Abstract
We investigated the electrical properties of Fe3O4 films grown on MgO (001) substrates by pulsed-laser deposition for use as high-temperature resistors. By systematically examining the thickness-dependent resistivity and magnetoresistance, we found that a film thickness of approximately 200 nm was critical to obtaining a small, bulk-like temperature coefficient of resistance at high temperature. We improved the thermal stability of Fe3O4 films by surface passivation with an insulating Al2O3 layer. By combining these findings, we achieved a resistance change as small as 13% over a temperature range of −40 °C to 250 °C, satisfying a basic requirement of high-temperature resistors.
Export citation and abstract BibTeX RIS
Semiconductors with large electronic bandgaps can function in electronic devices at much higher ambient temperatures than silicon because of their lower intrinsic carrier concentration and higher breakdown voltage. SiC devices1,2) have already been used in power electronics modules, enabling efficient power conversion in various applications. One current challenge is achieving high thermal stability so that these devices can be employed in harsh environments at temperatures as high as 250 °C (523 K). To this end, all the elements in modules—such as resistors, capacitors, substrates, and packages—must be built with heat-resistant materials.
In this work, we study conductive oxides for use in high-temperature resistors. Resistors must have a low-temperature coefficient of resistance,  where ρ and T are the electrical resistivity and temperature, respectively. In commercial RuO2 thick-film chip resistors, TCR is adjusted by mixing in glass, which ensures that the resistance fluctuates little with changing T;3–5) however, such devices are not designed to withstand temperatures above 150 °C and cannot satisfy the benchmark resistance fluctuation of ±10% at temperatures of −40 °C to 250 °C.6) Thus, finding oxides with intrinsically small TCR has drawn great interest. Nearly T-independent ρ–T characteristics often appear near the metal-to-insulator (semiconductor) transition; solid solutions made from 3d transition metal oxides7–9) and degenerate oxide semiconductors10) can exhibit such characteristics near room temperature.
where ρ and T are the electrical resistivity and temperature, respectively. In commercial RuO2 thick-film chip resistors, TCR is adjusted by mixing in glass, which ensures that the resistance fluctuates little with changing T;3–5) however, such devices are not designed to withstand temperatures above 150 °C and cannot satisfy the benchmark resistance fluctuation of ±10% at temperatures of −40 °C to 250 °C.6) Thus, finding oxides with intrinsically small TCR has drawn great interest. Nearly T-independent ρ–T characteristics often appear near the metal-to-insulator (semiconductor) transition; solid solutions made from 3d transition metal oxides7–9) and degenerate oxide semiconductors10) can exhibit such characteristics near room temperature.
Rather than following these approaches, we focused on a simple binary system, Fe3O4, whose contributions of band conduction and hopping conduction with two opposite TCR signs produce a very weak T dependence of ρ near room temperature.11,12) However, to use Fe3O4 in film-type high-temperature resistors, two issues must be addressed. First, a high density of antiphase boundaries (APBs)13–18) are generated in Fe3O4 films, giving rise to a strongly T-dependent ρ contribution17) and altering the TCR to be semiconductor-like. APBs inevitably form because the inverse spinel structure of Fe3O4 has a cation sublattice, permitting fractional unit-cell translations with in-plane and out-of-plane shift vectors in the layer-by-layer growth.13,14,18) Although APBs have been studied extensively for anomalous magnetic and magneto-transport behavior not seen in the bulk,13–16) bulk-like ρ–T characteristics remain elusive, especially at high T. Second, at high ambient temperatures, chemical reactions of Fe3O4 films easily occur,19,20) making it difficult to reliably measure electrical properties at high temperature. In this paper, we fabricate Fe3O4 devices with bulk-like TCR and high thermal stability. Our high-temperature resistance measurement demonstrates a resistance change of only 13% across the required T range from −40 °C to 250 °C.
Epitaxial films of Fe3O4 were grown on MgO (001) substrates by pulsed-laser deposition using an ArF excimer laser.21) Prior to deposition, the MgO substrates were annealed at 700 °C using an oxygen pressure of 1 atm to obtain atomically smooth surfaces.22) During deposition, the substrate temperature was 350 °C, the oxygen partial pressure was 1.0 × 10−4 Pa. Some of the Fe3O4 films were transferred to a separate sputtering system and were covered with 130 nm thick Al2O3 films for the surface passivation. The film thickness was measured with an atomic force microscope, and the crystal structure was characterized by X-ray diffraction (XRD) with Cu Kα radiation. The electrical properties were measured by a four-probe method with a Physical Property Measurement System (Quantum Design, Inc.) at temperatures of 100–400 K and by a two-probe method using a probe station equipped with a hot chuck at temperatures of 323–523 K in air. Planar electrodes were made with a bilayer film of Au/Ti prepared by e-beam evaporation and a silver paint.
