Beyond RuBisCO: convergent molecular evolution of multiple chloroplast genes in C4 plants
- Published
- Accepted
- Received
- Academic Editor
- Carmen Arena
- Subject Areas
- Agricultural Science, Evolutionary Studies, Genomics, Plant Science
- Keywords
- C4 photosynthesis, Grasses, PACMAD, Molecular evolution, Convergent evolution, Poaceae, C3 photosynthesis
- Copyright
- © 2022 Casola and Li
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. Beyond RuBisCO: convergent molecular evolution of multiple chloroplast genes in C4 plants. PeerJ 10:e12791 https://doi.org/10.7717/peerj.12791
Abstract
Background
The recurrent evolution of the C4 photosynthetic pathway in angiosperms represents one of the most extraordinary examples of convergent evolution of a complex trait. Comparative genomic analyses have unveiled some of the molecular changes associated with the C4 pathway. For instance, several key enzymes involved in the transition from C3 to C4 photosynthesis have been found to share convergent amino acid replacements along C4 lineages. However, the extent of convergent replacements potentially associated with the emergence of C4 plants remains to be fully assessed. Here, we conducted an organelle-wide analysis to determine if convergent evolution occurred in multiple chloroplast proteins beside the well-known case of the large RuBisCO subunit encoded by the chloroplast gene rbcL.
Methods
Our study was based on the comparative analysis of 43 C4 and 21 C3 grass species belonging to the PACMAD clade, a focal taxonomic group in many investigations of C4 evolution. We first used protein sequences of 67 orthologous chloroplast genes to build an accurate phylogeny of these species. Then, we inferred amino acid replacements along 13 C4 lineages and 9 C3 lineages using reconstructed protein sequences of their reference branches, corresponding to the branches containing the most recent common ancestors of C4-only clades and C3-only clades. Pairwise comparisons between reference branches allowed us to identify both convergent and non-convergent amino acid replacements between C4:C4, C3:C3 and C3:C4 lineages.
Results
The reconstructed phylogenetic tree of 64 PACMAD grasses was characterized by strong supports in all nodes used for analyses of convergence. We identified 217 convergent replacements and 201 non-convergent replacements in 45/67 chloroplast proteins in both C4 and C3 reference branches. C4:C4 branches showed higher levels of convergent replacements than C3:C3 and C3:C4 branches. Furthermore, we found that more proteins shared unique convergent replacements in C4 lineages, with both RbcL and RpoC1 (the RNA polymerase beta’ subunit 1) showing a significantly higher convergent/non-convergent replacements ratio in C4 branches. Notably, more C4:C4 reference branches showed higher numbers of convergent vs. non-convergent replacements than C3:C3 and C3:C4 branches. Our results suggest that, in the PACMAD clade, C4 grasses experienced higher levels of molecular convergence than C3 species across multiple chloroplast genes. These findings have important implications for our understanding of the evolution of the C4 photosynthesis pathway.
Introduction
Convergent evolution represents the independent acquisition of similar phenotypic traits in phylogenetically distant organisms. Understanding the genomic changes underlying the recurrent emergence of phenotypes is a major goal of molecular evolution. The rapidly increasing taxonomic breadth of genomic resources combined with the development of rigorous frameworks to comparatively investigate molecular changes has accelerated the pace of discovery in this area. For instance, substitutions in coding regions of conserved genes have been implicated in phenotypic changes responsible for adaptation of marine mammals to an aquatic lifestyle (Foote et al., 2015; Zhou, Seim & Gladyshev, 2015). Other examples of convergent phenotypes whose molecular underpinnings have been investigated include adaptations in snake and agamid lizard mitochondria (Castoe et al., 2009), echolocation in mammals (Parker et al., 2013; Thomas & Hahn, 2015; Zou & Zhang, 2015; Storz, 2016), and hemoglobin function in birds (Natarajan et al., 2016). Convergent traits can evolve via changes toward the same derived state (similar phenotype) from the same initial state, which is known as parallelism, or through changes of different initial states, referred to as convergence (Zhang & Kumar, 1997; Storz, 2016). For the sake of simplification, we will refer to these two processes using the general terminology ‘convergence’ and ‘convergent replacements’ throughout the manuscript, unless differently stated.
Several traits are also known to have convergently evolved in land plants (Li et al., 2018; Lu et al., 2018; Preite et al., 2019). One of the most notable examples is represented by the repeated evolution of the C4 photosynthetic pathway in flowering plants. The C4 pathway is a complex functional adaptation that allows for better photosynthesis efficiency under certain environmental conditions, such as dry and warm climates, high light intensity, low CO2 concentration, and limited availability of nutrients (Knapp & Medina, 1999; Long, 1999). The C4 pathway involves cytological, anatomical and metabolic modifications thought to have evolved multiple times independently in various lineages from the C3 type (Kellogg, 1999; Sage, 2004; Sage, Christin & Edwards, 2011). According to phylogenetic, anatomical and biochemical evidence, the few slightly different variants of the C4 photosynthesis type evolved more than 60 times in angiosperms (Sage, Sage & Kocacinar, 2012; Heyduk et al., 2019). In grasses (family Poaceae) alone, the C4 pathway has evolved independently ∼20 times (Grass Phylogeny Working Group II, 2012).
Transitions from C3 to C4 plants resulted from genetic changes that include nonsynonymous substitutions, gene duplications and gene expression alterations (Christin et al., 2007; Christin et al. 2013a; Christin et al., 2015; Goolsby et al., 2018; Heyduk et al., 2019). It has been suggested that the evolution of the C4 pathways proceeded throughout a series of evolutionary steps wherein the Kranz leaf anatomy typical of this pathway originated first, followed by changes in the expression patterns of key genes and finally by adaptive modifications of protein sequences (Sage, Sage & Kocacinar, 2012; Christin et al. 2013b; Williams et al., 2013). A model of the adaptive steps leading to C4 photosynthesis showed that key biochemical components of this pathway evolved modularly along a trajectory that was likely very similar across lineages with C3 to C4 transitions (Heckmann et al., 2013). Overall, these scenarios suggest that enzymes involved in C3 to C4 transitions experienced similar selective pressures that resulted in the convergent evolution of the same amino acid replacements across C4 lineages.
