Mycology, Plant Science, Taxonomy
- Keywords
- Melanoleuca, Agaricales, Morphology, Multiple-genes phylogeny
- Copyright
- © 2022 Qi et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. Melanoleuca subgriseoflava and M. substridula—two new Melanoleuca species (Agaricales, Basidiomycota) described from China. PeerJ 10:e13807 https://doi.org/10.7717/peerj.13807
Abstract
Two new Melanoleuca species, Melanoleuca subgriseoflava and M. substridula, are originally reported and described in China based on both morphological and molecular methods. Melanoleuca subgriseoflava, collected in Liaoning province, is mainly characterized by its greyish-brown to yellowish-grey pileus, creamy to light orange lamellae, greyish-yellow context, round and warted basidiospores and fusiform hymenial cystidia. Melanoleuca substridula, discovered in Sichuan province, is mainly characterized by its light brown to dark brown pileus, whitish lamellae, light brown to greyish-brown stipe, round and warted basidiospores and lack of any forms of cystidia. The phylogenetic relationships as well as divergence-time estimation were analyzed using the combined data set (ITS-nrLSU-RPB2), and the results showed that the two Melanoleuca species formed two distinct lineages. Based on the combination of morphological and molecular data, M. subgriseoflava and M. substridula are confirmed as two new species to science. A theoretical basis is provided for the species diversity of Melanoleuca.
Introduction
Melanoleuca Pat. is distributed worldwide, containing around 423 validly published names (Index Fungorum, http://www.indexfungorum.org/, accessed on 7 April 2022), 12 of which were considered as edible by Dai et al. (2010), including Melanoleuca arcuata (Bull.) Singer, Melanoleuca brevipes (Bull.) Pat., Melanoleuca cognata (Fr.) Konrad & Maubl etc. Recently, many new species of Melanoleuca have been reported throughout the world (Vizzini et al., 2010; Vizzini et al., 2011; Sánchez-García, Cifuentes-Blanco & Matheny, 2013; Antonín et al., 2014; Antonín et al., 2017; Antonín et al., 2021; Yu et al., 2014; Nawaz, Jabeen & Khalid, 2017; Xu et al., 2019; Pei et al., 2021). Melanoleuca is a taxonomically complicated genus because many species in the genus are very similar in macroscopical characteristics and only present subtle differences (Bon, 1991; Boekhout, 1999; Vizzini et al., 2011). The genus is typified by a dull-colored pileus; amyloid and warted basidiospores; two types of cystidia (urticiform or fusiform to lageniform) and all hyphae without clamp connections (Singer, 1986; Bon, 1991; Boekhout, 1988; Vizzini et al., 2011).
Despite considerable evidence that the Melanoleuca genus belongs to a monophyletic group, the infrageneric classification system of the genus has always been controversial. Singer (1986) divided the genus into four sections circumscribed only by pileus color and stipe ornamentations, i.e., sect. Alboflavidae Singer, sect. Humiles Singer, sect. Oreinae Singer and sect. Melanoleuca Pat. Bon (1978), the first to take micro-morphological characters into consideration, divided the genus into seven sections. As Boekhout (1988) emphasized the crucial role of cystidia, the genus was divided into three subgenera according to the absence/presence and shape of cystidia, i.e., subgen. Macrocystis Boekhout, subgen. Melanoleuca Pat. and subgen. Urticocystis Boekhout. However, these taxonomical units are not supported by molecular data. Vizzini et al. (2011), using a large number of ITS sequences to construct phylogenetic relationships of Melanoleuca, defined only two subgenera, i.e., subgen. Urticocystis Vizzini and subgen. Melanoleuca Vizzini. Then, follow-up studies on species of Melanoleuca support the classification opinion proposed by Vizzini et al. (2011) (Yu et al., 2014; Kalmer, Acar & Dizkirici, 2018; Xu et al., 2019).
Only 31 species of Melanoleuca have been reported in China (Bau & Li, 1999; Zhang, Li & Song, 2001; Chen, 2007; Mao, 2009; Sun et al., 2012; Wang, 2013; He et al., 2014; Yu et al., 2014; Zhao et al., 2014; Wei, Fan & Yan, 2015; Du et al., 2016; Tian et al., 2018; Xu et al., 2019; Pei et al., 2021). Although China has a complex climate and geographical conditions, species resources of Melanoleuca remain scarce. This study reports and describes two Melanoleuca species collected from Liaoning province and Sichuan province in China from 2019 to 2020. In order to confirm the two collections as new to science, both morphological and method analyses were conducted. The morphological similarities and differences between the two species and other related species are also discussed.
