Direct multicomponent synthesis of benzocoumarins
- Published
- Accepted
- Received
- Academic Editor
- Robert Smith
- Subject Areas
- Natural Products, Organic Chemistry (other), Organic Compounds, Synthetic Organic Chemistry
- Keywords
- Cycloadditions, Multicomponent reactions, Isocyanides, Benzocoumarins, Natural products, Diels-alder reaction, Tandem reactions
- Copyright
- © 2019 Bornadiego et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ Organic Chemistry) and either DOI or URL of the article must be cited.
- Cite this article
- 2019. Direct multicomponent synthesis of benzocoumarins. PeerJ Organic Chemistry 1:e1 https://doi.org/10.7717/peerj-ochem.1
Abstract
A straightforward and versatile protocol for the synthesis of dibenzo[b,d]pyran-6-ones from readily available 3-carbonylcoumarins is reported. Our strategy is based on a reaction cascade of successive [4+1] and [4+2] cycloadditions that occur in one single operation. This work illustrates the unprecedented use of a multicomponent reaction of isocyanides for the preparation of this biologically relevant type of compounds. Notably, in this highly convergent and atom-economic process, one new single and two new double carbon-carbon bonds are formed in a simple synthetic operation.
Introduction
Coumarins or 2H-chromen-2-ones represent an important class of compounds, which are ubiquitous structures as secondary metabolites in plants and many species of fungi and bacteria. The parent member of this class, coumarin itself, was first isolated by Vogel in 1820 from the tonka bean (Dipteryx odorata) (Vogel, 1820). Since then, many different simple and polycyclic coumarins have been discovered and extensively studied due to their potent and singular biological activities (Stefanachi et al., 2018; Medina et al., 2015; Borges et al., 2005; Lavoie et al., 2019) and their photophysic properties (Wagner, 2009; Trenor et al., 2004). Among them, benzo[c]coumarins (1; Fig. 1) have emerged as privileged structures in drug discovery (Garazd & Garazd, 2016; Mao et al., 2014). Relevant examples include mycotoxin alternariol (2), (Solfrizzo, 2017) antioxidant and anticancer ellagic acid (3), (Ceci et al., 2018) synthetic cannabinoid agonists cannabilactones (4), (Khanolkar et al., 2007) antimalarial dioncolactone (5) (François et al., 2016) and the glucoside derivatives with antitumor properties gilvocarcins (6), (Tomita, Takahashi & Tamaoki, 1982) chrysomycins (7), (Matson et al., 1989) and ravidomycins (8; Fig. 1) (Yamashita et al., 1998).
Figure 1: The structure of benzo[c]coumarin and some natural and synthetic bioactive analogues.
Thus, in the last few years, several research groups have directed their efforts to develop efficient syntheses of benzo[c]coumarins. Most approaches rely upon the construction of B ring of benzo[c]courmarin (1), either by lactonization of a biaryl (9; Fig. 2, path a) (Morack, Metternich & Gilmour, 2018; Ramirez, Bosque & Gonzalez-Gomez, 2015; Luo et al., 2013) or by the formation of biaryl bond starting from a bicyclic ester (10; Fig. 2, path b) (Ortiz Villamizar et al., 2017). These methods usually involve the use of UV light, transition metal catalysts, and toxic oxidizing agents, limiting their actual applicability. Other efficient alternative approaches reported in literature involve tandem cycloaddition reactions to form C ring of benzo[c]courmarin 1 (Fig. 2, path c) (Pottie et al., 2011; He et al., 2012). In this latter case, the Diels-Alder reaction is especially useful, as it offers the greatest potential for diversity (Pottie et al., 2011). Unfortunately, harsh reaction conditions and the use of starting materials that are difficult to synthesize are generally required (Pottie et al., 2011).
Figure 2: Strategies for the synthesis of benzo[c]coumarins.
Herein we describe a facile synthesis of derivatives of benzo[c]coumarins (1a-n) by the Diels-Alder reaction of aminofuranocoumarins (11) prepared in situ from 3-carbonylcoumarins (12) and isocyanides (13). This approach has the advantages of an easy and concise construction of 2-aminofuranes by the [4+1] cycloaddition reaction of α, β-unsaturated carbonyl compounds with isocyanides, (Chatani et al., 2003; Ito, Kato & Saegusa, 1982; Bornadiego, Díaz & Marcos, 2019; Bornadiego, Díaz & Marcos, 2015; Bornadiego, Díaz & Marcos, 2014; Neo et al., 2013; Bornadiego, Díaz & Marcos, 2019) and the high reactivity of 2-aminofuranes in Diels-Alder reactions (Padwa et al., 1997).
Materials & Methods
General synthetic techniques
Materials. Methanol was dried by distillation over CaH2, immediately prior to use. Ethanol was freshly distilled from magnesium ethoxide, prepared from magnesium turnings in the presence of iodine. All other reagents were purchased from commercial sources and used as received.
Liquid reagents were measured using positive-displacement micropipettes with disposable tips and pistons. Thin layer chromatography was performed on aluminum plates, using 254 nm UV light or a mixture of p-anisaldehyde (2.5%), acetic acid (1%) and H2SO4 (3.4%) in 95% ethanol as developer.
Instrumentation. Melting points are uncorrected. IR spectra were recorded as KBr pellets. Proton and carbon-13 nuclear magnetic resonance (1H NMR or 13C NMR) spectra were obtained on a 400 MHz or 500 MHz spectrometer. The assignments of signals in 13C NMR were made by DEPT. High Resolution Mass Spectra (HRMS) were recorded using a 6520 Accurate Mass QTOF LC/MS Spectrometer. Experiments under microwave irradiation were performed in closed vials, using a focused single-mode microwave reactor CEM Discover BenchMate, provided with an IR internal thermal probe.