Evidence of convergent changes in proteins associated with photosynthetic processes has steadily accumulated since genomic data from multiple C4 lineages have become available in the past couple of decades. Most of these studies have focused on the ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO), a large multimeric enzyme that catalyzes the carboxylation of ribulose-1,5-bisphosphate (RuBP), allowing plants to fix atmospheric carbon (Andersson & Backlund, 2008). RuBisCO also catalyzes oxygenation of RuBP, which leads to loss of carbon in the process of photorespiration (Andersson & Backlund, 2008; Maurino & Peterhansel, 2010). RuBisCO’s limited ability to discriminate between CO2 and O2 has been attributed to the much higher CO2 to O2 atmospheric partial pressure until ∼400 million years ago (Sage, 1999; Sage, 2004; Sage, Sage & Kocacinar, 2012).
Previous studies have revealed multiple convergent amino acid replacements in the large RuBisCO subunit in C4 lineages, encoded by the chloroplast gene rbcL (Kapralov & Filatov, 2007; Christin et al., 2008; Kapralov et al., 2011; Kapralov, Smith & Filatov, 2012; Piot et al., 2018). Some of these convergent replacements have been associated to positive selection of the corresponding codons in C4 monocot and eudicot lineages (Kapralov & Filatov, 2007; Christin et al., 2008; Kapralov, Smith & Filatov, 2012; Piot et al., 2018). Notably, biochemical analyses have demonstrated that some recurrent amino acid changes in the large RuBisCO subunit of C4 plants critically alter the kinetics of RuBisCO, resulting in an accelerated rate of CO2 fixation at the beginning of the Calvin-Benson cycle (Studer et al., 2014; Bouvier et al., 2021). Convergent amino acid changes have also been described in enzymes that are encoded by nuclear genes and play a primary role in the C 4pathway, including the phosphoenolpyruvate carboxylase PEPC (Christin et al., 2007; Besnard et al., 2009), the NADP-malic enzymes NADP-me (Christin et al. 2009b), the phosphoenolpyruvate carboxykinase PEPCK (Christin et al. 2009a) and the small RuBisCO subunit (Kapralov et al., 2011).
Given the number of biochemical, physiological and anatomical traits that were affected in each evolutionary transition from C3 to C4 photosynthesis (Heyduk et al., 2019), it is likely that many genes experienced analogous selective pressures across taxa that include C4 plants. This has been shown to be the case by Huang et al. (2017), who have developed an approach to identify potential genes involved in the transition to C4 photosynthesis using a genome-wide scan for selection along a phylogeny of PACMAD grasses. Of the 88 genes showing signatures of positive or relaxed selection in C4 species, several were not previously known to have a role in C4 photosynthesis. Although this study did not focus on finding convergent replacements, it provided a comprehensive strategy and statistical testing framework to identify novel genes that have likely played a role in the evolution of C4 grasses. It is possible that a significant fraction of these genes accumulated convergent amino acid replacements during C3-to-C4 transitions.
Another recent, important work has produced the first analysis of convergent replacements across multiple proteins involved in the metabolism of C4 and crassulacean acid metabolism (CAM) among species belonging to the portullugo clade (Caryophyllales). Goolsby and colleagues (2018) compared evolutionary patterns in 19 gene families with critical roles in metabolic pathways of both C4 and CAM plants, also known as carbon-concentration mechanisms (CCMs) genes, and in 64 non-CCM gene families. They found convergent replacements in proteins from C4 and CAM lineages, as well as higher levels of convergent replacements in CCM vs. non-CCM gene families (Goolsby et al., 2018). Additionally, several amino acid replacements that are prevalent among C4 and CAM taxa compared to C3 lineages were identified in this study (Goolsby et al., 2018).
Altogether, the results of this and other studies demonstrated that convergent molecular evolution occurred across multiple genes in both C4 and CAM groups. While significant progress has been made towards the detection of signatures of selection associated to the evolution of CCMs (Huang et al., 2017; Piot et al., 2018), a rigorous framework to assess the full extent of molecular convergence in C3 to CCMs transitions has yet to be presented. For example, analyses of convergent evolution should include null hypotheses that assume no differences between taxa with and without convergence. In the case of CCMs evolution, a plausible null hypothesis consists in statistically equivalent numbers of convergent replacements between C4 (or CAM) lineages and C3 lineages.
Additionally, nonadaptive replacements should be used to normalize convergent replacements, in order to account for variation in the rates of nonsynonymous substitutions across lineages. This approach has been successfully applied in studies of molecular convergent evolution in vertebrates by assessing both convergent replacements and protein sequence changes that result in different amino acids, or divergent replacements (Castoe et al., 2009; Thomas & Hahn, 2015; Zou & Zhang, 2015). A broader definition of the latter group incorporates all replacements leading to different amino acids, regardless of their ancestral state. We refer to such changes as non-convergent replacements.
Furthermore, testing hypotheses about the extent of convergent molecular evolution remains particularly challenging for many nuclear genes, because of the prevalence of duplicated copies, particularly in plants (Christin et al., 2007; Goolsby et al., 2018). Single-copy nuclear or organelle genes allow to more easily recognize convergent changes and overcome possible confounding compensatory effects due to the presence of paralogous copies.
Given these premises, we sought to test if convergent amino acid changes occur more frequently in proteins encoded by chloroplast genes in a taxon that includes multiple well-characterized lineages of C4 and C3 grasses. Chloroplast proteins represent an ideal set of targets to study the role of convergent evolution in C3 to C4 transitions for a variety of reasons. First, most chloroplast proteins are involved in biochemical and biophysical processes that are critical to photosynthesis. For instance, out of ∼75 functionally annotated protein-coding genes in the maize chloroplast genome, 45 genes are implicated in photosynthesis-related processes, including rbcL, 17 genes coding for subunits of the photosystems I and II (PS I and PS II), 12 genes coding for subunits of the NADH dehydrogenase complex, 6 genes coding for chloroplast ATPase subunits, 4 genes coding for cytochrome b6f complex subunits, and a few more genes implicated in the assembly of other protein complexes (Maier et al., 1995). Second, nonannotated orthologous copies of chloroplast genes can be readily identified across plants through sequence homology searches, taking advantage of the thousands of complete chloroplast genome sequences currently available for green plants. Third, comparative studies of convergent evolution in C4 photosynthesis are facilitated by detailed reconstruction of phylogenetic relationships within groups with both C4 and C3 lineages. Fourth, signatures of positive selection have been found in multiple chloroplast genes in taxa that contain both C3 and C4 plants, although only the genes rbcL and psaJ, which encodes a small subunit of the Photosystem I complex, showed evidence of adaptive changes exclusively in C4 lineages (Christin et al., 2008; Goolsby et al., 2018; Piot et al., 2018). Finally, most chloroplast genes occur as single copy loci, as opposed to the multiple paralogs typically present for plant genes encoded in the nucleus.
