Abstract
Background:
Measles, Rubella, and Hepatitis B are vaccine-preventable infectious diseases that cause several complications and impose a burden on health systems worldwide. Medical students are exposed to these viruses during their clinical training period and are more susceptible to these infections. Therefore, it is imperative to assess their immunity against these three viruses.Methods:
In this cross-sectional study in 2013, 75 blood samples were collected from the medical students of Shiraz University of Medical Sciences, Iran. The sample included 53 female and 22 male students with a mean age of 22 years. The IgG levels against measles, hepatitis B, and rubella were measured by the ELISA method. To analyze the data, chi-square test was applied using SPSS 22 software.Results:
The protective levels of IgG against measles, rubella, and hepatitis B were detected in 88%, 97.3%, and 94.7% of the students, respectively. We found a significant correlation between a positive history of measles and hepatitis B vaccination and protection against these two viruses (P value = 0.016 and P value = 0.000, respectively). A significant correlation was detected between the number of hepatitis B vaccination doses and immunity against this virus (P value = 0.016).Conclusions:
Immunity of medical students to measles, hepatitis B, and rubella was at an acceptable level. However, it is prudent to address any possible problems in measles vaccines. It is suggested that health planners evaluate medical students regarding their immunity to these viruses before they start their clinical training at hospitals.Keywords
Measles Rubella Hepatitis B Shiraz University of Medical Sciences
1. Background
Measles, rubella, and hepatitis B are among infectious diseases for which vaccines have been available since many years ago. Medical students are in constant exposure to these infectious agents in their clinical training period. According to the literature, immunity to these viruses in medical students is suboptimal. In a previous study in Southern Iran, only 52% of the students were found to be immune to measles. In the same study, 68% of the students were immune against hepatitis B virus (1). Reports from other parts of the world confirm this pattern. In a previous study by Mosaad et al. in Saudi Arabia, only 15.2% of medical students had protective levels of antibody to hepatitis B (2). In another study in Japan, 38.1% and 67.9% of the students were serologically protected against measles and rubella, respectively (3). Based on these data, assessing the immunity of medical students against these viruses is a necessity.
Measles is a highly contagious disease caused by an RNA virus transmitted by airborne spread or via droplets (4). Before the advent of the measles vaccine, 8 million people died of measles each year (5). Despite the significant control of measles in many parts of the world, measles was still the most common cause of vaccine-preventable deaths in children in the year 2000 and the fifth leading cause of all deaths in children less than 5 years of age. Outbreaks of measles can occur with the increasing proportion of susceptible individuals in the population (6). In the year 2012 in Iran, 7 cases of measles were reported in a village in Fars province, southwest of Iran. The primary case was an Afghan refugee and the secondary cases were fully vaccinated cases except for one case (7). The probable reasons for the lack of immunity to measles in vaccinated individuals include the drop in the level of antibodies after a few years, improper maintenance of the vaccine cold chain, and the inhibitory effect of maternal antibodies on antibody responses in infants (8, 9).
Rubella is caused by an RNA virus and its major route of transmission in the postnatal period is the direct contact with nasopharyngeal secretions of the patients. In the first trimester of pregnancy, rubella infection can have teratogenic effects on the fetus and lead to congenital rubella syndrome with complications ranging from blindness, deafness, and heart defects to stillbirth and abortion (10, 11). The immunity conferred by the rubella vaccine is variable as reported by different studies. In a previous study, it was estimated that the vaccine confers immunity against rubella viremia for more than 16 years (12-14). However, a small percentage of the vaccines either do not confer any immunity or produce such low levels of antibodies that the serum titer disappears after 5 to 8 years (15-17). Similar to measles, the reason for vaccine failure in people vaccinated against rubella could be the improper maintenance of the vaccine cold chain and the fact that the IgG level starts to decline after a few years.
More than two billion people in the world are infected with the hepatitis B virus as a DNA virus (18). Around 350 to 400 million people have hepatitis B chronic infection (19). In Iran, data on the prevalence of hepatitis B are variable (20-22), ranging from 1.3% (23, 24) to 2.14% (25) in different provinces. Approximately, 5% of those who have been vaccinated with the standard three-dose hepatitis B vaccine regimen display an inadequate response and are thus called non-responders (26).
