Abstract
A high production rate of negative hydrogen ions (H−) was observed from a nanoporous 12CaO  7Al2O3 (C12A7) electride surface immersed in hydrogen/deuterium low-pressure plasmas. The target was negatively biased at 20–130 V, and the target surface was bombarded by H3+ ions from the plasma. The production rate was compared with that from a clean molybdenum surface. Using the pseudo-exponential work-function dependence of the H− production rate, the total H− yield from the C12A7 electride surface bombarded at 80 V was evaluated to be 25% of that from a cesiated molybdenum surface with the lowest work-function. The measured H− energy spectrum indicates that the major production mechanism is desorption by sputtering. This material has potential to be used as a production surface of cesium-free negative ion sources for accelerators, heating beams in nuclear fusion, and surface modification for industrial applications.
7Al2O3 (C12A7) electride surface immersed in hydrogen/deuterium low-pressure plasmas. The target was negatively biased at 20–130 V, and the target surface was bombarded by H3+ ions from the plasma. The production rate was compared with that from a clean molybdenum surface. Using the pseudo-exponential work-function dependence of the H− production rate, the total H− yield from the C12A7 electride surface bombarded at 80 V was evaluated to be 25% of that from a cesiated molybdenum surface with the lowest work-function. The measured H− energy spectrum indicates that the major production mechanism is desorption by sputtering. This material has potential to be used as a production surface of cesium-free negative ion sources for accelerators, heating beams in nuclear fusion, and surface modification for industrial applications.
Export citation and abstract BibTeX RIS
Large-scale negative ion beams of hydrogen isotopes have been intensively developed as plasma heating and current-drive tools in nuclear fusion projects.1,2) At present, medium-size negative ion beams are widely used as injectors for particle accelerators, such as those of J-PARC, CERN, SNS-Oak Ridge, China Spallation Neutron Source (CSNS), Los Alamos Neutron Science Center (LANSCE)/PFR, ISIS Spallation Neutron and Muon Facility, etc.3) Small-size negative ion sources are now under development for applications in accelerators in medical fields.4) Negative ion sources have recently received a significant attention owing to their application in microelectronics fields, such as proton implantation for surface modification, or smart-ion-beam cut of wafers,5,6) as the charge-up problem of positive ion injection can be avoided.
It is known that H− ions can be produced in a plasma volume,7) and on a low-work-function surface covered by a half (or less) monolayer of alkali metals.8,9) In most large- or medium-size ion sources, cesium vapor is introduced not only for an efficient H− surface production, but also for a remarkable reduction of the co-extracted electron current. However, it has many disadvantages, such as a limited life-time of the coverage, i.e., cesium leakage. Cesium contamination on surfaces to be treated, and surroundings is a large drawback for applications to microelectronics. Studies are ongoing to develop cesium-free negative-ion sources. Alternative materials, such as carbon materials, and, in particular, highly oriented pyrolytic graphite (HOPG) or diamond, are studied to enhance the negative-ion production on a plasma grid of ion sources.10,11)
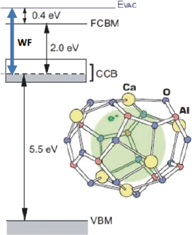
In 2003, Hosono et al. revealed that a refractory oxide 12CaO  7Al2O3 (C12A7) can be transformed to an electride with a low work function,12–14) by exchanging clathrated oxygen ions as the counter anions from the positively charged crystallographic cages of C12A7 leading to formations of high-density electrons in the cages. When 100% of the clathrated oxygen ions were removed, a conductivity of approximately 1,000 S/cm at 300 K was observed, demonstrating that the encaged electron behaves as an anion.15) They reported that the obtained [Ca24Al28O64]4+(4e−) could be regarded as an "electride", and demonstrated the unique properties including a very low work function of 2.4 eV,16) which is comparable to that of metal potassium; however, it is chemically and thermally stable. Figure 1 shows the schematic energy diagram of the C12A7 electride. In our previous study, H− formation from a sample of C12A7 electride was indirectly shown by exposing the sample to an atomic hydrogen (H0) flux and detecting the negative current generated on it.17) The observed current level was similar with that obtained from a low-work-function bi-alkali-covered molybdenum surface (∼2.3 eV) at the same condition.