In this study, we analyzed 67 chloroplast genes from 64 grass species, including 43 C4 and 19 C3 species belonging to the PACMAD clade, named after six of its most representative subfamilies: Panicoideae, Arundinoideae, Chloridoideae, Micrairoideae, Aristidoideae and Danthonioideae. Using published information, we placed thirteen known independent C3 to C4 transitions in the reconstructed phylogeny of these 64 species. We applied a series of tests based on convergent vs. non-convergent amino acid replacements and determined that convergent molecular evolution occurred at a higher rate in chloroplast genes of C4 lineages compared to C 3lineages, a pattern that remained largely unchanged after excluding the RbcL protein from the convergence analyses. Our findings suggest that the evolutionary trajectories of multiple chloroplast genes have been affected during the emergence of the C4 adaptation in the PACMAD clade, a result that has significant implications for our understanding of C4 photosynthesis evolution.
Methods
Data source and filtering
We queried NCBI GenBank (Sayers et al., 2019) for complete chloroplast genome sequences of grass species that were included in phylogenetic analyses by the Grass Phylogeny Working Group II (2012) and downloaded the corresponding coding sequences. Each species was assigned to either C3 or C4 type following the results of the Grass Phylogeny Working Group II (2012). Additionally, we downloaded the coding chloroplast sequences for Dichanthelium acuminatum, Thyridolepis xerophila, Sartidia dewinteri and Sartidia perrieri (C3 species) (Brown & Smith, 1972; Smith & Brown, 1973; Hattersley & Stone, 1986; Hattersley et al., 1986; Besnard et al., 2014). We used the standalone blastn ver. 2.2.29+ (Camacho et al., 2009) with the Expect value (E) cutoff of 1e-10 to determine putative sequence orthology with coding sequences of the Zea mays chloroplast genes (Maier et al., 1995). Single copy putative orthologs that were present in more than 95% of the species were retained for further analysis (Table S1).
Multiple sequence alignment
We aligned the individual sequences using TranslatorX ver. 1.1 (Abascal et al., 2010) and the multiple sequence aligner MUSCLE with default parameters. Alignments were further adjusted manually using BioEdit ver. 7.0.9.0 (Hall, 1999). Stop codons and sites that could not be aligned unambiguously were removed.
Phylogeny reconstruction
We concatenated the individual sequence alignments and extracted third codon position sites for phylogeny reconstruction. We ran PartitionFinder ver. 1.1.1 (Lanfear et al., 2012) to identify the best partitioning scheme (partitioning by gene) for the downstream analysis using both Akaike information criterion (AIC) (Akaike, 1973) and Bayesian information criterion (BIC) (Schwarz, 1978). We then used maximum likelihood framework as implemented in RAxML ver. 8.2.10 (Stamatakis, 2014) to reconstruct the phylogeny. Branch support was estimated using 1,000 bootstrap replicates. Oryza sativa and Brachypodium distachyon from the BOP (Bambusoideae, Oryzoideae and Pooideae) clade were used as outgroup, whereas all ingroup species belonged to the PACMAD clade. We used FigTree ver. 1.4.0 (Rambaut, 2012) to rearrange and visualize the phylogeny, and the figures were edited further to improve readability and to indicate C4/C3 classification.
Ancestral state reconstruction
We reconstructed ancestral states at each phylogenetic node for each individual gene using the program codeml from the software package PAML ver. 4.9a (Yang, 2007) and the basic codon substitution model (model = 0, NSsites = 0). The guide tree consisted of the cladogram of all species with available sequences for each individual gene. Sites with gaps in one or more PACMAD species were excluded.
Definition and characteristics of “reference branches”
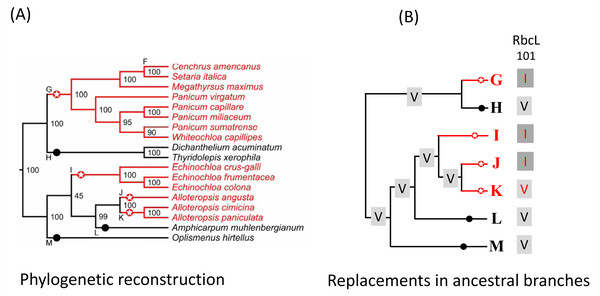
In the reconstructed PACMAD phylogeny, we identified the branches including the most recent common ancestors of C4-only clades and C3-only clades. We refer to these branches as “C4 reference branches” and “C3 reference branches”, respectively (see Figs. 1 and 2). We then compared the inferred protein sequence of each reference branch with the inferred sequence in their ancestral branch (next branch toward the root), in order to identify individual site changes that occurred along reference branches.
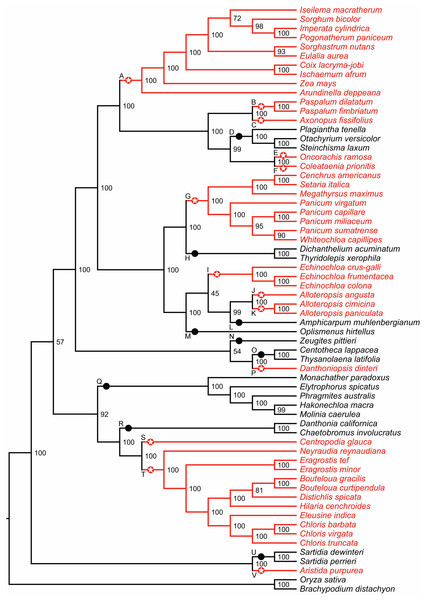
Figure 1: Phylogenetic relationships among 64 C4 and C3 grass species.
The phylogeny tree was obtained using RAxML (GTR+ Γ model) based on the third codon position sites in 67 chloroplast genes. The partitioning scheme was selected according to Akaike information criterion (AIC). C4 and C3 reference branches are shown in red and black, respectively. Red stars and black circles (labels A–V) indicate C4 and C3 reference branches, respectively. Numbers represent bootstrap support.To assess the number of convergent and non-convergent replacements, amino acid changes were compared in all possible pairs of reference branches. Replacements in two reference branches that resulted in the same state (amino acid) at a given site were considered convergent, regardless of whether the corresponding ancestral states were the same or different (Castoe et al., 2009). After identifying convergent replacements, we separated them into parallel and convergent changes (Zhang & Kumar, 1997; Storz, 2016). Likewise, two replacements were considered non-convergent if states at the descendant orthologous sites were different, regardless of the corresponding ancestral states (Castoe et al., 2009).
Figure 2: Example of C4 and C3 reference branches and convergent changes in C4 reference branches.