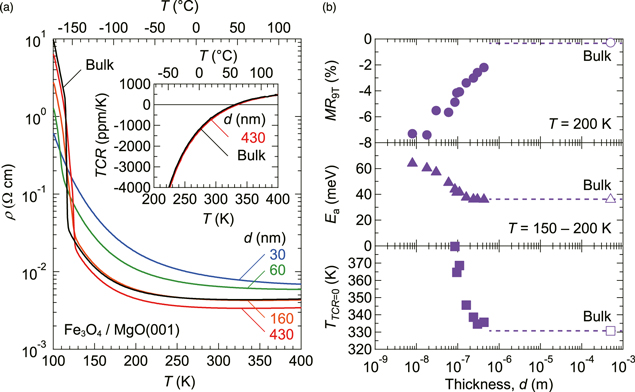
Figure 1(a) shows ρ–T curves of Fe3O4 films measured on heating for various thicknesses (d), together with bulk reference data measured for a Fe3O4 single crystal (SurfaceNet GmbH). In the films with d = 30 and 60 nm, the conduction was dominated by a negative TCR over the entire measured T range, and there is no obvious Verwey transition at low T (Fe valence ordering). As d was increased to a few hundreds of nanometers, the ρ at high T decreased and a broad ρ minima appeared near room temperature. As compared in the inset, the film with d = 430 nm exhibited a virtually bulk-like TCR with a sign-inversion T (TTCR=0) of approximately 50 °C. Also, the Verwey transition temperature of 124 K is very close to the highest bulk value of 125 K,23,24) implying that our Fe3O4 films are nearly stoichiometric and have small residual stresses.
Fig. 1. (Color online) (a) Resistivity versus temperature (ρ–T) curves of Fe3O4 films with various thicknesses (d). Data for a Fe3O4 single crystal are included for comparison. The inset shows the temperature coefficient of resistance (TCR) around room temperature. TCR is defined in the text. (b) Thickness dependence of magnetoresistance at 9 T (MR9T), activation energy estimated from ρ–T curves, and sign-inversion temperature of TCR (TTCR=0). Open symbols show the bulk data. Dashed lines are guides for the eye.
Download figure:
Standard image High-resolution imageFigure 1(b) shows the magnetoresistance measured at T = 200 K in a perpendicular magnetic field of 9 T (MR9T), the activation energy (Ea) estimated from ρ–T curves between 150 and 200 K, and TTCR=0 for various d. At antiferromagnetic APBs, spin-polarized electrons are scattered, which increases ρ at 0 T and produces the negative magnetoresistance (supplementary information is available online at stacks.iop.org/APEX/12/011003/mmedia).15,16) Because this additional ρ contribution has a large negative TCR,17) TTCR=0 smears out in the thin-film regime (d ≪ 100 nm).16) With increasing d, both Ea and TTCR=0 approach the bulk limit at a critical d of ∼200 nm. In agreement with previous papers,15,16) MR9T also decreases with increasing d, but still remains in the thick-film regime (supplementary information for the analysis of magnetoresistance data). For a more quantitative understanding of the relationship between magnetoresistance and ρ–T characteristics, it will be necessary to measure the domain size and the density of APBs by microscopy.
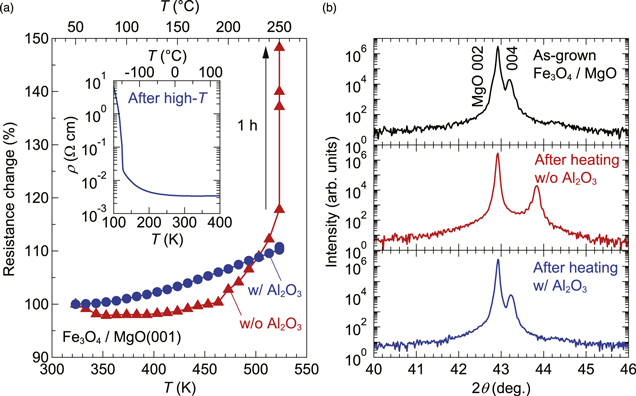
Having obtained the bulk-like TCR and TTCR=0, we examined the thermal stability of the Fe3O4 films. Figure 2(a) shows the resistance in air when heating from room temperature to 532 K (250 °C) for a bare Fe3O4 film (without Al2O3). The resistance rapidly increased above ∼450 K, and holding the sample at 532 K caused an irreversible change in resistance accompanied by the disappearance of the Verwey transition at low T (not shown). The upper and middle panels of Fig. 2(b) show the XRD patterns of bare Fe3O4 films before and after the high-temperature measurement, respectively. The shift of the Fe3O4 (004) reflection to higher angle corresponds to the c-axis shrinkage from 8.378 to 8.262 Å (a = b = c = 8.378 Å in Fe3O4 bulk: JCPDS, PDF No. 01-071-6336). In the presence of water (humid air), Fe3O4 oxidizes into cation-deficient spinel γ-Fe2O3 at moderately high T.20) Although the reduced c-axis parameter is even smaller than c = 8.352 Å of γ-Fe2O3 bulk (JCPDS, PDF No. 00-039-1346), this result and the disappearance of the Verwey transition suggest a substantial change in the crystal structure. We completely suppressed such changes by depositing an amorphous insulating Al2O3 layer on the Fe3O4 surface, as shown in the lower panel of Fig. 2(b), which let us perform high-temperature measurement without concern about heat-induced damage [Fig. 2(a)]. In the Fe3O4 films passivated with Al2O3 (and also Si3N4), the Verwey transition characteristics showed no significant differences before and after high-temperature measurement (Figs. 1(a) and 2(a) inset), indicating that the Fe valence states responsible for the Verwey transition remained intact.