Synthesis of 3-carbonylcoumarins (12a-d)
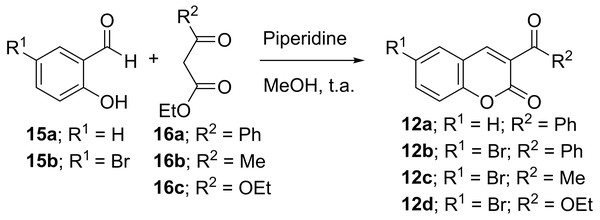
Acyl acetate 16 (3.3 mmol) and piperidine (15% mol) were added to a solution of salicylaldehyde (15, three mmol) in dry methanol (three mL). The mixture was stirred at rt and the progress of the reaction was followed by tlc. After 24–72 h the solvent was evaporated and the resulting solid was washed with cold cyclohexane to give chromones (12a-d), which were used in the following reactions without further purification.
3-Benzoyl-2H-chromen-2-one (12a); (Specht, Martic & Farid, 1982) (72 h, 661 mg, 88%). Obtained as a white solid; mp: 137–138 °C (lit (Specht, Martic & Farid, 1982) 137–139 °C); IR (cm−1): 3408, 1714, 1657, 1609, 1567; 1H-NMR (CDCl3, 500 MHz) δ 8.09 (s, 1H), 7.90 (d, J = 7.23 Hz, 2H), 7.67–7.60 (m, 3H), 7.49 (t, J = 7.1 Hz, 2H), 7.42 (d, J = 8.1 Hz, 1H), 7.36 (t, J = 7.2 Hz, 1H) ppm; 13C{1H}-NMR (CDCl3, 101 MHz) δ 191.8 (C), 158.5 (C), 154.9 (C), 145.6 (CH), 136.3 (C), 134.0 (CH), 133.8 (CH), 129.7 (CH), 129.3 (CH), 128.7 (CH), 127.2 (C), 125.1 (CH), 118.3 (C), 117.1 (CH) ppm.
3-Benzoyl-6-bromo-2H-chromen-2-one (12b); (Wang et al., 2012) (24 h, 465 mg, 47%). Obtained as a white solid; mp: 171–176 °C (lit (Wang et al., 2012) 171–172 °C); IR (cm−1): 3413, 3069, 1717, 1656, 1619, 1598; 1H-NMR (CDCl3, 500 MHz) δ 7.97 (s, 1H), 7.87 (d, J = 7.43 Hz, 2H), 7.72 (d, J = 7.13 Hz, 2H), 7.63 (t, J = 7 Hz, 1H), 7.49 (t, J = 7.4 Hz, 2H), 7.30 (d, J = 9.06 Hz, 1H) ppm; 13C{1H}-NMR (CDCl3, 101 MHz) δ 191.2 (C), 157.8 (C), 153.6 (C), 143.9 (CH), 136.4 (CH), 136.0 (C), 134.2 (CH), 131.4 (CH), 129.7 (CH), 128.8 (CH), 128.2 (C), 119.8 (C), 118.8 (CH), 117.7 (C) ppm.
3-Acetyl-6-bromo-2H-chromen-2-one (12c); (Parveen, Khan & Ahmed, 2019) (24 h, 360 mg, 45%). Obtained as a light yellow solid; mp: 224–228 °C (lit (Parveen, Khan & Ahmed, 2019). 232–233 °C); IR (cm−1): 3435, 3042, 1735, 1675, 1608, 1550; 1H-NMR (CDCl3, 500 MHz) δ 8.40 (s, 1H), 7.78 (s, 1H), 7.73 (d, J = 8.27 Hz, 1H), 7.27 (s, 1H), 2.72 (s, 3H) ppm; 13C{1H}-NMR (CDCl3, 126 MHz) δ 195.1 (C), 158.7 (C), 154.3 (C), 146.1 (CH), 137.2 (CH), 132.3 (CH), 125.7 (C), 119.9 (C), 118.6 (CH), 117.7 (C), 30.6 (CH3) ppm.
Ethyl 6-bromo-2-oxo-2H-chromene-3-carboxylate (12d); (Volmajer et al., 2005) (24 h, 197 mg, 22%). Obtained as a white solid; mp: 177 °C (lit. 180–181 °C; (Volmajer et al., 2005) 172 °C (Santos-Contreras et al., 2007)); 3449, 3070, 2974, 1753, 1704, 1617, 1599; 1H-NMR (CDCl3, 500 MHz) δ 8.43 (s, 1H), 7.75 (s, 1H), 7.72 (d, J = 8.79 Hz, 1H), 7.25 (d, J = 8.79 Hz, 1H), 4.42 (q, J = 7.12 Hz, 2H), 1.41 (t, J = 7. 11 Hz, 3H) ppm; 13C{1H}-NMR (CDCl3, 126 MHz) δ 162.8 (C), 156.0 (C), 154.1 (C), 147.1 (CH), 137.0 (CH), 131.7 (CH), 119.7 (C), 119.5 (C), 118.7 (CH), 117.5 (C), 62.3 (CH2), 14.3 (CH3) ppm.