(A) PACMAD phylogeny and identification of reference branches. The C4 reference branches (highlighted by red circles with stars) contain the common ancestor of a clade with only C4 species (red lines). The C3 reference branches (highlighted by black circles) contain the common ancestor of a clade with only C4 species (black lines). C4 reference branches that are next to each other represent lineages that independently acquired the C4 pathway and are separated by species with the C3 pathway that were no included in this study because of the lack of complete chloroplast genomes. For each species, the C4 or C3 photosynthesis type was obtained from the Supplementary figure 1 in the Grass Phylogeny Working Group II (2012). (B) Amino acid replacements in the reference branches. The sequence of chloroplast proteins was inferred in each reference branch and compared to the inferred sequence in the branch ancestral to the reference branch. In this example, the amino acid 101 in the protein RbcL is represented by a Valine (V) in branches ancestral to all reference branches, but a convergent V->I amino acid replacement occurred along the C4 reference branches G, I and J.The pairwise comparisons between reference branches are akin to the phylogenetically independent contrast (PIC) method developed by Felsenstein (Felsenstein, 1985). In the PIC approach, the values to compare are represented by differences between branches. The differences between two branches are independent of the differences between two other branches. Therefore, pairwise comparisons of these values are independent and can be tested using 2 × 2 contingency table tests (see also below). In our study, pairwise comparisons are independent from each other, i.e., replacements in each pair of branches are independent of replacements in each other pairs of branches. The difference from the PIC method is that we compare both differences (non-convergent replacements) and similarities (convergent replacements). A similar approach have been used in studies of convergent amino acid replacements (Castoe et al., 2009; Foote et al., 2015; Thomas & Hahn, 2015).
Reference branch lengths were extracted from the RAxML phylogeny obtained on the AIC partitioning scheme (Fig. S2). Testing was performed on the sum of pairs of branch lengths for each photosynthesis type using the R package exactRankTests (Table S2).
Inference of convergent and non-convergent replacements and statistical testing
Using the approach described above, we identified putative convergent and non-convergent amino acid changes in each gene product individually. We summarized those data within each of the three categories: (1) two C4 reference branches (C4:C4), (2) C3 reference branch and C4 reference branch (C3:C4), and (3) two C3 reference branches (C3:C3).
To test the significance of replacement differences between categories we used the
Boschloo’s exact unconditional test (Boschloo, 1970) implemented in the SciPy library ver. 1.7.1 in python3 (Virtanen et al., 2020). In the Boschloo’s test, the p-value from the Fisher’s exact test represents the test statistic of the exact unconditional test. It has been shown that Boschloo’s test is more powerful than Fisher’s exact test (Mehrotra, Chan & Berger, 2003). There is no restriction to using contingency table tests, including Boschloo’s test, on categories with different sample size, as long as the categories are independent (Mehrotra, Chan & Berger, 2003), as in the case of reference branches in our phylogeny.
Data availability
Raw data, including alignments, fasta sequences, and phylogenetic analyses data, are available through the following Figshare repository: https://figshare.com/articles/dataset/Convergence-chlorplast-genes-C4-Casola-Li-2021/15180690.
Results
Phylogeny reconstructions
We examined 63 grass chloroplast genomes to identify gene orthologs for Zea mays chloroplast genes and extracted the corresponding coding and protein sequences. The resulting dataset included up to 67 DNA/protein sequences in 64 grass species that were retained for further analysis (Table S1). One to four sequences were absent in thirteen species. Out of 64 species, 43 were classified as C4 and 21 (including two outroup species) as C3. The reconstructed phylogeny is well supported, except for three branches with low to moderate bootstrap values, and it is consistent for both AIC and BIC partitioning schemes (Fig. 1 and Figs. S1–S3). We identified thirteen C4 reference branches that represent putative C3 to C4 transitions, and nine C3 reference branches (Fig. 1). Four pairs of reference branches corresponding to C3 to C4 transitions—B–C, E–F, J–L and S–T—are sister to each other in Fig. 1. Phylogenetic inferences from deep-taxonomic sampling of the PACMAD clade has shown that each of the these four pairs of reference branches is separated by at least one clade of C3 species (Grass Phylogeny Working Group II, 2012), supporting the independent origin of C4 photosynthesis in all reference branches shown in Fig. 1. However, no high-quality chloroplast genomes are available for any of the C3 species between these pairs of reference C4 branches, precluding their inclusion in our study.
Overall, the reference branches A-V showed support values that were in close agreement with those reported in the Grass Phylogeny Working Group II (2012), including the three branches with low statistical support in our tree. Importantly, the species topology was identical between the two phylogenies downstream these three branches. We also noticed three other branches that shared higher statistical support in our phylogeny compared to the Grass Phylogeny Working Group II tree. Two of these branches occurred in the subtribe Boivinellinae and correspond to the split between the group J/K and the branch L, and the split between the group I/J/K/L and the branch M (Fig. 1). The third node with higher support in our phylogeny correspond to the reference branch Q (tribe Arundoideae).
Convergent and non-convergent amino acid replacements across chloroplast proteins
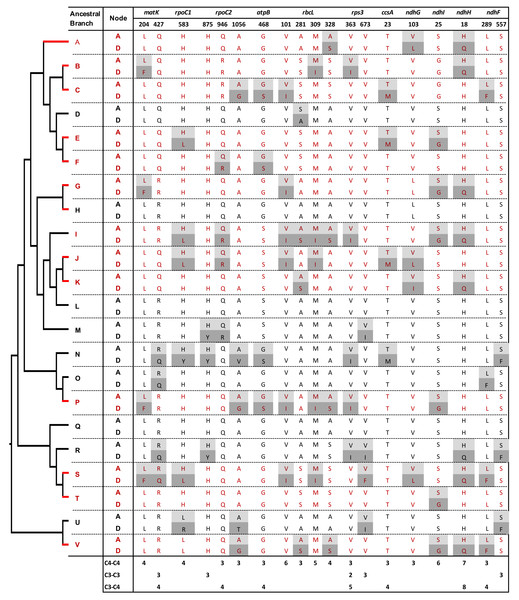
We assessed the level of molecular convergence in C3 to C4 transitions by quantifying convergent and non-convergent amino acid replacements across the PACMAD phylogeny by performing pairwise comparisons of reconstructed sequences in reference branches (Figs. 2 and 3, Table S3; see Methods). A total of 217 sites showed at least one convergent replacement: 104 in C4:C4, 120 in C3:C4 and 34 in C3:C3 pairs. A further 201 sites exhibited one or more non-convergent replacements: 96 in C4:C4, 121 in C3:C4, and 39 in C3:C3 pairs (Table 1). The difference in convergent/non-convergent site distributions between the three photosynthesis types was not statistically significant (P ≥ 0.05, Boschloo’s test; Table 1). The vast majority of convergent replacements shared the same ancestral state and should thus be considered parallel replacements according to widely accepted definitions of convergence (Zhang & Kumar, 1997; Storz, 2016). Only two sites, one in MatK (T205S/K205S in two C4 reference branches) and the other in NdhF (L636I/K636I in one C4 and three C3 reference branches), shared replacements with different ancestral states, representing true convergent sites (Table S3).
