Pyramidal Neurons: Looking for the origins of axons
Pyramidal neurons are easily recognizable because their soma – the part of the neuron that contains the nucleus – has a characteristic triangular shape (hence their name). On closer examination, however, it becomes clear that the size of the soma can vary, as can the size and shape of the ‘arbor’ formed by the dendrites that carry signals to the soma (DeFelipe and Fariñas, 1992). Moreover, it has been reported that the axons of some pyramidal neurons in the mammalian cortex emerge from dendrites rather than from the base of the soma (Triarhou, 2014; Figure 1).
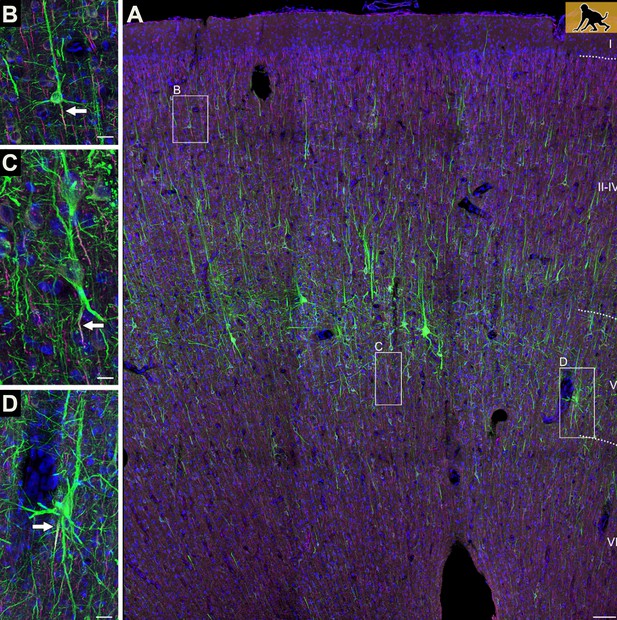
Axons can emerge from different parts of pyramidal neurons.
(A) The neocortex of an infant macaque monkey that has been stained with dyes that label the dendrites and axons (initial segment only) of pyramidal neurons (shown in green). Branching off the base of the soma are several basal dendrites, and a single apical dendrite protrudes from its apex. Most pyramidal neurons contain an axon (indicated by the white arrow) that exits from the base of the soma (B). In some neurons, however, the axon emerges from a dendrite (C), or initially co-joins with a dendrite as it exits from the soma (D). Scale bars represent 100 micrometers for panel A, and 25 micrometers for the other panels.
Image credit: Adapted from Figure 1 in Wahle et al., 2022
These ‘axon carrying dendrites’ are unusual because the signals dendrites receive are usually processed in the soma before they are sent out via the axon to other neurons (Förster, 2014). These kinds of morphological differences are important because they influence how individual neurons and neuronal groups compute information. Researchers are particularly interested in features that only occur in humans and primates, as these may be associated with cognitive behaviors as well as neurological and psychiatric conditions. Now, in eLife, Petra Wahle from Ruhr University Bochum and co-workers in Germany, Austria and Spain report that the proportion of axon carrying dendrites (AcDs) varies between mammalian species and different areas of the brain (Wahle et al., 2022).
Wahle et al. used a range of histological techniques to compare the morphology and structure of pyramidal neurons in postmortem tissue samples extracted from six cortical areas at different stages of the animals’ development (Figure 2). This revealed that the proportion of pyramidal neurons with an AcD was around 10–20% in non-primate mammals (rat, cat and ferret), but much lower (typically a few percent) in macaque monkeys and humans. Moreover, AcDs were rarely found in the upper layers of the neocortex: these layers are thicker in non-human primates and humans, and are associated with complex behaviors and higher cortical functions.
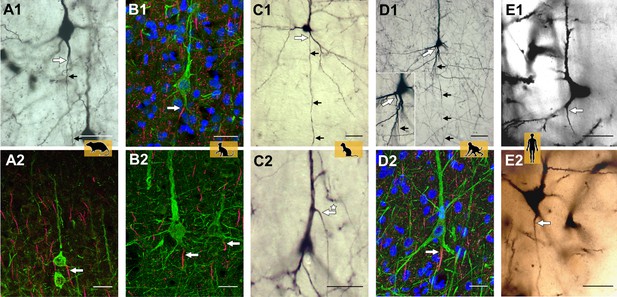
Axon carrying dendrites in different mammalian species.