General procedure for the synthesis of 6H-benzo[c]chromen-6-ones (1a-n)
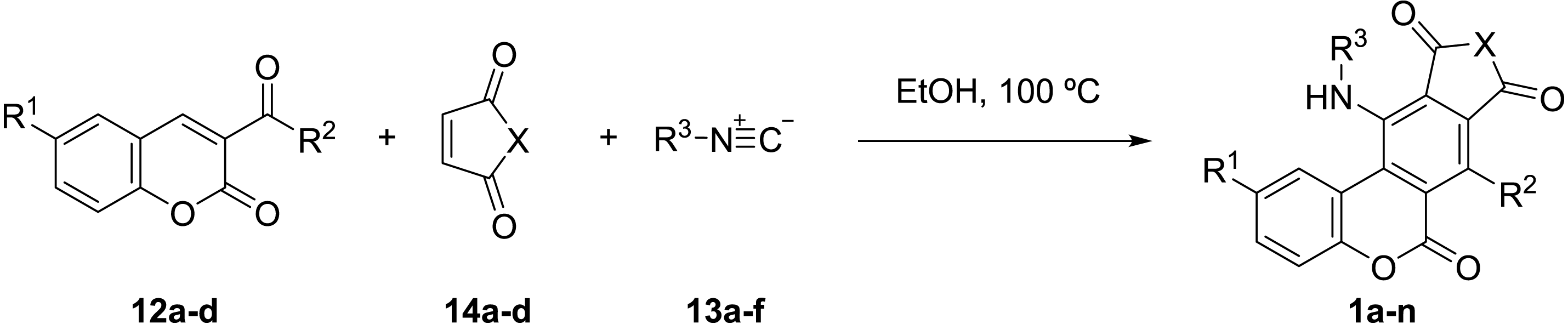
Maleimide derivative (14, 0.36 mmol) and isocyanide (13, 0.36 mmol) were successively added to a solution of coumarin (12, 0.30 mmol) in dry ethanol (two mL). The reaction mixture was stirred under N2 atmosphere, at 100 °C until completion, as judged by tlc. Then, 1 N HCl (20 mL) was added and the mixture stirred 1 h to hydrolyse any excess of isocyanide. The resulting mixture was extracted with CH2Cl2 and the organic phase was dried (Na2SO4) and concentrated in the rotary evaporator. In the case of obtaining a solid, this was washed with cold cyclohexane to give the pure products 1i, 1k and 1l. In all the other cases, the crude was subjected to flash column chromatography (SiO2 12 g cartridge, cyclohexane to cyclohexane-ethyl acetate 8:2), to give the pure products 1a-h; 1j and 1m-n.
11-(Cyclohexylamino)-7,9-diphenylchromeno[3,4-f]isoindole-6,8,10(9H)-trione (1a); (15 h, 124 mg, 80%). Obtained as a fluorescent orange solid; mp: 243–244 °C; IR (cm−1): 3311, 2930, 2850, 1746, 1706, 1600, 1501; 1H-NMR (CDCl3, 500 MHz) δ 8.89 (dd, J = 8.2, 1.2 Hz, 1H), 7.51 (t, J = 8.5 Hz, 1H) , 7.47–7.42 (m, 5H), 7.41–7.39 (m, 2H), 7.38–7.34 (m, 2H), 7.33 (m, 1H), 7.30–7.26 (m, 2H), 6.70 (d, J = 11.0 Hz, 1H), 3.43–3.27 (m, 1H), 1.90 (m, 2H), 1.73 (m, 2H), 1.39–1.30 (m, 2H), 1.27–1.13 (m, 4H) ppm; 13C{1H}-NMR (CDCl3, 101 MHz) δ 168.7 (C), 164.7 (C), 158.6 (C), 150.4 (C), 145.6 (C), 136.3 (C), 136.2 (C), 133.3 (C), 131.5 (CH), 131.2 (C), 129.1 (CH), 128.3 (CH), 128.2 (CH), 128.0 (CH), 127.9 (CH), 126.6 (CH), 126.4 (C), 126.0 (CH), 124.1 (CH), 120.8 (C), 118.5 (C), 117.3 (CH), 56.1 (CH), 33.9 (CH2), 25.5 (CH2), 24.8 (CH2) ppm; HRMS (ESI+-QTOF) m/z: [M + H]+ Calcd for C33H27N2O4: 515.1966; found: 515.1975.
2-Bromo-11-(cyclohexylamino)-7,9-diphenylchromeno[3,4-f]isoindole-6,8,10(9H)-trione (1b); (23 h, 142 mg, 79%). Obtained as a fluorescent orange solid; mp: 142–146 °C; IR (cm−1): 3308, 3061, 2927, 2851, 1750, 1707, 1597; 1H-NMR (CDCl3, 500 MHz) δ 9.11 (s, 1H), 7.60 (dd, J = 8.7, 2.3 Hz, 1H), 7.47–7.44 (m, 2H), 7.44 (d, J = 2.5 Hz, 3H), 7.41–7.34 (m, 3H), 7.26 (dd, J = 5.3, 4.2 Hz, 2H), 7.22 (d, J = 8.7 Hz, 1H), 6.69 (d, J = 11.1 Hz, 1H), 3.36–3.25 (m, 1H), 1.97 (m, 2H), 1.76 (m, 2H), 1.42–1.33 (m, 2H), 1.25 (m, 4H) ppm; 13C{1H}-NMR (CDCl3, 126 MHz) δ 168.5 (C), 164.6 (C), 158.0 (C), 149.4 (C), 145.6 (C), 136.4 (C), 136.0 (C), 134.1 (CH), 131.8 (C), 131.2 (C), 129.1 (CH), 128.8 (CH), 128.5 (C), 128.4 (CH), 128.2 (CH), 128.1 (CH), 126.6 (CH), 121.4 (C), 120.0 (C), 118.8 (CH), 117.1 (C), 57.0 (CH), 33.9 (CH2), 25.5 (CH2), 24.9 (CH2) ppm; HRMS (ESI+-QTOF) m/z: [M + H]+ Calcd for C33H26BrN2O4: 593.1071; found: 593.1093.