Figure 3: Amino acid replacements shared by at least three C4 or C3 reference branches.
Ancestral (A) and derived (D) amino acids at replacement sites are shown. Site numbers correspond to the Zea mays orthologous sequence annotation. Red and black letters and branches represent C4 and C3 reference branches, respectively (see also Figs. 1 and 2).To control for possible biases in the counting of convergent replacements due to branch length variation, we tested whether reference branch lengths in the three photosynthesis types C4:C4, C3:C4 and C3:C3 were different (Table S2). We found no significant difference among types (P > 0.5 for each of the three pairwise comparisons, Mann–Whitney U test). We performed the same test only on branches with convergent and non-convergent replacements and found no significant difference between categories (P > 0.5, Mann–Whitney U test; Table S2). Therefore, branch length variation between the three types is not expected to affect our results.
Among the C4 reference branches, several individual sites showed high contrast in the number of branches involved in convergent and non-convergent replacements (Fig. 3, Tables S3 and S4). For example, seven C4 branches (54%) shared the H18Q replacement in the product of ndhH, with no non-convergent replacements. Six, five, and four C4 branches (46%, 38%, and 31%) showed convergent replacements at three sites in the RbcL protein (V101I, M309I, and A328S, respectively). Furthermore, six C4 branches shared the S25G replacement in the product of ndhI and four L204F changes in the protein encoded by matK. In all these cases, there were no other convergent or non-convergent replacements in C3:C3 or C3:C4 branch comparisons, except for one H18Q change in NdhH in a C3:C3 branch. Two sites with convergent replacements in the proteins encoded by ndhF (L557F) and rpoC2 (H875Y) were found uniquely in C3:C3 pairs, and only one site in the protein Rps3 showed convergence independently in C4:C4 and C3:C3 pairs (Fig. 3).
| C4:C4 | C3:C4 | C3:C3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Con | NC | Ratio | Con | NC | Ratio | Con | NC | Ratio | |
| Sites | 104 | 96 | 1.08 | 120 | 121 | 0.99 | 34 | 39 | 0.87 |
| Sites* | 80 | 64 | 1.25 | 82 | 69 | 1.19 | 17 | 16 | 1.06 |
| Genes | 24 | 23 | 1.04 | 26 | 32 | 0.81 | 13 | 17 | 0.76 |
| Genes* | 24 | 20 | 1.2 | 25 | 29 | 0.86 | 9 | 10 | 0.9 |
Notes:
- Con
-
convergent
- NC
-
non-convergent
We then searched for convergent replacements that occurred along more than two C4 branches at sites that remained otherwise conserved in C3 and C4 lineages, arguing that such changes could result from selective pressure rather than drift. We identified twelve C4-specific convergent sites in proteins from 7 genes: matK, ndhF, ndhG, ndhI, rbcL, rpoC1 and rpoC2 (Table S4). Five of these sites were found in RbcL, whereas two sites were identified in NdhI. We also observed two convergent sites NdhF and one in RpoC2 that were uniquely found in three C3 branches.
Molecular convergence in individual chloroplast proteins
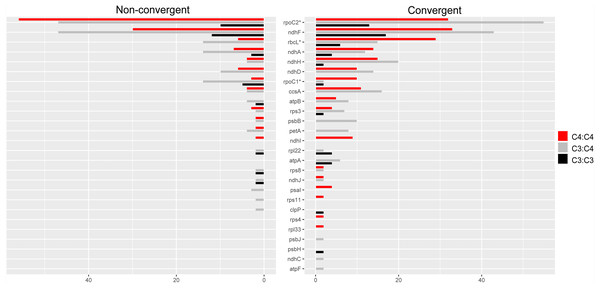
Convergent and non-convergent amino acid replacements were detected in the products of 45 chloroplast genes, thirteen of which had at least one site with four or more replacements (Fig. 4, Tables 1 and S3). Twenty-four genes had convergent changes in C4:C4, 26 in C3:C4, and 13 in C3:C3 types of pairs (Table 1). Although the convergent/ non-convergent replacement ratio was higher in C4:C4 pairs than C3:C4 and C3:C3 pairs, the differences between the three photosynthesis types were not statistically significant (P ≥ 0.05, Boschloo’s test; Table 1). The lack of replacements was the single most common state for chloroplast proteins across photosynthesis types; however, in C4:C4 there were more genes with a higher number of convergent vs. non-convergent replacements (Fig. 4 and Table S5).
Figure 4: Distribution of convergent and non-convergent amino acid replacements in pairs of reference branches.
(A) C4:C4 pairs. (B) C3:C3 pairs. (C) C3:C4 pairs. NC: non-convergent.Overall, 26 proteins showed a higher number of convergent vs. non-convergent sites, of which 16, 13 and 10 were found in C4:C4, C3:C4 and C3:C3 pairs, respectively (Fig. 5 and Table S5). We found statistically significant differences in the number of convergent vs. non-convergent replacements between C4:C4 and C3:C4 pairs, but not C3:C3 pairs, in the products of the genes rbcL, rpoC1 and rpoC2 (P < 0.05, Boschloo’s test; Table S5). In RbcL and RpoC1, C4:C4 pairs shared much higher proportion of convergent vs. non-convergent replacements, whereas the opposite was true in RpoC2. RpoC1 was also the only protein showing more convergent than non-convergent replacements in C4:C4 pairs compared to C3:C3 and C3:C4 pairs. In C4:C4 pairs, RpoC1 shared 4 convergent and 1 non-convergent replacement, compared to 1 and 2 in C3:C3 pairs and 1 and 5 in C3:C4 pairs, respectively. Additionally, the proteins NdhG, NdhI, PsaI, RpoA, Rps4 and Rps11 exhibited convergent replacements only in C4:C4 pairs (Table S5). When considering the number of affected sites rather than the number of replacements, no genes showed a significantly different pattern between photosynthesis types (P ≥ 0.05, Boschloo’s test; Table S5).
Figure 5: Amino acid replacements in chloroplast proteins with more convergent than non-convergent changes.