Neurons with axon carrying dendrites (AcDs) in the rat visual cortex (A1, A2), the cat visual cortex (B1, B2), the ferret visual cortex (C1, C2), the macaque premotor cortex (D1, D2), and the human auditory cortex (E1, E2). Tissue samples were stained using one or two of the following techniques: biocytin (black and white images), immunofluorescence (multi-color images), or the Golgi method (human auditory cortex only). The site where the axons emerge is indicated by a large white arrow, with smaller black arrows highlighting the direction the axon takes through the neocortex. In most pyramidal neurons, the AcD emerges from the base of the soma. It is very rare for the AcD to emerge from the apical dendrite: an example of this is indicated by the asterisk in panel C2. Fewer than ten examples of apical AcDs were identified in the rat, ferret and macaque tissue samples studied, and none were identified in the human tissue samples. Scale bars represent 25 micrometers for all panels.
Image credit: Adapted from Figure 2 in Wahle et al., 2022.
So, what might be the reason for humans and non-human primates having fewer AcDs? It is thought that AcDs enhance the electrical behavior of pyramidal neurons by allowing signals to bypass the soma and flow directly from the dendrite to the axon. This is supported by prior studies showing that AcDs generate stronger and more frequent electrical spikes, and also require a lower threshold to trigger an action potential (Thome et al., 2014; Kole and Brette, 2018). Wahle et al. propose that humans and non-human primates have fewer AcDs because they already have other cellular specializations that can boost the strength of the electrical signal sent through pyramidal neurons.
The careful quantification and systematic survey of pyramidal neurons from different species and brain regions also yielded three other observations. First, Wahle et al. found that inhibitory interneurons do not follow the same trend as pyramidal cells: primates have similar numbers of AcD interneurons as other mammalian species. Second, humans have an unexpectedly high number of AcDs (8.86–13.23%) in the white matter compartment below the cortex, which largely contains bundles of axons intermixed with a population of scattered neurons. Among other features, these neurons are born early in development and have less distinct axon and dendritic regions compared to neurons at the cortical surface (Suárez-Solá et al., 2009). This weakened polarity may be the reason why a higher proportion of neurons in white matter have AcDs.
Third, consistent with previous work on the hippocampus (Benavides-Piccione et al., 2020), Wahle et al. found that humans had more pyramidal neurons with AcDs in this region of the brain than mice – which is the opposite of what happens in other parts of the cortex. Wahle et al. suggest that this could be because the hippocampus of humans and non-human primates requires extra features to carry out its complex memory-related processes. One way to test this could be to expand the histological analysis to other parts of the brain that are also associated with higher order functions, such as the amygdala.
In a broader context, the work by Wahle et al. adds to an already long list of pyramidal neurons that are morphologically distinct (DeFelipe and Fariñas, 1992). This includes inverted pyramidal neurons where apical dendrites (which usually emerge from the top of the soma) orientate down towards the white matter rather than up towards the surface of the brain (Banovac et al., 2021). An anatomical study in rabbits showed that the axons of inverted neurons can exit from multiple places (the base or side of the cell body, or off the downward-facing dendrite), and can project to a variety of cortical structures (Mendizabal-Zubiaga et al., 2007). This mixed population of standard and inverted pyramidal neurons has also been found in the brains of non-primate mammals and the hippocampus of non-human primates, suggesting that they may lead to different axonal outputs.
Another possibility is that differences in morphology may result from mis-steps during development. Notably, inverted pyramidal neurons are abundant in the cortex of mutant reeler mice, which have disorganized cortical layers owing to disruptions in neuronal migration during development (Landrieu and Goffinet, 1981; Förster, 2014).
Organization of the cytoskeleton (the scaffold of proteins that maintains the cell’s structure) may also play a role in these morphological differences, particularly the location of the initial segment of the axon, which varies across vertebrates and invertebrates (Prokop, 2020). Species variations are becoming increasingly easy to investigate due to the advent of super-resolution microscopy (Xu et al., 2013), and AcDs offer an interesting assay for examining ultrastructural specializations in normal and abnormal brains.
The findings of Wahle et al. highlight how the morphology of pyramidal neurons, and potentially other cells in the brain, does not always follow a uniform pattern and can vary between species. The study also offers several new avenues of research. For instance, the mechanisms involved in the formation and function of AcDs remain to be explored. Furthermore, it is still unclear why in the samples studied by Wahle et al. it is more common for dendrites at the base of the pyramidal soma to have AcDs than the apical dendrite (Figure 2C). In addition, other mechanisms – such as gene expression and metabolism – might play a role in this unusual axon location. AcDs could be an effective assay to identify neurons with distinct roles in the brain (Höfflin et al., 2017).
Answering these questions will require expanding the histological approach used by Wahle et al. to other areas of the brain, especially regions outside the cortex, and examining more species and stages of development. In addition, computer simulations could be used to model the consequences of having different proportions of AcDs. This could provide new insights into how and why cognitive behavior differs so much between species, particularly humans and non-human primates.