Material and Methods
Specimens and morphological description
Fresh basidiomata were photographed in the field. Specimens were dried with an electric drier and deposited with silica gel. Dried specimens were preserved in the Fungal Herbarium of Shenyang Agricultural University (SYAU-FUNGI), Liaoning, China and Herbarium of Microbiology Institute of Guangdong (GDGM), Guangdong, China. Tissue blocks were removed from the inner part of the dried specimens for DNA analyses. Color abbreviations followed Kornerup & Wanscher (1963). Methods for morphological observation followed Pei et al. (2021). For observation of surface of the basidiospores, SEM microphotographs were performed using a scanning electron microscope (REGLUS 8100; Hitachi, Tokyo, Japan).
Nomenclature
The electronic version of this article in Portable Document Format (PDF) will represent a published work according to the International Code of Nomenclature for algae, fungi, and plants, and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone. In addition, new names contained in this work have been submitted to MycoBank from where they will be made available to the Global Names Index. The unique MycoBank number can be resolved and the associated information viewed through any standard web browser by appending the MycoBank number contained in this publication to the prefix “http://www.mycobank.org/MycoTaxo.aspx?Link=T&Rec=”. The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central SCIE, and CLOCKSS.”
Phylogenetic construction
Genomic DNA was extracted from the dried specimens using the CTAB method (Doyle & Doyle, 1987). PCR protocol and sequencing were conducted as described by Wang et al. (2019). Primer pairs used in this study are listed in Table 1. The newly generated sequences were submitted to GenBank.
| Regions | Primer | Sequence (5′–3′) | Reference |
|---|---|---|---|
| ITS | ITS5 | GGAAGTAAAAGTCGTAACAAGG | White et al. (1990) |
| ITS4 | TCCTCCGCTTATTGATATGC | White et al. (1990) | |
| nLSU | LROR | GTACCCGCTGAACTTAAGC | Michot, Hassouna & Bachellerie (1984) |
| LR5 | ATCCTGAGGGAAACTTC | Michot, Hassouna & Bachellerie (1984) | |
| RPB2 | b7.1R | TGGGGYATGGTNTGYCCYGC’ | Matheny et al. (2007) |
| b6F | CCCATRGCYTGYTTMCCCATDGC | Matheny et al. (2007) |
Representative sequences of Melanoleuca species in former studies (Sánchez-García, Cifuentes-Blanco & Matheny, 2013; Yu et al., 2014; Antonín et al., 2014; Antonín et al., 2015; Antonín et al., 2017; Nawaz, Jabeen & Khalid, 2017; Xu et al., 2019; Antonín et al., 2021; Pei et al., 2021) were retrieved from GenBank. These sequences were aligned with those obtained from this study using Bioedit v7.0.9 (Hall, 1999) and MAFFT v7.313 (Katoh & Standley, 2013). The data partition homogeneity test (Farris et al., 1995) performed in PAUP (Swofford, 2003) allowed combining three regions (ITS, nrLSU and RPB2) (P-value 0.43). A combined data set was then completed with Pluteus romellii as an outgroup. The data matrix includes a total of 2,073 characters of 71 samples. Bayesian Inference (BI) and Maximum Likelihood (ML) were performed as previously described in Pei et al. (2021). Specifically, the combined data set was run for 2 million generations under the GTR+I+G mode using MrBayes v.3.2.6 (Ronquist et al., 2012). RAxML−8.2.10-WIN was performed under the GTR-GAMMA model of evolution (Stamatakis, 2014). The resulting files were viewed using Figtree v1.4.4 (Rambaut, 2018) and were compiled in Adobe Illustrator CC.
Divergence time estimation within Melanoleuca
Divergence time was estimated using BEAST v2.6.3 (Bouckaert et al., 2014). BEAUTI v2.6.3 was used to construct an XML file. ModelFinder (Kalyaanamoorthy et al., 2017) was used to infer the best substitution model. The clock model and substitution model were chosen following Pei (2021) and Zhao et al. (2016). On the calibrated nodes, the offset ages of 98 and 110 Ma were set for the genus Melanoleuca and Pluteus, respectively (Pei, 2021). We ran four independent Monte Carlo Markov Chains (MCMC) of 50 million generations, logging states every 5,000 generations. The checking for convergence was completed in Tracer v1.6 (Rambaut, 2018). TreeAnnotator v.1.8 was used for summarizing tree files. The resulting files were viewed and compiled in Figtree v1.4.4 (Rambaut, 2018) and Adobe Illustrator CC, respectively.