2. Objectives
In this study, we aimed to measure the IgG titer against measles, rubella, and hepatitis B in the medical students of Shiraz University of Medical Sciences, Iran, before they enter their clinical rotations at hospitals. The immunity status of the students has important implications for health policy-makers in terms of evaluating both vaccine quality and vaccine efficacy and providing the non-immune students with booster doses and follow-up visits.
3. Methods
In this cross-sectional study, 75 blood samples were collected from the medical students of Shiraz University of Medical Sciences, Iran, in 2013. We used the census method of sampling since we included all the members of a class who were to begin their clinical rotations in the year 2013. Therefore, no inclusion or exclusion criteria were applied. The population under study included 53 female and 22 male students with a mean age of 22 years. No missing data were detected in this study. Consent forms were filled out by all the students. Then, information was recorded in a questionnaire regarding the measles, rubella, and hepatitis B vaccination status and socio-demographic variables including age, sex, history, and place of vaccination, and the number of received doses for hepatitis B. If they were not sure about the number of hepatitis B doses, we considered it as incomplete vaccination. The study protocol was approved by the Ethics Committee of Shiraz University of Medical Sciences (No. IR.sums.med.rec.1395.s216).
3.1. Serological Tests
The sera were centrifuged and stored at -20º and transferred to the Virology and Bacteriology Department of Shiraz University of Medical Sciences. Then, IgG against rubella, measles, and hepatitis B surface antigen was measured by the enzyme-linked immunosorbent assay (ELISA) test using an ELISA reader (Stat Fax-2100, Awareness Technology Inc.). The REF numbers were RE58341 for rubella IgG ELISA kits and 20099 for HBsAb.
According to the ELISA kit, an antibody titer of above 12 IU/mL was considered protective against measles and rubella while a titer of below 8 IU/mL was considered insufficient. Antibody titers in the range of 8 - 12 IU/mL were considered equivocal. For hepatitis B, an antibody titer of below 10 IU/mL and above 20 IU/mL were considered insufficient and protective, respectively. The levels from 10 IU/mL to 20 IU/mL were considered equivocal.
3.2. Statistical Analysis
The collected data were analyzed by SPSS 22 software. Descriptive statistics including frequency and mean were used for data description. The chi-square test (at one degree of freedom) was used to determine whether a significant correlation existed between the variables. P values of less than 0.05 were considered significant.
4. Results
The participants of this study included 75 medical students. All of the students completed the questionnaires and were tested for the presence of antibodies. The mean age of the subjects was 22 years (range: 21 - 23). There were 53 (70.7%) female and 22 (29.3%) male students in the sample. The participants originated from various regions of Iran. There were 47 (62.7%) students who were from Fars province who had received their vaccines in the province, while 28 (37.3%) were from other provinces of Iran. All of the students with a positive history of measles and rubella vaccination had been vaccinated in December 2003 according to Iran’s nationwide mass vaccination program. As for hepatitis B, the vaccinated students had received the hepatitis B vaccine in 2009 mass vaccination campaign.
4.1. Measles, Rubella, and Hepatitis B Vaccination Status
In terms of vaccination history, 71 (94.7%) students had a positive history of measles-rubella vaccination and 4 (5.3%) either did not receive the vaccine or did not remember their vaccination status. As for hepatitis B, 72 (96%) had received the hepatitis B vaccine. Only one (1.3%) student had not received the vaccine while 2 (2.7%) did not remember their vaccination history. Among those who had received hepatitis B vaccine, 66 (88%) had completed the three dose-regimen while 9 (22%) had received fewer than three doses.
4.2. Immunity to Measles, Rubella, and Hepatitis B
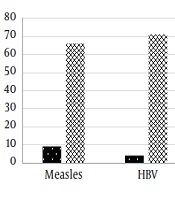
The results of the immunity status of the students are depicted in Figure 1. Of the 75 students, 66 (88%) had a measles IgG titer of above 12 IU/mL that was considered protective and 9 (12%) had an IgG level that was insufficient or equivocal. Moreover, 73 (97.3%) subjects had sufficient anti-rubella IgG and 2 (2.7%) had an antibody titer of below 12 IU/mL that was insufficient. Regarding hepatitis B, 71 (94.7%) had an IgG titer of above 20 IU/mL that was the ELISA kit index of being protective. On the other hand, 4 (5.3%) students did not have the protective levels of the antibody.