References
-
Von Economo neurons - primate-specific or commonplace in the mammalian brain?Frontiers in Neural Circuits 15:714611.https://doi.org/10.3389/fncir.2021.714611
-
Heterogeneity of the axon initial segment in interneurons and pyramidal cells of rodent visual cortexFrontiers in Cellular Neuroscience 11:332.https://doi.org/10.3389/fncel.2017.00332
-
The electrical significance of axon location diversityCurrent Opinion in Neurobiology 51:52–59.https://doi.org/10.1016/j.conb.2018.02.016
-
Inverted pyramidal neurons and their axons in the neocortex of reeler mutant miceCell and Tissue Research 218:293–301.https://doi.org/10.1007/BF00210345
-
The underside of the cerebral cortex: layer V/VI spiny inverted neuronsJournal of Anatomy 211:223–236.https://doi.org/10.1111/j.1469-7580.2007.00779.x
-
Cytoskeletal organization of axons in vertebrates and invertebratesThe Journal of Cell Biology 219:1–19.https://doi.org/10.1083/jcb.201912081
-
Neurons in the white matter of the adult human neocortexFrontiers in Neuroanatomy 3:7.https://doi.org/10.3389/neuro.05.007.2009
-
Axons emanating from dendrites: phylogenetic repercussions with Cajalian huesFrontiers in Neuroanatomy 8:133.https://doi.org/10.3389/fnana.2014.00133
Article and author information
Author details
Publication history
- Version of Record published: June 1, 2022 (version 1)
Copyright
© 2022, Rockland
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,946
- views
-
- 232
- downloads
-
- 1
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Biochemistry and Chemical Biology
- Evolutionary Biology
Stramenopiles form a clade of diverse eukaryotic organisms, including multicellular algae, the fish and plant pathogenic oomycetes, such as the potato blight Phytophthora, and the human intestinal protozoan Blastocystis. In most eukaryotes, glycolysis is a strictly cytosolic metabolic pathway that converts glucose to pyruvate, resulting in the production of NADH and ATP (Adenosine triphosphate). In contrast, stramenopiles have a branched glycolysis in which the enzymes of the pay-off phase are located in both the cytosol and the mitochondrial matrix. Here, we identify a mitochondrial carrier in Blastocystis that can transport glycolytic intermediates, such as dihydroxyacetone phosphate and glyceraldehyde-3-phosphate, across the mitochondrial inner membrane, linking the cytosolic and mitochondrial branches of glycolysis. Comparative analyses with the phylogenetically related human mitochondrial oxoglutarate carrier (SLC25A11) and dicarboxylate carrier (SLC25A10) show that the glycolytic intermediate carrier has lost its ability to transport the canonical substrates malate and oxoglutarate. Blastocystis lacks several key components of oxidative phosphorylation required for the generation of mitochondrial ATP, such as complexes III and IV, ATP synthase, and ADP/ATP carriers. The presence of the glycolytic pay-off phase in the mitochondrial matrix generates ATP, which powers energy-requiring processes, such as macromolecular synthesis, as well as NADH, used by mitochondrial complex I to generate a proton motive force to drive the import of proteins and molecules. Given its unique substrate specificity and central role in carbon and energy metabolism, the carrier for glycolytic intermediates identified here represents a specific drug and pesticide target against stramenopile pathogens, which are of great economic importance.
-
- Evolutionary Biology
- Genetics and Genomics
A protein’s genetic architecture – the set of causal rules by which its sequence produces its functions – also determines its possible evolutionary trajectories. Prior research has proposed that the genetic architecture of proteins is very complex, with pervasive epistatic interactions that constrain evolution and make function difficult to predict from sequence. Most of this work has analyzed only the direct paths between two proteins of interest – excluding the vast majority of possible genotypes and evolutionary trajectories – and has considered only a single protein function, leaving unaddressed the genetic architecture of functional specificity and its impact on the evolution of new functions. Here, we develop a new method based on ordinal logistic regression to directly characterize the global genetic determinants of multiple protein functions from 20-state combinatorial deep mutational scanning (DMS) experiments. We use it to dissect the genetic architecture and evolution of a transcription factor’s specificity for DNA, using data from a combinatorial DMS of an ancient steroid hormone receptor’s capacity to activate transcription from two biologically relevant DNA elements. We show that the genetic architecture of DNA recognition consists of a dense set of main and pairwise effects that involve virtually every possible amino acid state in the protein-DNA interface, but higher-order epistasis plays only a tiny role. Pairwise interactions enlarge the set of functional sequences and are the primary determinants of specificity for different DNA elements. They also massively expand the number of opportunities for single-residue mutations to switch specificity from one DNA target to another. By bringing variants with different functions close together in sequence space, pairwise epistasis therefore facilitates rather than constrains the evolution of new functions.