Results
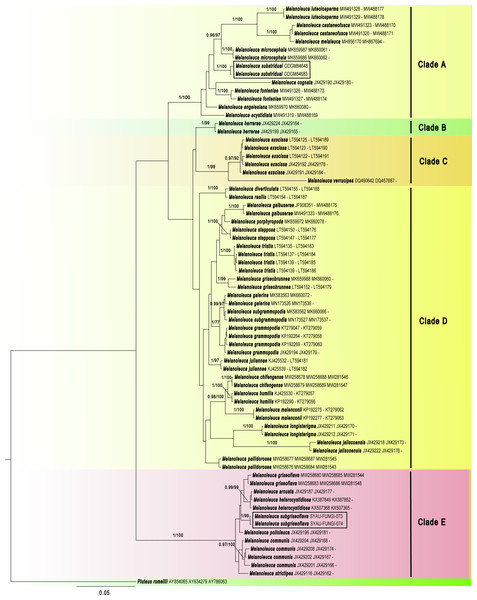
Phylogenetic analyses
The GenBank accession numbers of the sequences, determined in this study, are from ON262569 to ON262573 and ON220896 to ON220899 (Table 2). Maximum likelihood (ML) and Bayesian Inference (BI) showed almost identical topologies and the BI tree was selected for display (Fig. 1). The phylogenetic result suggested that the Melanoleuca should belong to a monophyletic group (PP = 1, BS = 100), which is consistent with the previous studies (Yu et al., 2014; Vizzini et al., 2011). A total of five clades (Clade A to Clade E) can be recognized within Melanoleuca, which is in line with the result of Pei et al. (2021). Additionally, the collections (SYAU-FUNGI-073 and SYAU-FUNGI-074) named M. subgriseoflava formed an independent lineage with strong statistical support (PP = 1.00, BS = 100), located within clade E. And these specimens are closely related to a clade containing sequences of http://www.indexfungorum.org/names/Names.asp?strGenus=Melanoleuca griseoflava X.D. Yu & H.B. Guo, M. arcuata (Bull.) Singer and M. heterocystidiosa (Beller & Bon) Bon. In clade A, the collections (GDGM 84648 and GDGM 84683) named M. substridula group together with well support (PP = 1.00, BS = 100) and far away from the other species in Melanoleuca.
| Species | Voucher collection | Origin | GenBank accession No. | ||
|---|---|---|---|---|---|
| ITS | nLSU | RPB2 | |||
| M. subgriseoflava | SYAU-FUNGI-073 | Shenyang City, Liaoning Province, China | ON262573 | ON262569 | ON220896 |
| M. substridula | GDGM 84648 | Jiuzhaigou valley, Sichuan Province, China | ON262575 | ON262571 | ON220898 |
| M. subgriseoflava | SYAU-FUNGI-074 | Shenyang City, Liaoning Province, China | ON262574 | ON262570 | ON220897 |
| M. substridula | GDGM 84683 | Jiuzhaigou valley, Sichuan Province, China | ON262576 | ON262572 | ON220899 |
Figure 1: Phylogenetic positions of the two new Melanoleuca species, inferred from the combined regions (ITS-nrLSU-RPB2) using MrBayes.
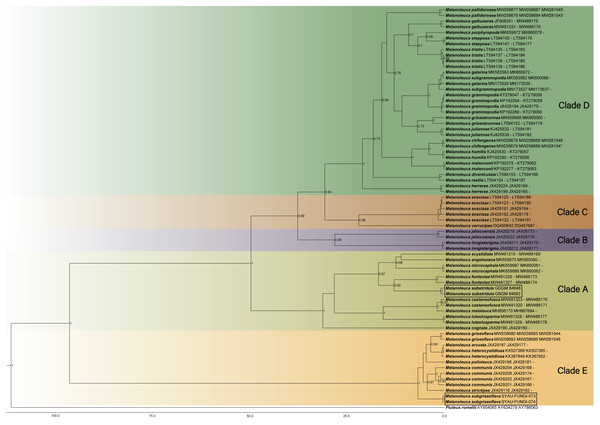
The lineages with new species were shown in boxes. PP ≥ 0.95 and BS ≥ 75% were indicated around the branches. Accession numbers in GenBank (ITS, nrLSU, RPB2) follow the fungal names.Maximum Clade Credibility (MCC) tree for Melanoleuca (Fig. 2) generated a topology similar to those of the phylogenetic analyses. Two new species of Melanoleuca also formed two separate clades with high support (PP = 1.00).