Immunity to measles, rubella, and hepatitis B

4.3. Association Between Different Variables and Immunity
We detected no association between gender and immunity against measles, rubella, and hepatitis B viruses. With regard to measles, 64 students with a positive history of vaccination had protective levels of anti-measles IgG while 7 students who were vaccinated against measles had insufficient antibody titers. A positive correlation was found between the vaccination history for measles and immunity against measles (P value = 0.016). Regarding rubella, there were 69 vaccinated students who were immune against rubella and 2 students who were vaccinated but did not have protective levels of the antibody. No correlation was found between the positive rubella vaccination history and protection against rubella (P value = 0.734). As for hepatitis B, there were 70 students who had received the hepatitis B vaccine, whether complete or incomplete, and had protective levels of IgG against the hepatitis B surface antigen. On the other hand, there were 2 students who had received the hepatitis B vaccine but had low antibody titers. We found a significant correlation between receiving the hepatitis B vaccine and having a high IgG titer against the hepatitis B surface antigen (P value = 0.000). Since the three doses of hepatitis B vaccine are required to confer immunity, we investigated the relationship between immunity and the vaccine doses that the subjects had received. There were 64 subjects who had been vaccinated fully and were immune against hepatitis B. There were 7 whose vaccinations were not complete but had sufficient antibody titers while 2 students were not immune despite having received three hepatitis B vaccine doses. A significant correlation was detected between the number of hepatitis B vaccination doses and immunity against this virus. In other words, those with full vaccination history were more likely to be immune to hepatitis B than those who had not received the three doses of the vaccine (P value = 0.016).
5. Discussion
The present study was performed to assess the immunity of medical students against measles, rubella, and hepatitis B viruses. The study showed that the measles-rubella vaccination rate among medical students under study was 94.7%. This is an acceptable coverage rate and is in accordance with the fact that the national coverage for the first and second doses of measles vaccine is 95% (7). In a previous study on health care workers in Uganda, the positive vaccination history for measles was found to be 49.2% (27). As for hepatitis B, the vaccination rate in our study was found to be 96% including those who had received less than three doses of the vaccine. This good coverage is due to the hepatitis B mass vaccination campaign that took place in 2009. There was only one student who had received the hepatitis B vaccine at birth. Contrary to our results, in a previous study in Saudi Arabia by Mosaad et al. in medical students, 36.2% of the students were sure to have received the hepatitis B vaccine (2) and in a previous study in Cameroon, only 18% of the medical students had completed the three doses of hepatitis B vaccine (28).
Immunity against measles was the least (88%) among the other two viruses whereas protection against rubella was the highest (97.3%). This low protection rate for measles in comparison with the corresponding rate of other viruses is worrying. Although the incidence of measles has significantly decreased in recent years in Iran (7) and other parts of the world (29), occasional outbreaks occur that can involve the non-immune population (6). These outbreaks usually happen with the introduction of the infection from abroad, as happened in a village in Fars province in 2012 in which 7 cases of measles were reported (7). The measles immunity rate in our study was in agreement with the study by Wicker et al. in Germany 2007 in which, 91.5% were immune (17). In a previous study performed on health care workers in Uganda in 2005, the immunity rate of 100% to measles was detected (27). In another previous study in Iran, 79.2% of the medical students under study had protective levels of antibody against measles (30). Compared to the results of a study undertaken at Shiraz University of Medical Sciences, two years before our study in which 52% of the students were immune to measles (1), the obtained rate of 88% in our study seems to be an improvement. This could be due to the lower vaccination rates in the previous study or due to improvements in the vaccine quality over the years. Therefore, attention should be paid to the measles vaccine to address any possible problems in the preservation and administration of the vaccine.
Immunity against rubella is crucial in women at their childbearing age due to the risk of congenital rubella syndrome with maternal rubella infection. In our study, 93.3% of the students were serologically protected against rubella. This is in accordance with the previous study at Shiraz University of Medical Sciences, Iran, that showed the rubella antibody level to be present in 100% of the students (1). In another previous study in Iran, 96.2% of the medical students were found to be immune against rubella (30).