7Al2O3 (C12A7) can be transformed to an electride with a low work function,12–14) by exchanging clathrated oxygen ions as the counter anions from the positively charged crystallographic cages of C12A7 leading to formations of high-density electrons in the cages. When 100% of the clathrated oxygen ions were removed, a conductivity of approximately 1,000 S/cm at 300 K was observed, demonstrating that the encaged electron behaves as an anion.15) They reported that the obtained [Ca24Al28O64]4+(4e−) could be regarded as an "electride", and demonstrated the unique properties including a very low work function of 2.4 eV,16) which is comparable to that of metal potassium; however, it is chemically and thermally stable. Figure 1 shows the schematic energy diagram of the C12A7 electride. In our previous study, H− formation from a sample of C12A7 electride was indirectly shown by exposing the sample to an atomic hydrogen (H0) flux and detecting the negative current generated on it.17) The observed current level was similar with that obtained from a low-work-function bi-alkali-covered molybdenum surface (∼2.3 eV) at the same condition.
Fig. 1. Energy diagram of the C12A7 electride. Anionic electrons are accommodated in the sub-nanometer-sized cages, which are connected with each other. The connected cages form a new conduction band referred to as cage conduction band (CCB), below the cage flame conduction band minimum (FCBM). The work function (WF) is 2.4 eV, comparable to that of metal potassium; however, it is chemically and thermally stable as the electrons are within the cages composed of a rigid Ca–Al–O network. EVAC: vacuum level, VBM: valence band maximum. The band gap is 7.5 eV.
Download figure:
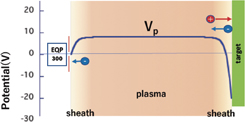
Standard image High-resolution imageIn this study, a direct measurement of H− formation was performed using an experimental apparatus at the Aix-Marseille University.10) It consisted of an upper plasma source chamber and lower spherical diffusion chamber. The plasma was initiated by a Huttinger PFG 1600 radio-frequency (RF) generator followed by a Huttinger matchbox. The sample, which could be retrieved into a separate vacuum chamber for change, was fixed on a rotatable target holder and inserted into the center of the lower diffusion chamber. The temperature of the target holder and target was controllable, from room temperature up to 600 °C, with a heating arrangement. An electron cyclotron resonance (ECR) plasma source was also employed; plasma could be injected in the lower chamber towards the target. The target was biased negatively against the electrically grounded chamber. Figure 2 shows the electrical potential profile between the target sample holder and mass spectrometer with respect to the chamber potential. Owing to the electrical potential drop, e(Vp − Vs), in front of the target sample, positive ions were accelerated and bombarded the surface; Vp and Vs denote the plasma potential and bias voltage on the target sample, respectively. Most of the incident ions were dissociated on the surface if they were molecular ions, and implanted in the sample or stopped near the surface (adsorption); but some of them were reflected from it. However, owing to the potential drop in front of the target only few positive ions could return to the plasma once recoiled at the surface. Neutral particles and negative ions including those reflected and sputtered escaped from the surface. The negative ions were accelerated by e(Vp − Vs) and reached the sheath region in front of the grounded entrance electrodes of the mass spectrometer, and decelerated by eVp. Consequently, the total energy gain of the negative ions was −eVs. In the presented experiment, the energies of the negative ions were analyzed using a Hiden EQP300 mass spectrometer equipped with an energy filter.18) Its aperture plate was placed 36 mm away from the target surface; the center of the sample was located on the axis of the spectrometer. Details of the experimental setup and conditions are provided in Ref. 19.
Fig. 2. Electrical potential profile between the sample holder and mass spectrometer EQP 300, when the target sample was biased at −20 V. The horizontal axis is not scaled.
Download figure:
Standard image High-resolution imageThe presented measurement was performed at a fixed hydrogen (or deuterium) gas pressure of 2 Pa, injected power for the RF plasma of 20–250 W, and injected power for ECR conditions of 60 W (1 Pa). In all of the plasmas, the dominant ion species was H3+. The target was kept at room temperature during the measurement. The plasma parameters were measured by a Langmuir probe in a separate experiment under the same conditions. The typical values were: Te ∼ 1 eV and ne ∼ 109/cm3 for the ECR operation, and Te ∼ 3 eV, ne ∼ 108/cm3 for the RF operation when the excitation of the coils for a vertical magnetic field was turned off in order to avoid the interference with the spectrometer. The plasma potentials, Vp, were approximately 8 V for both cases. The major negative ion impurities were OH− and H−. During the experiment, the negative-ion energy distribution functions (NIEDFs) of these impurities were occasionally measured; their peak values were approximately 3 and 0.2% of that of H−, respectively.