11-(Cyclohexylamino)-9-methyl-7-phenylchromeno[3,4-f]isoindole-6,8,10(9H)-trione (1c); (59 h, 111 mg, 82%). Obtained as a fluorescent yellow solid; mp: 199–205 °C; IR (cm−1): 3304, 2924, 2850, 1745, 1698, 1611, 1422; 1H-NMR (CDCl3, 500 MHz) δ 8.90 (d, J = 8.2 Hz, 1H), 7.50 (t, J = 7.6 Hz, 1H), 7.45 (m, 3H), 7.32 (d, J = 8.0 Hz, 2H), 7.24 (m, 2H), 6.50 (d, J = 11.0 Hz, 1H), 3.29 (m, 1H), 3.09 (s, 3H), 1.87 (m, 2H), 1.72 (m, 2H), 1.54 (m, 1H), 1.37–1.11 (m, 5H) ppm; 13C{1H}-NMR (CDCl3, 101 MHz) δ 169.6 (C), 166.0 (C), 158.7 (C), 150.5 (C), 145.1 (C), 136.5 (C), 135.8 (C), 133.2 (C), 131.4 (CH), 128.2 (CH), 128.0 (CH), 127.9 (CH), 126.1 (CH), 125.9 (C), 124.1 (CH), 121.7 (C), 118.5 (C), 117.2 (CH), 56.2 (CH), 33.9 (CH2), 25.6 (CH2), 24.9 (CH2), 24.0 (CH3) ppm; HRMS (ESI+-QTOF) m/z: [M + H]+ Calcd for C28H25N2O4: 453.1809; found: 453.1816.
9-Methyl-11-(pentylamino)-7-phenylchromeno[3,4-f]isoindole-6,8,10(9H)-trione (1d); (59 h, 100 mg, 76%). Obtained as a fluorescent orange solid; mp: 177–179 °C; IR (cm−1): 3346, 2929, 1737, 1702, 1609, 1500; 1H-NMR (CDCl3, 500 MHz) δ8.69 (d, J = 8.23 Hz, 1H), 7.49 (t, J = 8.2 Hz, 1H), 7.46–7.44 (m, 3H), 7.33 (t, J = 8.2 Hz, 2H), 7.25–7.23 (m, 2H), 6.64 (bs, NH), 3.09 (m, 5H), 1.62 (q, J = 7.3 Hz, 2H), 1.32–1.24 (m, 4H), 0.89–0,86 (m, 3H) ppm; 13C{1H}-NMR (CDCl3, 101 MHz) δ 169.6 (C), 166.1 (C), 158.7 (C), 150.5 (C), 146.2 (C), 136.5 (C), 135.3 (C), 132.0 (C), 131.1 (CH), 128.5 (C), 128.2 (CH), 128.0 (CH), 127.9 (CH), 126.4 (CH), 126.1 (C), 124.2 (CH), 120.2 (C), 118.3 (C), 117.1 (CH), 49.4 (CH2), 30.6 (CH2), 29.0 (CH2), 24.0 (CH3), 22.5 (CH2), 14.0 (CH3) ppm; HRMS (ESI+-QTOF) m/z: [M + H]+ Calcd for C28H24N2O4: 441.1809; found: 441.1830.
11-((2,6-Dimethylphenyl)amino)-7-phenylchromeno[3,4-f]isoindole-6,8,10(9H)-trione (1e); (69 h, 43 mg, 31%). Obtained as a fluorescent dark orange solid; mp: 310–312 °C (dec); IR (cm−1): 3299, 1765, 1713, 1608, 1505; 1H-NMR (CDCl3, 500 MHz) δ 9.01 (s, 1H), 7.86 (s, 1H), 7.45 (m, 3H), 7.29 (s, 2H), 7.18 (t, J = 7.5 Hz, 1H), 7.16–7.09 (m, 2H), 6.89 (m, 3H), 6.64 (t, J = 7.5 Hz, 1H), 2.04 (s, 6H) ppm; 13C{1H}-NMR (CDCl3, 101 MHz) δ 169.6 (C), 165.4 (C), 158.9 (C), 149.6 (C), 141.2 (C), 137.1 (C), 136.1 (C), 135.2 (CH), 134.6 (C), 131.3 (C), 130.8 (C), 130.8 (C), 130.4 (CH), 129.3 (CH), 128.3 (C), 128.7 (CH), 128.4 (CH), 128.0 (CH), 126.4 (CH), 126.3 (CH), 125.5 (CH), 122.1 (CH), 117.5 (C), 116.1 (CH), 115.9 (C), 19.9 (CH3) ppm; HRMS (ESI−-QTOF) m/z: [M - H]− Calcd for C29H19N2O4: 459.1350; found: 459.1365.
2-Bromo-11-(tert-butylamino)-7,9-diphenylchromeno[3,4-f]isoindole-6,8,10(9H)-trione (1f); (>100 h, 19 mg, 11%). Obtained as a fluorescent yellow solid; mp: 107–111 °C; IR (cm−1): 3446, 2923, 1769, 1748, 1710, 1597; 1H-NMR (CDCl3, 500 MHz) δ 10.11 (s, 1H), 7.58 (d, J = 8.7 Hz, 1H), 7.46–7.43 (m, 5H), 7.38 (d, J = 7.5 Hz, 3H), 7.29 (m, 2H), 7.17 (d, J = 8.72 Hz, 1H), 5.83 (s, 1H), 1.24 (s, 9H) ppm; 13C{1H}-NMR (CDCl3, 101 MHz) δ 167.4 (C), 164.4 (C), 158.0 (C), 149.3 (C), 144.7 (C), 139.2 (C), 138.3 (C), 135.9 (C), 134.5 (CH), 131.1 (C), 130.2 (CH), 129.1 (CH), 128.5 (CH), 128.2 (CH), 128.1 (CH), 128.0 (CH), 127.1 (C), 126.6 (CH), 125.7 (C), 121.6 (C), 118.9 (CH), 116.6 (C), 59.8 (C), 30.1 (CH3) ppm; HRMS (ESI+-QTOF) m/z: [M + H]+ Calcd for C31H24BrN2O4: 567.0914; found: 567.0921.