Figure 2: Maximium Clade Credibility tree of Melanoleuca based on ITS, nrLSU, and RPB2 genes sequences with the outgroup Pluteus.
The lineages with new species were shown in boxes. PP ≥ 0.60 are annotated at the internodes.Taxonomy
Melanoleuca subgriseoflava X.D. Yu & H.B. Guo, sp. nov.
MycoBank No. MB843803 (Fig. 3)
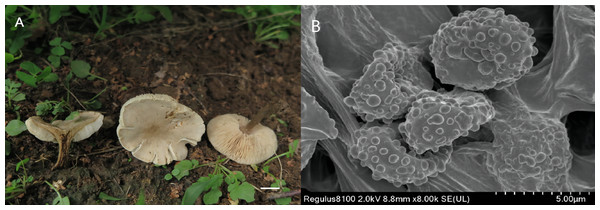
Figure 3: Melanoleuca subgriseoflava (Holotype, SYAU-FUNGI-073).
(A) macroscopic habitat (B) surface of basidiospores. Scale bars: 1 cm (A); 5 µm (B).Etymology The epithet “subgriseoflava” refers to the greyish-brown color of the pileus, which is similar to the species Melanoleuca griseoflava.
Diagnosis: Pileus convex to applanate to depressed at center, greyish-brown to yellowish-grey pileus; lamellae adnate to sinuate, creamy to light orange; stipe yellowish-brown to brown; context greyish-yellow; basidiospores with round and scattered warts and hymenial cystidia fusiform.
Holotype: CHINA. Liaoning Province: Shenyang City, Shenyang Agricultural University, on the soil in meadows, 2 Sep 2020, X.D. Yu (holotype: SYAU-FUNGI-073).
Description: Basidiomata medium-sized. Pileus 24–70 mm diam., convex to plano-convex at first, then gradually applanate, becoming depressed at center when mature; margin entire at first, and slightly lacerate when mature; surface greyish-brown (6D3 to 6F3) at first, then gradually becoming greyish-orange, greyish-yellow, yellowish-grey (5B3 to 5B5, 4B2 to 4B5, 4B2) when mature and dry, brownish-orange at centre (5C5 to 5C6). Lamellae adnate to sinuate, 3.0–7.0 mm broad, white to greyish-orange (5A1, 5B3) at first, becoming creamy, orange-white, light orange (4A3, 5A2, 5A3) when mature, often deeper at margin, crowded, with lamellulae of two or four lengths, edge entire. Stipe cylindrical and somewhat broadened downwards, 25–55 mm long × 2.0-−7.0 mm diam., central, solid, light brown to yellowish-brown (5D7 to 5D8) in upper part, often becoming brown (5F8 to 6E8) towards base, with whitish flocculose apex, striate. Pileus context up to 10 mm thick near stipe attachment, thinner at margin, greyish-yellow (4B3 to 4B6), unchanging when exposed. Smell slightly farinaceous, taste mild. Spore deposit creamy.
Basidiospores (88/10/8) (5.0) 7.0–8.0 (8.5) × 4.0-−5.0 (6.0) µm, av. 7.6 × 4.9 µm, Q = 1.55, broadly ellipsoid, some obovate, ornamentation of small to large warts, some warts with irregular ridges, amyloid. Basidia (40/10/8) (18) 23–27 (28) × (7.0) 8.0–10.0 (11.0) µm, av. 26 × 8.7 µm, clavate to subclavate, hyaline, four-spored. Cheilocystidia lageniform, fusiform to conical cystidia, (30/10/8) (40) 45–49 (50) × (9.0) 10.0–11.0 (12.0) µm, found both at the edge of lamellae, most thin-walled, less thick-walled without distinct upper part. Pleurocystidia have a small amount, similar to cheilocystidia. Trama hyphae thin-walled, regular, 4.0–10.0 µm wide, inamyloid. Pileipellis hyphae cylindrical, with numerous branched, thin-walled, up to 10.5 µm wide. Stipitipellis hyphae in parallel, 4–14.0 µm wide, thin-walled, somewhat slightly thick-walled. Caulocystidia absent. Clamp connections absent in all tissues.
Habitat and distribution: Solitary or in small groups, saprotrophic on the soil, on the grass, on roadsides, in woods. Known from north-eastern China.