Medical students should be considered as having the same high risk as health care providers for infection with hepatitis B during their clinical training period. In our study, 94.7% of the subjects had protective levels of antibody against hepatitis B virus. In the study undertaken at Shiraz University of Medical Sciences, Iran, two years before our study, 68% of the students were serologically protected against hepatitis B virus. In a meta-analysis study of the immunity of health care personnel and health care students against hepatitis B in Iran, the efficacy of the hepatitis B virus vaccine was found to be 93.1% (31). In contrast to the results of our study, in the study done by Mosaad et al., only 15.2% of the medical students had protective levels of HBsAb (2).
Gender did not seem to play a role in determining the immunity against measles, rubella, and hepatitis B viruses in our study.
Since vaccination is the only means of seroproetction other than natural infection, we investigated the relationship between positive vaccination history and seroproetction. We found a significant correlation between measles and hepatitis B vaccination history and protective levels of antibody; however, no correlation was found between rubella vaccination history and rubella seroprotection. This could be explained by either natural infection in those who had not been vaccinated or simply by the fact that vaccinated individuals have forgotten their vaccination history.
The low antibody titer in vaccinated individuals in this study can be explained partly by vaccine failure. Primary vaccine failure happens when the body cannot mount a protective immune response after vaccination. This can occur when the presence of maternal antibodies to measles inhibits seroconversion, especially in very young subjects (29). Secondary vaccine failure occurs due to the declining levels of antibodies over the years. This mechanism has been proposed for hepatitis B (32), measles (9), and rubella (15, 16, 27). It has been shown that approximately 5% of those who have been vaccinated with the standard three-dose hepatitis B vaccine show an inadequate response and are called non-responders. Walayat et al. proposed some risk factors for the hepatitis B virus vaccine non-response, among which were old age, male sex, certain HLA haplotypes, renal failure, immunodeficiency, and intra-gluteal vaccine administration. However, most non-responders were healthy individuals without any known risk factors (26).
As for the rubella virus, it has been shown that antibodies in the serum can disappear after 5 to 8 years (15, 16, 27). Other causes of vaccine failure may be related to the problems in maintaining the cold chain of the vaccine (8), improper administration of the vaccine, etc. Interrupted maintenance of the vaccine cold chain especially applies to live attenuated vaccines such as measles and rubella vaccines since they are heat-sensitive.
That being said, we cannot safely assume that vaccine failure has occurred in all the students who were vaccinated and had low titers. Some studies have shown that despite the low antibody titer against HBs antigen or the non-responder state, immunity against hepatitis B is at least partially present in the presence of hepatitis B surface antibody-producing memory cells. According to a study, it is useless or even detrimental to immunize this population with the same vaccine and they may not produce more antibodies in this way (33). High-risk individuals such as healthcare workers should be considered for post-vaccination testing for HBs antibody 1 to 2 months after the last dose of the vaccine series (34).
To achieve our goal of maximum protection among medical students, we suggest that the students receive booster doses of hepatitis B vaccine if they have not received the three doses of hepatitis B vaccine and do not have sufficient antibody titer against hepatitis B. In any case, we recommend those who have not received the hepatitis B vaccine to become vaccinated and to be tested for hepatitis B surface antibody titer after 1 to 2 months.
To the best of our knowledge, our study is among the very few studies conducted in Iran to evaluate the immunity of medical students to these infectious agents. Measuring the antibody titer to these and possibly other infectious agents before the students enter the hospital for clinical rotations is a prudent action that health planners in the university must undertake.
One of the limitations in this study was the small population under study. Since the subjects were all the medical students of a class, no sampling was done. The small sample size makes it difficult to generalize the results of this study. Recall bias could also be present in this study as the students were vaccinated in their childhood and teen years and might not have remembered their vaccination history completely.
5.1. Conclusions
The majority of the medical students at Shiraz University of Medical Sciences, Iran, had protective levels of antibodies to measles, rubella, and hepatitis B. However, due to the constant exposure of medical students to these infectious agents and the complications and threats imposed by the infections, it is highly suggested that the university authorities take steps to assess the immunity of these future healthcare providers on a regular basis.
Acknowledgements
References
-
1.