2-Bromo-9-methyl-11-(pentylamino)-7-phenylchromeno[3,4-f]isoindole-6,8,10(9H)-trione (1g); (87 h, 98 mg, 63%). Obtained as a fluorescent yellow solid; mp: 164–169 °C; IR (cm−1): 3304, 3072, 2930, 2858, 1761, 1744, 1700, 1609, 1425; 1H-NMR (CDCl3, 500 MHz) δ 8.86 (s, 1H), 7.58 (d, J = 8.7 Hz, 1H), 7.47–7.44 (m, 3H), 7.23–7.19 (m, 3H), 6.65 (t, J = 6 Hz, 1H), 3.10 (s, 3H), 3.08 (t, J = 6.88 Hz, 2H), 1.68 (q, J = 7.4 Hz, 2H), 1.37–1.31 (m, 4H), 0.90 (t, J = 7.1 Hz, 3H) ppm;13C{1H}-NMR (CDCl3, 126 MHz) δ 169.4 (C), 165.9 (C), 158.1 (C), 149.4 (C), 146.2 (C), 136.2 (C), 135.6 (C), 133.8 (CH), 130.6 (C), 129.5 (C), 129.0 (CH), 120.2 (CH), 128.0 (CH), 128.0 (CH), 126.2 (C), 120.8 (C), 119.9 (C), 118.8 (CH), 117.2 (C), 49.7 (CH2), 30.5 (CH2), 29.1 (CH2), 24.1 (CH3), 22.5 (CH2), 14.1 (CH3) ppm; HRMS (ESI+-QTOF) [M + H]+ Calcd for C27H24BrN2O4: 519.0914; found: 519.0918.
2-Bromo-11-((2,6-dimethylphenyl)amino)-7-phenylchromeno[3,4-f]isoindole-6,8,10(9H)-trione (1h); (67 h, 50 mg, 31%). Obtained as an orange solid; mp: 322–325 °C (dec); IR (cm−1): 3319, 1765, 1737, 1710, 1607, 1477; 1H-NMR (CDCl3, 500 MHz) δ 8.96 (s, 1H), 7.77 (s, 1H), 7.46 (m, 3H), 7.28–7.25 (m, 4H), 7.00–6.92 (m, 4H), 2.07 (s, 6H) ppm; 13C{1H}-NMR (CDCl3, 101 MHz) δ 169.4 (C), 165.3 (C), 158.3 (C), 148.5 (C), 141.4 (C), 136.7 (C), 135.8 (C), 134.6 (C), 133.2 (CH), 131.1 (C), 130.0 (CH), 129.5 (C), 129.3 (CH), 129.2 (C), 128.4 (CH), 128.2 (CH), 128.1 (CH), 126.4 (C), 125.9 (CH), 117.8 (C), 117.6 (C), 117.4 (C), 115.8 (C), 20.1 (CH3) ppm; HRMS (ESI−-QTOF) [M - H]− Calcd for C29H18BrN2O4: 537.0455; found: 537.0428.
2-Bromo-11-(cyclohexylamino)-7-methyl-9-phenylchromeno[3,4-f]isoindole-6,8,10(9H)-trione (1i); (7 h, 158 mg, 99%). Obtained as an orange solid; mp: 241–244 °C; IR (cm−1): 3439, 2930, 2853, 1758, 1698, 1598, 1500; 1H-NMR (CDCl3, 500 MHz) δ 9.16 (s, 1H), 7.59 (d, J = 8.65 Hz, 1H), 7.54 (t, J = 7.6 Hz, 2H), 7.46–7.42 (m, 3H), 7.23 (d, J = 8.7 Hz, 1H), 6.50 (d, J = 11.14 Hz, 1H), 3.14 (m, 4H), 1.89 (m, 2H), 1.72–1.18 (m, 8H) ppm; 13C{1H}-NMR (CDCl3, 101 MHz) δ 168.4 (C), 166.5 (C), 159.2 (C), 149.2 (C), 144.3 (C), 136.4 (C), 134.0 (CH), 132.4 (C), 131.3 (C), 129.3 (CH), 128.9 (CH), 128.6 (CH), 128.3 (C), 127.3 (C), 126.7 (CH), 121.9 (C), 120.1 (C), 118.6 (CH), 117.1 (C), 56.8 (CH), 33.8 (CH2), 25.5 (CH2), 24.9 (CH2), 16.2 (CH3) ppm; HRMS (ESI+-QTOF) [M + H]+ Calcd for C28H24BrN2O4: 531.0914; found: 531.0885.