Twenty-six chloroplast proteins with more convergent than non- convergent changes in C4:C4, C3:C4 and C3:C3 pairs.The proteins encoded by matK, rpoC2 and ndhF shared much higher numbers of both convergent and non-convergent replacements than other chloroplast proteins across all photosynthesis type comparisons (Table S5). Both matK and ndhF are known to be rapidly evolving and have been consistently used in low taxonomic level phylogenetic studies in flowering plants (Patterson & Givnish, 2002; Barthet & Hilu, 2008). The gene rpoC2 has also been recently described as a useful phylogenetic marker in angiosperms (Walker et al., 2019).
Molecular convergence across reference branches
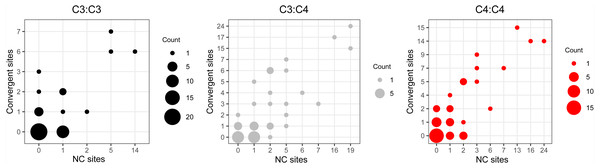
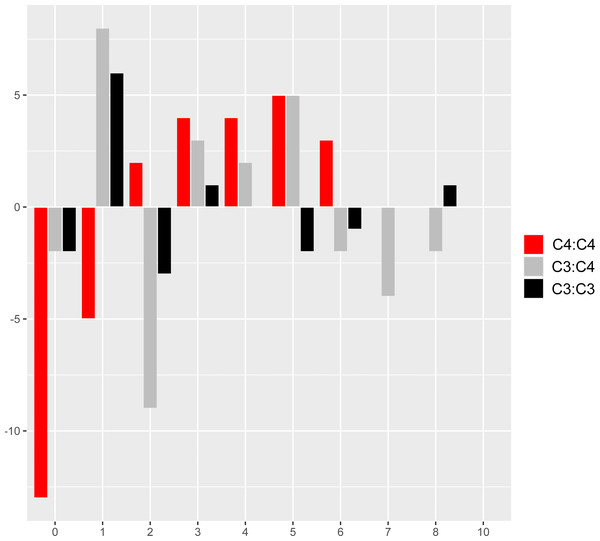
The comparison of reference branch pairs with convergent and non-convergent replacements revealed remarkable differences between photosynthesis types. Overall, C4:C4 pairs of reference branches showed a distribution skewed toward more convergent and non-convergent replacements than the two other categories (Fig. 6). There were significantly fewer pairs of C4:C4 reference branches with no replacements and with no convergent replacements than C3:C4 and C3:C3 pairs (P < 0.05, Boschloo’s test; Table 2). Conversely, significantly more C4:C4 pairs shared more convergent than non-convergent replacements, and at least two convergent changes compared to C3:C4 and C3:C3 pairs (P < 0.05, Boschloo’s test; Table 2). No significant difference was observed between pairs of C3:C4 and pairs of C3:C3. We found identical patterns when the same analyses were performed after excluding all replacements in the RbcL protein, except for the lack of a significant difference between C4:C4 and C3:C3 in the proportion of pairs with non-convergent replacements and pairs with more convergent than non-convergent changes (Table S6).
Figure 6: Pairs of reference branches by convergent and non-convergent replacements.
Difference in the number of pairs of reference branches for convergent and non-convergent categories (0–8 and 10 replacements).| C4:C4 | C3:C4 | C3:C3 | |
|---|---|---|---|
| No replacements | 6 (.08) | 30 (.26) | 12 (.33) |
| No Con | 12 (.15) | 48 (.41) | 16 (.44) |
| w/Con | 66 (.85) | 69 (.59) | 20 (.56) |
| w/NC | 63 (.81) | 67 (.57) | 18 (.50) |
| Con > NC | 40 (.51) | 36 (.31) | 10 (.28) |
| Con > 1 | 49 (.63) | 39 (.33) | 8 (.22) |
Notes:
- Con
-
convergent
- NC
-
non-convergent
- Con > NC
-
pairs of branches with more convergent than non-convergent replacements
- Con > 1
-
pairs of branches with more than one convergent replacement
Distribution of amino acid replacements across PACMAD lineages
Convergent and non-convergent replacements were preferentially found in specific pairs of reference branches. In C4 pairs, convergent sites were most abundant between Danthoniopsis dinteri and Aristida purpurea (ten sites, branches P and V in Fig. 1), whereas non-convergent sites were most common between Centropodia glauca and Aristida purpurea (ten sites, branches S and V in Fig. 1). In pairwise C3 branch comparisons, most convergent sites were identified between both Zeugites pittieri and Danthonieae (branches N and R in Fig. 1) and Danthonieae and Sartidia spp. (branches R and U in Fig. 1), whereas the most non-convergent site-rich pair was formed by Zeugites pittieri and Sartidia spp. (eight sites, branches N and U in Fig. 1; Table S7).
Molecular convergence in the RuBisCO large subunit
We further inspected the evolution of the RuBisCO large subunit across the PACMAD clade. A total of 4 out of 9 RbcL amino acids with convergent changes in C4 reference branches—V101I, A281S, M309I and A328S—have been identified in previous studies on PACMAD grasses (Christin et al., 2008; Piot et al., 2018) as sites that experienced adaptive evolution in C4 species (Table 3). A further site, T143A, was found to evolve under positive selection in C3 to C4 transitions in monocots (Studer et al., 2014). Interestingly, an adaptive S143A replacement has also been detected in the gymnosperm Podocarpus (Sen et al., 2011). Three more sites with convergent replacements—at positions 93, 94 and 461—correspond to amino acids that were reported to evolve under positive selection in different groups of seed plants by Kapralov & Filatov (2007). Thus, all of the rbcL codons that appear to have evolved convergently among the PACMAD C4 lineages we have examined are also known to have experienced adaptive evolution in seed plants, but not all of them have been shown to evolve adaptively in C4 grasses.
| Codon | Ancestral AA | Convergentchange/p.s.s. | #Convergenta.b. |
|---|---|---|---|
| 10 | S | G | 2 |
| 93 | E | D | 2 |
| 94 | A | P | 2 |
| 101*† | V | I | 6 |
| 142*† | P | Several | na |
| 143 | T | A | 3 |
| 145*† | S | A/V | na |
| 258* | R | K | na |
| 270* | L | I | na |
| 281*† | A | S | 3 |
| 282† | H | Several | na |
| 309*† | M | I | 5 |
| 328*† | A | S | 4 |
| 461* | V | I | 2 |
| 468† | E | D | na |
| 471† | E | Several | na |
| 476† | I | L/V | na |
Notes:
- Ancestral AA
-
ancestral amino acid
- Convergent change/p.s.s
-
derived amino acid in multiple C4 reference branches and positively selected sites from previous studies
- #Convergent a.b.