Moattari A, Asad AG, Mansouri A, Vaziri MM, Shammami FM. Evaluation of measles, rubella, mumps, hepatitis B and varicella zoster antibodies in medical and dental students in Shiraz, Iran. Novel Biomed. 2014;2(1):6-9. https://doi.org/10.22037/nbm.v2i1.5911.
-
2.
Mosaad M, Al Nozha OM, Yamany H, Amer S. A survey of hepatitis B immune status of Taibah University medical students. J Taibah Univ Med Sci. 2014;9(4):301-6. https://doi.org/10.1016/j.jtumed.2014.05.006.
-
3.
Katsuyama M, Koike Y, Hirohara T, Kogawa K. Immune status of representative infectious diseases among Japanese female university students. Int J Adolesc Med Health. 2016;30(2). [PubMed ID: 27508954]. https://doi.org/10.1515/ijamh-2016-0038.
-
4.
Al-Kobaisi MF. Jawetz, Melnick and Adelberg’s medical microbiology 24th edition. Sultan Qaboos Univ Med J. 2007;7(3):273-5. [PubMed Central ID: PMC3074881].
-
5.
Hilleman MR. Current overview of the pathogenesis and prophylaxis of measles with focus on practical implications. Vaccine. 2001;20(5-6):651-65. [PubMed ID: 11738730].
-
6.
Mansour-Ghanaei F, Rahimi H, Joukar F, Bagherzadeh AH, Heidarzadeh A, Rahbar A, et al. Mass vaccination of measles and rubella (MR) in Guilan, Northern Iran: Evaluation of coverage and complications. Iran Red Crescent Med J. 2008;10(3):173-9.
-
7.
Moghadam M, Afsarkazerooni P, Ebrahimi M, Soltani M, Razmpoor A, Pirasteh E, et al. Measles outbreak in South of Iran, where vaccine coverage was high: A case-series study. Iran J Public Health. 2014;43(3):375-80. [PubMed ID: 25988100]. [PubMed Central ID: PMC4419178].
-
8.
Kumru OS, Joshi SB, Smith DE, Middaugh CR, Prusik T, Volkin DB. Vaccine instability in the cold chain: Mechanisms, analysis and formulation strategies. Biologicals. 2014;42(5):237-59. [PubMed ID: 24996452]. https://doi.org/10.1016/j.biologicals.2014.05.007.
-
9.
Veit BC, Schydlower M, McIntyre S, Simmons D, Lampe RM, Fearnow RG, et al. Serological response to measles revaccination in a highly immunized military dependent adolescent population. J Adolesc Health. 1991;12(3):273-8. [PubMed ID: 2054370].
-
10.
Wicker S, Rabenau HF, Gottschalk R, Doerr HW, Allwinn R. Seroprevalence of vaccine preventable and blood transmissible viral infections (measles, mumps, rubella, polio, HBV, HCV and HIV) in medical students. Med Microbiol Immunol. 2007;196(3):145-50. [PubMed ID: 17273881]. https://doi.org/10.1007/s00430-007-0036-3.
-
11.
Trevisan A, Morandin M, Frasson C, Paruzzolo P, Davanzo E, Marco LD, et al. Prevalence of childhood exanthematic disease antibodies in paramedical students: Need of vaccination. Vaccine. 2006;24(2):171-6. [PubMed ID: 16154242]. https://doi.org/10.1016/j.vaccine.2005.07.062.
-
12.
Atrasheuskaya AV, Kulak MV, Neverov AA, Rubin S, Ignatyev GM. Measles cases in highly vaccinated population of Novosibirsk, Russia, 2000-2005. Vaccine. 2008;26(17):2111-8. [PubMed ID: 18343536]. https://doi.org/10.1016/j.vaccine.2008.02.028.
-
13.
Chamat S, Salameh P, Haddad N, Berry A, Chedid P, Bouharoun-Tayoun H. Protection of medical and paramedical university students in Lebanon against measles, mumps, rubella and varicella: Active measures are needed. J Infect Public Health. 2011;4(3):125-34. [PubMed ID: 21843858]. https://doi.org/10.1016/j.jiph.2011.02.003.
-
14.
Yalcin SS, Kanra G, Pehlivan T. Outbreak of measles in medical students and determination of immune status to measles-mumps-rubella viruses. Int J Adolesc Med Health. 2006;18(4):615-22. [PubMed ID: 17340852].