Fig. 2. (Color online) (a) Resistance change versus T, measured in air. The resistance at 323 K (50 °C) is used as the standard (100%). The inset shows ρ–T curves of an Al2O3-covered Fe3O4 film taken after the high-T measurement, showing that its Verwey transition temperature and the magnitude of the resistive jump do not change from those in the pristine sample [Fig. 1(a)]. (b) Out-of-plane XRD patterns of a bare Fe3O4 film before (upper panel) and after (middle panel) heating at 250 °C for 3 h in air, and an Al2O3-covered Fe3O4 film after the identical heating process (lower panel).
Download figure:
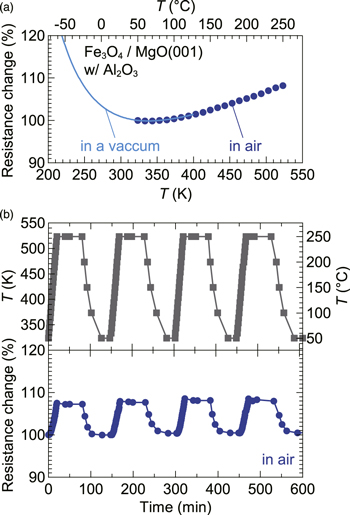
Standard image High-resolution imageFigure 3(a) shows merged data of the low-temperature (in a vacuum) and high-temperature (in air) resistance characteristics measured for a 430 nm thick Fe3O4 film covered with Al2O3. At an operating T from −40 °C to 250 °C, the resistance change was as small as 13%. Although this change is not monotonic due to the characteristic ρ upturn, it falls well within the benchmark value of 20% (±10%) required for high-T resistors. A thermal cycling test, shown in Fig. 3(b), confirms that the resistances are reproducible, validating the high thermal stability of the Al2O3/Fe3O4/MgO structure. We believe that the improved thermal stability at high temperature came from the low oxygen diffusion coefficient of Al2O3 (Ref. 25) and the protection from contact with air.
Fig. 3. (Color online) (a) Resistance change of an Al2O3-covered Fe3O4 film. The Fe3O4 film thickness was 430 nm. Two data sets collected with different measurement setups are combined. An excitation current of 1 mA was used for the low-temperature measurement. The resistance at 323 K (50 °C) is used as the standard (100%). (b) Resistance change in air through four thermal cycles.
Download figure:
Standard image High-resolution imageVarious applications of Fe3O4 films have been proposed, including spintronic devices based on their half-metallic nature26,27) and resistive memory devices exhibiting a reversible change in resistance by applying an electrical bias.28) The operation of the thermally stable resistor demonstrated in this work suggests that Fe3O4-based devices might also be used in power electronics modules. Exploring such applications would require fabrication of oxide devices with CMOS-compatible processes.
In summary, we tested Fe3O4 for use in high-temperature resistors. Using thick Fe3O4 films and appropriate passivation layers was important to suppress the effects of APBs and heat-induced degradation, and to correctly evaluate the resistance characteristics at high T. The small resistance change of 13% between −40 °C and 250 °C demonstrated by a simple binary, thus cheap, and environmentally friendly Fe3O4 system may offer a new route in the search for materials to use in high-temperature resistors.
Acknowledgments
We thank K. Matsumoto and Y. Kanai for their assistance with Si3N4 deposition, and thank K. Shinoda for our discussion. This work was partly supported by JSPS KAKENHI (No. 25790041) and Kumagai Foundation for Science and Technology. We also thank Joshua Yearsley, MS, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.