-
number of reference branches with convergent changes
Bold indicates sites with convergent changes identified in this study.
Discussion
The recurrent emergence of carbon-concentration mechanisms (CCMs) across multiple angiosperm clades in the past 35 million years represents one of the most striking examples of convergent evolution of a complex phenotypic trait (Sage, Christin & Edwards, 2011; Heyduk et al., 2019). Several investigations have shown that the phenotypic parallelism across C4 lineages is to some extent mirrored by convergent changes in the sequence of proteins with key metabolic roles in the biochemistry of C4 photosynthesis, both in monocots and eudicots (Christin et al., 2007; Besnard et al., 2009; Christin et al. 2009a; Christin et al. 2009b; Kapralov et al., 2011; Goolsby et al., 2018). Furthermore, biochemical analyses have determined that some of these changes reflect adaptive shifts, as in the case of the increased availability of CO2 at the RuBisCO site (Studer et al., 2014). Substantial changes in several RuBisCO kinetic traits associated to C3 to C4 transitions have recently been described (Bouvier et al., 2021). Further evidence of changes in the selective pressure associated to the C3 to C4 transitions have emerged from the detection of several positively selected sites in multiple genes associated with photosynthetic processes (Christin et al., 2008; Studer et al., 2014; Goolsby et al., 2018; Piot et al., 2018). These and other discoveries have paved the way to a more nuanced understanding of the molecular basis of phenotypic convergence in CCM plants and may accelerate the development of crop varieties with augmented resistance to high temperature and low water availability.
For these aims to be fully realized, a robust framework to assess the extent and phenotypic impact of convergent molecular changes is necessary. Along the lines of strategies applied in vertebrates research (Castoe et al., 2009; Foote et al., 2015; Thomas & Hahn, 2015; Zou & Zhang, 2015), we presented here the results of a novel methodological approach to the study of molecular convergence in C4 grasses. We investigated patterns of convergent and non-convergent amino acid changes in nearly 70 chloroplast proteins across multiple C4 and C3 lineages in the PACMAD clade, with the goal of testing a specific hypothesis: is the evolution of chloroplast proteins showing stronger signatures of convergent amino acid replacements in C4 lineages compared to C3 lineages? This analysis also allowed us to establish if proteins other than enzymes involved in the CCM biochemistry underwent parallel amino acid changes in C4 lineages. Our reasoning is that many proteins expressed in the chloroplast could have experienced similar selective pressure across multiple C3 to C4 transitions and might have accumulated convergence replacements as a result. In agreement with our expectation, dozens of nuclear genes sharing signatures of positive or relaxed selection and likely associated with the evolution of C4 PACMAD grasses have been recently described, albeit these analyses relied on a limited number of species (Huang et al., 2017).
We based our analysis on the identification of amino acid replacements shared by pairs of reference C4 branches, defined here as branches corresponding to C3 to C4 transitions in the PACMAD phylogeny. We compared these changes to those identified in reference C3 branches, namely all C3 lineages that include only C3 species (Figs. 1 and 2), and to changes found between reference C3 and C4 branches. For each of the three possible pairs of photosynthesis types, C4:C4, C3:C4 and C3:C3, we determined the number of amino acid sites, genes and pairs of reference branches with convergent replacements.
We detected signatures of convergent evolution in all types of datasets. First, we identified many individual replacements that emerged repeatedly and uniquely in C4 reference branches, particularly in the proteins RbcL, NdhH, NdhI and MatK. We also observed C3-specific convergent replacements in NdhF and RpoC2, and a case of multiple C4 and C3 convergent changes in Rps3. Additionally, we identified 7 chloroplast genes with one or more C4-specific convergent sites and 3 chloroplast genes with at least one C3-specific convergent site. Second, we found evidence of significantly higher rates of convergent replacements in C4 lineages in both RbcL and RpoC1, and several convergent replacements that occurred exclusively in C4:C4 pairs in proteins encoded by ndhG, ndhI, psaI, rpoA, rps4 and rps11. These genes are involved in a variety of biological processes in the chloroplast, from the cyclic electron transport in (ndhG and ndhI) and the stabilization of (psaI) the photosystem I, to transcription (rpoA and rpoC1), translation (rps4 and rps11) and CO2 fixation (rbcL). Third, we identified statistically significant differences in pairs of C4 branches with convergent replacements (Table 2). Crucially, we observed more pairs with higher convergent than non-convergent replacements in C4:C4 compared to both C3:C3 and C3:C4, even after removing replacements identified in the RuBisCO large subunit, RbcL.
Altogether, these findings suggest that multiple biochemical processes occurring in the chloroplast might have experienced recurrent adaptive changes associated with the emergence of C4 photosynthesis. Notably, some of these proteins are not directly involved in the light-dependent or light-independent reactions of the photosynthesis, implying that processes such as regulation of gene expression and protein synthesis in the chloroplast are also experiencing significant selective pressures during the transition from C3 to C4 plants. These results should motivate further studies to determine the prevalence of convergent amino acid replacements in transitions to CCMs among the thousands of proteins encoded by nuclear genes but expressed in the chloroplast (Jarvis & Lopez-Juez, 2013). Although such analyses are currently hindered by the limited number of sequenced nuclear genomes in taxa with multiple C3 and C4 lineages, including the PACMAD clade, genome-wide investigations of convergent replacements will be possible in the near future given the current pace of DNA sequencing in plants.
A further important conclusion drawn from these results is that convergent replacements are not uncommon between C3:C3 and C3:C4 lineages. This is possibly due to some environmental factors affecting the evolution of chloroplast genes that are shared across grass lineages regardless of their photosynthesis type.
The analysis of individual convergent replacements in the RuBisCO large subunit both confirmed previous findings (Christin et al., 2008; Studer et al., 2014; Piot et al., 2018) and highlighted novel potentially adaptive changes among PACMAD species. Importantly, these novel convergent replacements are known to evolve under positive selection in non-PACMAD seed plants (Kapralov & Filatov, 2007; Sen et al., 2011). This underscores the potential of our approach to identify novel changes with functional significance in the transition to CCMs in grasses, as opposed to standard statistical tests of positive selection. Alternatively, some RbcL sites could experience convergence across a variety of seed plants because of selective pressure other than those associated with C3 to C4 transitions.