2-Bromo-11-(tert-butylamino)-7,9-dimethylchromeno[3,4-f]isoindole-6,8,10(9H)-trione (1j); (27 h, 8 mg, 6%). Obtained as a fluorescent yellow solid; mp: 210–213 °C; IR (cm−1): 3305, 2960, 1760, 1741, 1697, 1433;1H-NMR (CDCl3, 500 MHz) δ 10.11 (s, 1H), 7.55 (d, J = 8.72 Hz, 1H), 7.16 (d, J = 8.71 Hz, 1H), 5.52 (s, 1H), 3.19 (s, 3H), 3.14 (s, 3H), 1.13 (s, 9H) ppm; 13C{1H}-NMR (CDCl3, 126 MHz) δ 168.9 (C), 167.5 (C), 159.3 (C), 149.2 (C), 142.6 (C), 138.8 (C), 138.4 (C), 134.2 (CH), 130.6 (CH), 128.6 (C), 128.4 (C), 126.2 (C), 121.7 (C), 118.6 (CH), 116.5 (C), 59.1 (C), 29.9 (CH3), 24.2 (CH3), 16.4 (CH3) ppm; HRMS (ESI+-QTOF) [M + H]+ Calcd for C21H20BrN2O4: 443.0601; found: 443.0585.
2-Bromo-7,9-dimethyl-11-(pentylamino)chromeno[3,4-f]isoindole-6,8,10(9H)-trione (1k); (13 h, 72 mg, 52%). Obtained as a fluorescent orange solid; mp: 139–142 °C; IR (cm−1): 3324, 2931, 1740, 1698, 1606, 1436; 1H-NMR (CDCl3, 500 MHz) δ 8.91 (s, 1H), 7.56 (d, J = 8.58 Hz, 1H), 7.21 (d, J = 8.67 Hz, 1H), 6.45 (t, J = 5.3 Hz, 1H), 3.19 (s, 3H), 3.08 (s, 3H), 3.01–2.96 (m, 2H), 1.61 (m, 2H), 1.31–1.30 (m, 4H), 0.88 (m, 3H) ppm; 13C{1H}-NMR (CDCl3, 101 MHz) δ 169.3 (C), 167.7 (C), 159.3 (C), 149.2 (C), 145.0 (C), 135.4 (C), 133.7 (CH), 131.2 (C), 129.0 (CH), 128.9 (C), 126.9 (C), 121.4 (C), 119.9 (C), 118.5 (CH), 117.2 (C), 49.5 (CH2), 30.3 (CH2), 29.0 (CH2), 24.1 (CH3), 22.5 (CH2), 16.2 (CH3), 14.0 (CH3) ppm; HRMS (ESI+-QTOF) [M + H]+ Calcd for C22H22BrN2O4: 457.0758; found: 457.0755.
11-(Benzylamino)-2-bromo-7-methylchromeno[3,4-f]isoindole-6,8,10(9H)-trione (1l); (18 h, 107 mg, 77%). Obtained as a fluorescent orange solid; mp: 234–238 °C; IR (cm−1): 3431, 1757, 1737, 1709, 1606, 1424; 1H-NMR (CDCl3, 500 MHz) δ 9.02 (s, 1H), 7.78 (s, 1H), 7.54 (d, J = 8.66 Hz, 1H), 7.24–7.17 (m, 4H), 7.12 (d, J = 6.96 Hz, 2H), 6.73 (t, J = 6.3 Hz, 1H), 4.14 (d, J = 6.49 Hz, 2H), 3.02 (s, 3H) ppm; 13C{1H}-NMR (CDCl3, 101 MHz) δ 168.5 (C), 167.0 (C), 159.1 (C), 149.4 (C), 144.6 (C), 137.5 (C), 136.8 (C), 134.2 (CH), 131.9 (C), 129.2 (C), 129.1 (CH), 129.1 (CH), 128.1 (CH), 127.9 (CH), 127.4 (C), 122.4 (C), 119.8 (C), 118.7 (CH), 117.5 (C), 52.9 (CH2), 16.2 (CH3) ppm; HRMS (ESI+-QTOF) Calcd for C23H15BrN2O4H+: 463.0288; found: 463.0289.
2-Bromo-11-((2,6-dimethylphenyl)amino)-7-methylchromeno[3,4-f]isoindole-6,8,10(9H)-trione (1m); (18 h, 60 mg, 42%). Obtained as a fluorescent orange solid; mp: 337–342 (dec); IR (cm−1): 3441, 2919, 2850, 1739, 1713, 1634; 1H-NMR (CDCl3, 500 MHz) δ 8.77 (s, 1H), 7.72 (s, 1H), 7.30 (s, 1H), 7.01 (d, J = 8.69 Hz, 1H), 6.95–6.88 (m, 4H), 3.12 (s, 3H), 2.01 (s, 6H) ppm; due to the insolubility of compound 1m, it was not possible to record its 13C-NMR spectrum. HRMS (ESI−-QTOF) [M - H]− Calcd for C24H16BrN2O4: 475.0299; found: 475.0326.
2-Bromo-11-((4-methoxyphenyl)amino)-7-methylchromeno[3,4-f]isoindole-6,8,10(9H)-trione (1n); (32 h, 78 mg, 54%). Obtained as a light red oil; IR (cm−1): 3441, 2919, 2850, 1759, 1732, 1702, 1509; 1H-NMR (CDCl3, 500 MHz) δ 8.54 (s, 1H), 8.42 (d, J = 2.26 Hz, 1H), 7.73 (s, 1H), 7.37 (d, J = 8.7 Hz, 1H), 7.09 (d, J = 8.69 Hz, 1H), 6.75–6.68 (m, 4H), 3.71 (s, 3H), 3.16 (s, 3H) ppm; due to the insolubility of compound 1n, it was not possible to record its 13C-NMR spectrum. HRMS (ESI+-QTOF) [M + H]+ Calcd for C23H16BrN2O5: 479.0237; found: 479.0234.