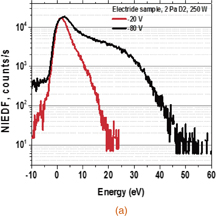
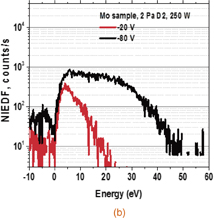
The NIEDFs shown in Figs. 3(a) and 3(b) were measured by the energy-analyzing mass spectrometer from the C12A7 electride and molybdenum target, respectively, with D2 plasmas with an RF power of 250 W. The vertical axis represents counts per second detected by the mass spectrometer, while the horizontal axis represents the negative-ion energy after subtraction of the energy gain by the target bias voltage, so that it corresponds to the initial energy of the negative ions leaving the target. The two curves shown in the figures are NIEDFs with bias voltages of −20 V (red) and −80 V (black). The target was placed normal to the spectrometer axis, and most of the detected particles were emitted almost normally. The effects of the angular distribution of the ions from the target, their trajectories, and collection probability by the spectrometer on the measured NIEDF were studied by simulations and discussed in Refs. 10 and 11. A large peak in the low-energy region below 10 eV was observed only in the C12A7 electride NIEDF. It has been known that low-energy negative hydrogen ions are produced through volume production, where vibrational-excited H2(X,ν) molecules are dissociated into H− and H0 in conjunction with electron attachment.7) It was revealed that some metallic surfaces exhibited an enhancing effect on highly vibrationally excited hydrogen molecules H2(X,ν) in gas-phase experiments.20) However, the potential profile shown in Fig. 2 prevented the negative ions produced in the plasma from coming into the sheath region in front of the analyzer. This was also confirmed by signals of the noise level at a bias voltage of Vs = 0. Therefore, the low-energy peaks presented in this study originated from the surface production on the electride.
Download figure:
Standard image High-resolution imageFig. 3. Negative-ion spectra from the (a) C12A7 electride and (b) molybdenum in the RF plasma, obtained at Vs = −20 V (red curve) and −80 V (black curve).
Download figure:
Standard image High-resolution imageIn order to confirm that the low-energy negative ions from the C12A7 electride were produced by desorption, in which implanted H/D particles were sputtered out, two measurements were performed. In the first experiment, the energy spectra (NIEDF) of the hydrogen negative ions from the C12A7 electride bombarded by hydrogen and argon ions were compared, as shown in Fig. 4. In both measurements, the target was pre-treated with the same procedure with an exposure to a hydrogen ECR plasma (1 Pa H2, 60 W) at Vs = −130 V for 10 min. The hydrogen plasma at 1 Pa, 60 W, Vs = −60 V or argon plasma at 0.1 Pa, 30 W, Vs = −60 V were then switched on and negative-ion spectra were recorded. The dominant peak in the low-energy region below 10 eV was attributed to the sputtering of pre-implanted hydrogen particles.
Fig. 4. Comparison of the normalized NIEDF spectra measured in an ECR Ar plasma (black) under 0.1 Pa, 30 W, and in an ECR H2 plasma (red) under 1 Pa, 60 W at Vs = −60 V after the sample was treated for 10 min in an ECR H2 plasma (1 Pa, 60 W, Vs = −130 V).