Additional material studied: CHINA. Liaoning Province: Shenyang City, Dongling Park, on the soil in meadows, 21 Jul 2019, H.B. Guo (SYAU-FUNGI-074); CHINA. Liaoning Province: Shenyang City, Dongling Park, on the grass in woods, 21 Jul 2019, X.D. Yu (SYAU-FUNGI-075); CHINA. Liaoning Province: Shenyang City, on the campus of Shenyang Agricultural University, on roadsides, 2 Sep 2020, X.D. Yu (SYAU-FUNGI-076); CHINA. Liaoning Province: Shenyang City, on the campus of Shenyang Agricultural University, on the soil in meadows, 8 Sep 2019, X.D. Yu (SYAU-FUNGI-077); CHINA. Liaoning Province: Shenyang City, on the campus of Shenyang Agricultural University, on roadsides, 8 Sep 2019, H.B. Guo (SYAU-FUNGI-078).
Remarks: The main features of M. subgriseoflava are its greyish-brown to yellowish-grey pileus, creamy to light orange lamellae, greyish-yellow context, basidiospores with scattered warts and fusiform hymenial cystidia. On account of the pileus color, M. subgriseoflava is closely related to M. griseoflava originally described in northeastern China by Pei et al. (2021). Nevertheless, M. griseoflava, differs from M. subgriseoflava by its adnate to adnexed and white lamellae. Besides, M. griseoflava differs by the presence of whitish tomentum at the stipe base (Pei et al., 2021). Micromorphologically, M. griseoflava is also distinct from M. subgriseoflava by its almost reticulate surface ornamentations of basidiospores and the presence of caulocystidia (Pei et al., 2021).
Melanoleuca substridula M. Zhang & X.D. Yu, sp. nov.
MycoBank No. MB 843804 (Fig. 4)
Figure 4: Melanouca substridula (Holotype, GDGM 84648).
(A) Macroscopic habitat; (B) surface of basidiospores. Scale bars: 1 cm (A) 5 µm (B).Etymology: The epithet “substridula” refers to the ochre brown color of the pileus, which is similar to the species Melanoleuca stridula.
Diagnosis: Basidiomata slightly small; pileus umbonate, brown to dark brown; lamellae sinuate to adnate, white; stipe light brown in upper part and grey-brown in lower part; basidiospores with round and scattered warts and lack of any forms of cystidia.
Holotype: CHINA. Sichuan Province: Jiuzhaigou valley reserve, on the soil in meadows, 19 Sep 2020, Ming Zhang (GDGM 84648).
Description: Basidiomata slightly small-sized. Pileus 21–38 mm diam.; umbonate at first; margin first slightly inflexed, soon becoming straight, depressed when mature and dry; surface glabrous, light brown at first (5D5 to 5D7), becoming brown to dark brown (7E8 to 7F8) when mature, often darker at margin, with a conspicuous dark brown (6F8 to 7F8) umbo at centre. Lamellae crowded, sinuate to adnate, 3.0–4.0 mm broad, white, edge entire and concolorous, with lamellulae of two or four lengths. Stipe cylindrical and somewhat broadened downwards, 26–38 mm long × 4.0-−5.0 mm diam., central, solid, light brown (6D4 to 6D8) in upper part, becoming grey-brown (5E3 to 6E3) towards base, with whitish flocculose apex, longitudinally striate, with whitish basal tomentum. Context up to 30–50 mm thick near stipe attachment, thinner at margin, white. Odor faint. Spore deposit whitish.
Basidiospores (86/6/2) 7.0–8.0 (9.0) × 5.0-−6.0 (6.5) µm, av. 7.4 × 5.2 µm, Q = (1.40) 1.42–1.45 (1.50), obovate to ellipsoid, subhyaline, ornamentation of somewhat regular warts, less warts with ridges, amyloid. Basidia (43/6/2) (25) 27–30 (31) × (6.0) 7.0–9.0 (10.0) µm, av. 30 × 8.0 µm, clavate, subhyaline. All types of cystidia absent. Lamella edge sterile. Trama hyphae thin-walled, regular, 5.5–16.5 µm wide. Pileipellis hyphae cylindrical, with numerous branched, thin-walled, up to 7.5 µm wide. Stipitipellis hyphae in parallel, with few branches, 3.5–8.0 µm wide, thin-walled. Caulocystidia absent. Clamp connections absent in all tissues.
Habitat: Solitary or in small groups, saprotrophic on the soil, on the grass in woods. Known from south-western China.
Material studied: CHINA. Sichuan Province: Jiuzhaigou valley reserve, 19 Sep 2020, Ming Zhang (GDGM 84683).