Results and Discussion
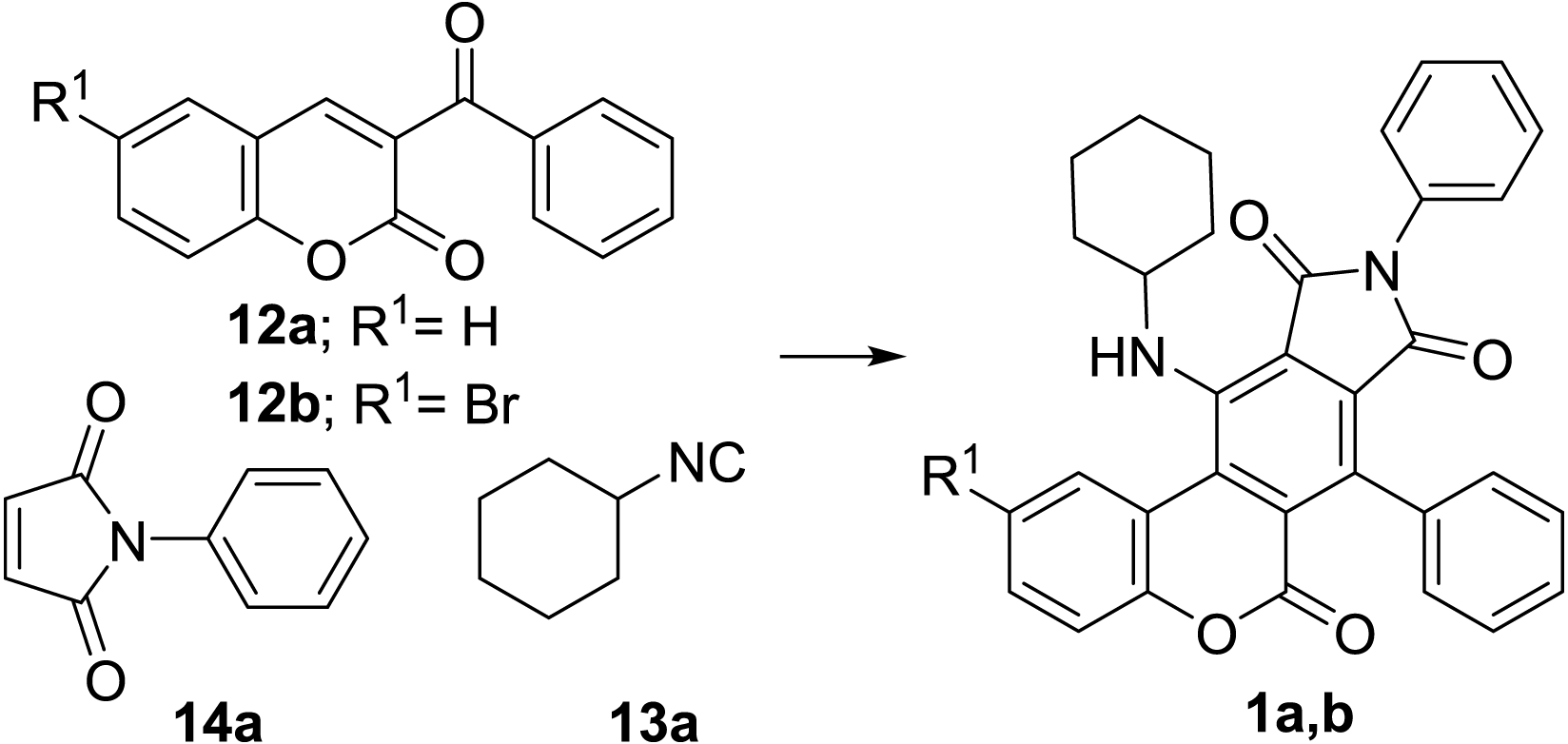
Aminofurans are very reactive dienes that are known to undergo Diels-Alder cycloadditions in relatively mild reaction conditions. We have recently shown that 2-aminofurans, readily synthesized by a [4+1] cycloaddition of isocyanides and α, β-unsaturated carbonyl compounds, can react with dienophiles in a single operation to give anilines (Bornadiego, Díaz & Marcos, 2019; Bornadiego, Díaz & Marcos, 2015; Bornadiego, Díaz & Marcos, 2014; Neo et al., 2013; Bornadiego, Díaz & Marcos, 2019). Henceforth, we reason that 1-amino-4H-furo[3,4-c]chromen-4-ones (11) would be suitable intermediate dienes for the Diels-Alder construction benzo[c]coumarin C ring. Accordingly, we propose a multicomponent synthesis of benzo[c]coumarins (1) by the sequential [4+1] / [4+2] cycloaddition of isocyanides (13), 3-carbonylcoumarins (12) and dienophiles (14, Fig. 3).
The starting carbonylcoumarins (12) were synthesized by the Knoevenagel condensation of salicylaldehydes (15) with different β-ketoesters (16), followed by cyclization, according to slightly modified Farid’s method (Specht, Martic & Farid, 1982). In our case, the reaction was optimally carried out at room temperature in methanol, using piperidine as basic catalyst (Fig. 4).
In order to prove our strategy for the synthesis of benzo[c]coumarins (1), we reacted 3-benzoylcoumarin 12a with 1.2 equivalents of cyclohexyl isocyanide 13a and N-phenylmaleimide 14a in THF. After 12 h at 25 °C, the reaction medium became yellow-orange and a new highly fluorescent yellow product was evident on tlc. However, the reaction was extraordinarily sluggish at room temperature and it did not reach completion even after 30 days (Table 1, entry 1). The reaction was still slow when the temperature was raised to 80 °C, but it did conclude after 9 days, allowing the isolation of a new product, 1a, the identity of which was confirmed by 1H-RMN, 13C-RMN, IR and MS (Table 1, entry 2).