Download figure:
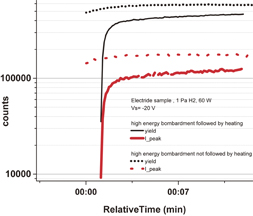
Standard image High-resolution imageIn the second experiment, the effect of hydrogen removal from the electride surface by baking was investigated. In the beginning of the experiment, an ECR H2 plasma of 60 W was applied for 10 min, 30 min, and 2 h at Vs = −130 V for hydrogen adsorption. After each bombardment, the NIEDF was measured at Vs = −20 V to confirm the hydrogen adsorption on the electride surface. The hydrogen adsorption saturated after 10 min of bombardment. The dotted curves in Fig. 5 represent the time behaviors of the integrated counts of the total negative-ion yield and peak counts of the NIEDF immediately after the application of a bias voltage of −20 V. A peak-intensity increase by a factor of 1.7 was observed. Further, a 520-°C baking was applied in vacuum after a 10-min-long bombardment at Vs = −130 V by an ECR H2 plasma. During the baking, a mass scan of the hydrogen H2 and H2O variations was recorded; the amounts of hydrogen and H2O increased 3 and 24 times during the heating, respectively. After cooling down, an ECR hydrogen plasma was ignited (1 Pa, 60 W, Vs = −20 V). Figure 5 shows the time behaviors of the yield and peak counts of the NIEDF immediately after the application of the bias voltage of −20 V, represented by the black and red curves, respectively. The negative-ion peak intensity increased by a factor of 11.7 in the first 5 min, followed by a slower increase. The rapid increase indicates that a substantial part of hydrogen adsorbed on the surface was removed by the baking and that most of the negative-ion counts can be attributed to desorption by sputtering.
Fig. 5. Time evolution of the negative-ion yield after 10 min of high-energy bombardment followed by heating in vacuum or without heating in vacuum.
Download figure:
Standard image High-resolution imageIn these experiments, a high negative-hydrogen-ion (H−) production rate from the C12A7 electride target surface was observed; negative ions were produced through (1) charge exchange (electron pick-up) reflection process and (2) desorption process by sputtering.21) The latter component was remarkably high compared with that from molybdenum. It has been reported that the negative-ion production ratio strongly depends on the surface work function in both processes. The high production yield of this material may be attributed to its low work function of 2.4–2.7 eV,16) but also to the specific nanostructure of the C12A7 electride. When this electride is exposed to a hydrogen environment, some of the encaged electrons are replaced by hydrogen negative ions.22) Although the nanocrystal structure is not preserved in the as-cleaved surface, the fractured cages can be easily restored upon heating at appropriate conditions.23)
Although an absolute production rate of negative hydrogen ions (H−) was not obtained in this study, it was compared with that from a clean molybdenum surface. Wada et al. measured the work-function dependence of the H− production rate of a cesiated molybdenum surface immersed in a hydrogen plasma, at bias voltages of −100, −200, and −300 V.24) Using their pseudo-exponential work-function (ϕ) dependence, the obtained ratio of the H− yield from the cesiated molybdenum surface with the lowest work-function to that from a clean molybdenum surface (ϕ = 4.3 eV) was approximately 40 at 100 V, while the ratio of the total H− yield from a C12A7 electride surface bombarded at 80 V to that from a clean molybdenum surface was approximately 10, as shown in Fig. 3. The ratio increased with the decrease of the negative bias voltage Vs, to 50 at Vs = −20 V. This value is consistent to our previous indirect measurement where the electric current corresponding to a negative hydrogen-ion current observed upon the exposure of a C12A7 electride to an atomic hydrogen (H0) flux was at a similar level to that obtained from a low-work-function bi-alkali-covered molybdenum surface.17) Together with the present direct observation of the negative hydrogen ions, it is likely that this material has a potential to produce a similar level of negative ions as that of the cesiated molybdenum surface in an H− source where a plasma grid is bombarded with relatively low-energy particles.
The C12A7 electride material is air-stable, mechanically robust, and machinable, with potentials to be used as a production surface of cesium-free negative ion sources for accelerators, plasma heating beams in nuclear fusion, and surface modification for industrial applications. Studies of extraction of H− from a compact ion source using a plasma grid obtained from the C12A7 electride are in progress.
Acknowledgments
The C12A7 electride was supplied from Asahi Glass Co., Ltd. We acknowledge the technical support and extensive discussions with Naomichi Miyakawa, Satoru Watanabe, and Kazuhiro Ito of Asahi Glass Co., Ltd. We would like to thank T. Yokoyama for the use of the C12A7 electride. H.H. acknowledges a fund from the JST ACCEL program and JSPS KAKENHI Grant Number JP 17H061253. J.E. acknowledges a support from the CDT-Fusion and EPSRC (No. EP/L01663X/1). The experimental setup at PIIM has been developed with a financial support of the French Research Federation for Fusion Studies (FR-FCM) and French Research Agency (ANR) under a grant 13-BS09-0017 H INDEX TRIPLED. This study was performed under the collaboration program of the National Institute for Fusion Science (NIFS15KBAR011 and NIFS15KLER041).