Remarks: The most distinctive characteristics of Melanoleuca substridula are its slightly small-sized basidiocarp, light brown to dark brown pileus with a prominent umbo, sinuate to adnate lamellae, light brown to greyish-brown stipe and lack of cystidia. On account of the pileus color, M. substridula is closely related to M. stridula originally described by Singer (1943). However, M. stridula is featured by a slightly larger pileus (15–60 mm in diameter) (Singer, 1943). Additionally, M. stridula is often characterized by a pileus with a center depression, which differs by the umbonate pileus of M. substridula. Microscopically, M. stridula can be distinguishable from M. substridula by the presence of subcylindrical cystidia-like cells at the apex of the stipe (Metrod, 1949).
Discussion
Two new species, Melanoleuca subgriseoflava and Melanoleuca substridula, discovered and collected in Liaoning province and Sichuan province respectively, were originally reported and described in this study. Morphologically, the most distinctive features of M. subgriseoflava are a grey-brown to yellowish-grey pileus, creamy lamellae, fusiform hymenial cystidia and warted basidiospores. According to the classification system of Boekhout (1988), M. subgriseoflava should belong to the section Strictipedes in the subgenus Macrocystidia because of the presence of grey-brown pileus and fusiform hymenial cystidia (Boekhout, 1988). Amongst the section Strictipedes, M. subgriseoflava mainly differs from the other species by its lack of caulocystidia, including M. turrita (Fr.) Sing, M. polioleuca (Fr.: Fr.) Kühn. & Maire, M. atripes Boekhout and M. albifolia Boekhout and (Boekhout, 1988). Furthermore, M. albifolia Boekhout differs from M. subgriseoflava by white lamellae. M. polioleuca (Fr.: Fr.) Kühn. & Maire can be distinguishable from M. subgriseoflava by a longer stipe, with a length of around 35–85 mm (Boekhout, 1988).
Melanoleuca substridula is easily recognized by its light brown to dark brown pileus, prominent umbo, adnate to sinuate lamellae, light brown to grey-brown stipe and acystidiate micromorphology. For lack of any forms of cystidia, M. substridula belongs to the subgenus Melanoleuca (Boekhout, 1988). Within the subgenus Melanoleuca, M. graminicola (Velen.) Kiihner& Maire, M. melaleuca (Pers.: Fr.) Murrill, M. stridula (Fr.) Metrod and M. striimarginata Metrod are characterized by adnate to subdecurrent lamellae and a depressed pileus center, making them easily distinguishable from M. substridula (Boekhout, 1988). The latter three species also differ by their larger basidiomata, with a pileus diameter of 35–65 mm. Moreover, M. striimarginat a differs by a striate margin of the pileus (Metrod, 1942).
Both the phylogenetic relationships and the divergence-time estimation, based on three regions (ITS-nrLSU-RPB2), showed that there are five clades in the genus Melanoleuca (Figs. 1 and 2), which was corroborated by Pei et al. (2021). According to the phylogram, M. subgriseoflava is closely related to the other three species with high support in clade E, i.e., M. arcuata, M. heterocystidiosa and M. griseoflava. Melanoleuca arcuata differs by its brick-red pileus and decurrent lamellae (Fries, 1821). Melanoleuca heterocystidiosa can be easily separated from M. subgriseoflava based on its smaller basidiomata, with a pileus diameter of 15 mm (Singer, 1939; Bon, 1984). Melanoleuca griseoflava differs from M. subgriseoflava as elaborated above. In clade A, with the exception of M. microcephala, M. substridula is far away from the other species of Melanoleuca. However, M. microcephala can easily distinguish from M. substridula by its longer stipe with a length of 105 mm. Besides, caulocystidia in groups can be observed in M. microcephala, but not any forms of cystidia in M. substridula (Antonín et al., 2021).
In the present study, five clades can be recognized in both the BI tree (Fig. 1) and the MCC tree (Fig. 2). However, using phylogenetic analyses, species of Melanoleuca were divided into two clades in former research (Vizzini et al., 2011; Yu et al., 2014; Nawaz, Jabeen & Khalid, 2017; Xu et al., 2019). The species of Melanoleuca within each clade have lacked uniform characteristics to work in identification. In order to clarify the infrageneric classification, taxonomic treatments should be performed based on additional materials and complete morphological descriptions in later studies. Two new Melanoleuca species have been confirmed and a key for further studies on the Melanoleuca genus has been provided in this study.