Figure 3: Schematic representation of sequential [4+1] and [4+2] cycloaddition reactions leading to the synthesis of 3-carbonylcoumarins.
With the purpose of finding better reaction conditions, we explored the use of different solvents, temperatures, and the addition of catalysts (Table 1). Using a solvent with a higher boiling point, such as dibutyl ether, resulted in a complex mixture, difficult to purify. Fortunately, when we carried out the reaction in ethanol at 100 °C, benzocoumarin 1a was obtained in just 15 h with an 80% yield (Table 1, entry 5). Similarly, the reaction with bromocoumarin 12b afforded the corresponding benzocoumarin 1b with a 79% yield (entry 7). In consequence, the reaction, which is very slow in THF, seems to be favored in ethanol, suggesting the occurrence of charged intermediates that may be stabilized in protic polar solvents. This is in accordance with our theoretical studies (Wu, Xu & Xie, 2005) that show that related syntheses of 2-aminofuranes proceed through a [4+1] stepwise cycloaddition involving a zwitterionic intermediate (Bornadiego, Díaz & Marcos, 2019). On the other hand, the use of proton donors (entries 8 and 9) or Lewis acid catalysts (entry 3) did not significantly improve the reaction.
Figure 4: Synthesis of 3-carbonylcoumarins.
Thus, the optimal reaction conditions were applied to different combinations of isocyanides (13a-f), dienophiles (14a-d) and 3-carbonylcoumarins (12a-d; Table 2).
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Coumarin | R1 | R2 | Dienophile | X | Isocyanide | R3 | Time, h | Product (yield, %) |
| 1 | 12a | H | Ph | 14a | N-Ph | 13a | cC6H11 | 15 | 1a (80) |
| 2 | 12a | H | Ph | 14b | N-CH3 | 13a | cC6H11 | 59 | 1c (82) |
| 3 | 12a | H | Ph | 14b | N-CH3 | 13b | C5H11 | 59 | 1d (76) |
| 4 | 12a | H | Ph | 14c | N-H | 13c | 2,6-diMe-Ph | 69 | 1e (31) |
| 5 | 12b | Br | Ph | 14a | N-Ph | 13a | cC6H11 | 23 | 1b (71) |
| 6 | 12b | Br | Ph | 14a | N-Ph | 13d | tBu | >100 | 1f (11) |
| 7 | 12b | Br | Ph | 14b | N-CH3 | 13d | tBu | >100 | NIa |
| 8 | 12b | Br | Ph | 14b | N-CH3 | 13b | C5H11 | 87 | 1g (63) |
| 9 | 12b | Br | Ph | 14c | N-H | 13c | 2,6-diMe-Ph | 67 | 1h (31) |
| 10 | 12c | Br | CH3 | 14a | N-Ph | 13a | cC6H11 | 7 | 1i (99) |
| 11 | 12c | Br | CH3 | 14b | N-CH3 | 13d | tBu | 27 | 1j (6) |
| 12 | 12c | Br | CH3 | 14b | N-CH3 | 13b | C5H11 | 13 | 1k (52) |
| 13 | 12c | Br | CH3 | 14d | O | 13a | cC6H11 | 11 | CMb |
| 14 | 12c | Br | CH3 | 14c | N-H | 13e | CH2C6H5 | 18 | 1l (77) |
| 15 | 12c | Br | CH3 | 14c | N-H | 13c | 2,6-diMe-Ph | 18 | 1m (42) |
| 16 | 12c | Br | CH3 | 14c | N-H | 13f | 4-MeO-Ph | 32 | 1n (54) |
| 17 | 12d | Br | OEt | 14a | N-Ph | 13a | cC6H11 | >100 | NRc |
In most of the cases the products are obtained with moderate to excellent yields. The reaction proceeds equally well with different aromatic and aliphatic substituted 3-carbonylcoumarins. Conversely, as expected, coumarin ester 12d (Table 2, entry 17) does not react. N-substituted and non-substituted maleimides can be used as dienophiles; however, the reaction with maleic anhydride produces a complex mixture of products (entry 13). Aliphatic isocyanides (entries 1–3, 5, 8, 10, 12, 14) require shorter reaction times and result in better yields than less reactive aromatic isocyanides (entries 4, 9, 15, 16). An exception is tert-butyl isocyanide (entries 6, 7, 11), possibly due to steric hindrance.
Conclusions
In conclusion, we have successfully designed a highly convergent and atom-economic synthesis of benzo[c]coumarins. Our strategy is based on the trapping with dienophiles of reactive 2-aminofurans generated in a [4+1] cycloaddition of isocyanides and readily available 3-carbonyl coumarins. In this way, the target compounds are readily obtained in one-pot, in mild conditions with no need of catalysis. Furthermore, different substitution patterns can be easily accessed, as the reaction tolerates a wide choice of the three starting materials. Therefore, this multicomponent approach provides a flexible method to fine tune the properties of the products.


![The structure of benzo[c]coumarin and some natural and synthetic bioactive analogues.](https://dfzljdn9uc3pi.cloudfront.net/2019/ochem-1/1/fig-1-1x.jpg)
![Strategies for the synthesis of benzo[c]coumarins.](https://dfzljdn9uc3pi.cloudfront.net/2019/ochem-1/1/fig-2-1x.jpg)
![Schematic representation of sequential [4+1] and [4+2] cycloaddition reactions leading to the synthesis of 3-carbonylcoumarins.](https://dfzljdn9uc3pi.cloudfront.net/2019/ochem-1/1/fig-3-1x.jpg)