Overall, our results are robust to several possible confounding factors. First, we analyzed branches that are strongly supported in our phylogeny reconstruction. The phylogenetic tree built using the 67 chloroplast genes is well supported, with the exception of three branches with fairly low bootstrap support. However, all three branches are short and have minimal impact upon our conclusions regarding C4 evolution (Fig. 1 and Figs. S1–S3). Moreover, the tree is largely consistent with a comprehensive recent study of 250 grasses based on complete plastome data (Saarela et al., 2018). Second, by focusing only on reference branches and ignoring amino acid replacements that may have occurred after the divergence of species within a given C4 clade, our strategy provided a conservative estimate of the number of convergent changes that could have occurred during the evolution of PACMAD grasses. Third, we eliminated genes with possible paralogous copies, which could have introduced false positive replacements.
We recognize some potential caveats in our approach. By relying on a relatively small sample of PACMAD species, our statistical power to detect signatures of convergent evolution was limited. Increasing the number of reference C4 and C3 lineages should provide a broader representation of convergent replacements in C4 clades. Furthermore, we applied a strict definition of convergence that ignores changes to amino acids with similar chemical properties. We think that a conservative approach was necessary given that amino acids with similar chemical properties might have a very different functional effect on protein activity given their size and tridimensional interactions with nearby residues. Third, we assumed that all the observed convergent replacements were the result of convergent phenotypic changes, which fall under the general category of homoplasy (Avise & Robinson, 2008). However, some of these replacements could instead represent hemiplasy, or character state changes due to introgression between different C4 lineages, incomplete lineage sorting (ILS) of reference alleles or horizontal gene transfer (Avise & Robinson, 2008). Recombination between chloroplast genomes, which is required for introgression to occur, has been documented but appears to be rare (Carbonell-Caballero et al., 2015; Greiner, Sobanski & Bock, 2015; Sancho et al., 2018). Introgression or horizontal gene transfer between congeneric species has been associated to the acquisition of part of the C4 biochemical pathway in the PACMAD genus Alloteropsis (Christin et al., 2012; Olofsson et al., 2016). However, these transfers were limited to a few nuclear genes. Moreover, only a very few cases of horizontal transfer between chloroplast genomes have been reported in plants (Stegemann et al., 2012). Therefore, the contribution of hemiplasy to the observed pattern of convergent replacements in C4 lineages is likely to be minimal. Finally, we treated C4 species regardless of their photosynthesis subtype (NAPD-ME, NAD-ME and PEPCK), which is known to vary among PACMAD subfamilies (Taylor et al., 2010). We argue that our results are conservative with regard to this aspect because convergent replacements should be expected to occur more often between C4 groups sharing the same photosynthesis subtype.
Conclusions
In this study, we showed that molecular convergent evolution in the form of recurrent amino acid replacements affected multiple chloroplast proteins in C4 lineages of the PACMAD clade of grasses. This finding significantly broadened the number of genes known to have evolved convergently in C4 species. We observed for the first time that genes not directly involved in photosynthesis-related processes experienced convergent changes, suggesting that future efforts should rely whenever possible on genome-wide analyses of amino acid changes rather than focus primarily on candidate key metabolic genes, similarly to previous investigations on gene expression patterns in C4 and CAM plants. Our methodological approach based on the comparison of convergent and non-convergent replacements among photosynthesis types underscores the importance of a more rigorous hypothesis-based testing of convergent evolution signatures in C4 plant evolution. Our results should inform more nuanced approaches to introduce CCM-like processes in C3 crops.
Supplemental Information
Species and chloroplast genome data
List of species, chloroplast genome versions, numbers of coding sequences used for each species, species photosynthesis type, and putative orthologous gene and gene product names (top and bottom header lines, respectively; annotation corresponds to the Zea mays orthologous sequence annotation). Sequences used for analysis are indicated with “X”.
Cumulative branch length of pairs of reference branches
C4 and C3 reference branches are shown in red and black, respectively. Branch lengths were obtained from the RAxML phylogeny based on the AIC partitioning schemes (Fig. S2).
Genes and amino acid sites with convergent and non-convergent replacements in thirteen C4 (red) and nine C3 (black) reference branches
Amino acid replacements are highlighted: light gray –ancestral node (A), dark gray –descendant node (D). Site numbers correspond to the Zea mays orthologous sequence annotation. Chloroplast genes with convergent and/or non-convergentreplacements are shown. Reference branch positions within the phylogeny are shown in Fig. 1. An asterisk is shown next to RbcL protein sites that were previously reported to evolve under positive selection.
Numbers of reference branches that form convergent (Con) and non-convergent (NC) amino acid replacements at a given amino acid site in C4:C4, C3:C3 and C3:C4 pairs of reference branch
Only chloroplast genes with convergent and/or non-convergent replacements are shown. Convergent and non-convergentreplacements unique to a given category are highlighted in green and pink, respectively. Twelve otherwise conserved sites with more than 2 convergent replacements in C4:C4 comparisons are underlined.
Numbers of amino acid replacements (gray-shaded) and amino acid sites with convergent (Con) and non-convergent (NC) replacements in each gene product in C4:C4, C3:C3 and C3:C4 pairs o
An asterisk is next genes with significant differences in replacements between C4:C4 and C3:C4 pairs (P < 0.05, Boschloo’s test). Genes with convergent replacements only in C4:C4 pairs are underlined.
Number of reference branches with convergent and non-convergent replacements with the exclusion of sites in RbcL
Proportions of pairs of reference branches over all branches by category are shown in parenthesis. One asterisk: significant differences between C4:C4 pairs and both C3:C3 and C3:C4 pairs (P < 0.05, Boschloo’s test). Two asterisks: significant differences between C4:C4 pairs and C3:C4 pairs only (P < 0.05, Boschloo’s test). Con: convergent. NC: non-convergent. Con > NC: pairs of branches with more convergent than non-convergent replacements. Con > 1: pairs of branches with more than one convergent replacement.
Numbers of amino acid sites with convergent (Con) and non-convergent (NC) replacements in each pair of reference branches
Reference branch positions within the phylogeny are shown in Fig. 1.
Phylogenetic relationships among 64 grass species using RAxML based on the third codon position sites in 67 chloroplast genes (cladogram)
The model GTR+ Γ was used. Partitioning scheme was selected using Bayesian Information Criterion (BIC). Numbers represent bootstrap support.
Phylogenetic relationships among 64 grass species using RAxML based on the third codon position sites in 67 chloroplast genes
The model GTR+ Γ was used. Partitioning scheme was selected using Akaike information criterion (AIC). Numbers represent bootstrap support.
Phylogenetic relationships among 64 grass species using RAxML based on the third codon position sites in 67 chloroplast genes
The model GTR+ Γ was used. Partitioning scheme was selected using Bayesian Information Criterion (BIC). Numbers represent bootstrap support.