-
15.
Botelho-Nevers E, Cassir N, Minodier P, Laporte R, Gautret P, Badiaga S, et al. Measles among healthcare workers: A potential for nosocomial outbreaks. Euro Surveill. 2011;16(2). [PubMed ID: 21251488].
-
16.
Baer G, Bonhoeffer J, Schaad UB, Heininger U. Seroprevalence and immunization history of selected vaccine preventable diseases in medical students. Vaccine. 2005;23(16):2016-20. [PubMed ID: 15734076]. https://doi.org/10.1016/j.vaccine.2004.03.073.
-
17.
Wicker S, Allwinn R, Gottschalk R, Rabenau HF. Reliability of medical students' vaccination histories for immunisable diseases. BMC Public Health. 2008;8:121. [PubMed ID: 18412957]. [PubMed Central ID: PMC2330143]. https://doi.org/10.1186/1471-2458-8-121.
-
18.
Merat S, Rezvan H, Nouraie M, Jamali A, Assari S, Abolghasemi H, et al. The prevalence of hepatitis B surface antigen and anti-hepatitis B core antibody in Iran: A population-based study. Arch Iran Med. 2009;12(3):225-31. [PubMed ID: 19400598].
-
19.
McMahon BJ. Natural history of chronic hepatitis B - clinical implications. Medscape J Med. 2008;10(4):91. [PubMed ID: 18504503]. [PubMed Central ID: PMC2390714].
-
20.
Ghavanini AA, Sabri MR. Hepatitis B surface antigen and anti-hepatitis C antibodies among blood donors in the Islamic Republic of Iran. East Mediterr Health J. 2000;6(5-6):1114-6. [PubMed ID: 12197336].
-
21.
Amini S, Mahmoodi MF, Andalibi S, Solati AA. Seroepidemiology of hepatitis B, delta and human immunodeficiency virus infections in Hamadan province, Iran: A population based study. J Trop Med Hyg. 1993;96(5):277-87. [PubMed ID: 8411302].
-
22.
Pourshams A, Nasiri J, Mohamadkhani A, Nasrollahzadeh D. Hepatitis B in Gonbad-e-Kavoos: Prevalence, risk factors, and intrafamilial spreading. Govaresh J. 2004;9(4):222-5.
-
23.
Ghadir MR, Belbasi M, Heidari A, Jandagh M, Ahmadi I, Habibinejad H, et al. Distribution and risk factors of hepatitis B virus infection in the general population of Central Iran. Hepat Mon. 2012;12(2):112-7. [PubMed ID: 22509188]. [PubMed Central ID: PMC3321318]. https://doi.org/10.5812/hepatmon.822.
-
24.
Nokhodian Z, Kassaian N, Ataei B, Javadi AA, Shoaei P, Farajzadegan Z, et al. Hepatitis B markers in Isfahan, Central Iran: A population-based study. Hepat Mon. 2009;9(1):12-6.
-
25.
Alavian SM, Hajarizadeh B, Ahmadzad-Asl M, Kabir A, Bagheri-Lankarani K. Hepatitis B virus infection in Iran: A systematic review. Hepat Mon. 2008;8(4).
-
26.
Walayat S, Ahmed Z, Martin D, Puli S, Cashman M, Dhillon S. Recent advances in vaccination of non-responders to standard dose hepatitis B virus vaccine. World J Hepatol. 2015;7(24):2503-9. [PubMed ID: 26523203]. [PubMed Central ID: PMC4621464]. https://doi.org/10.4254/wjh.v7.i24.2503.
-
27.
Lewis RF, Braka F, Mbabazi W, Makumbi I, Kasasa S, Nanyunja M. Exposure of Ugandan health personnel to measles and rubella: Evidence of the need for health worker vaccination. Vaccine. 2006;24(47-48):6924-9. [PubMed ID: 17014938]. https://doi.org/10.1016/j.vaccine.2006.05.126.
-
28.
Noubiap JJ, Nansseu JR, Kengne KK, Tchokfe Ndoula S, Agyingi LA. Occupational exposure to blood, hepatitis B vaccine knowledge and uptake among medical students in Cameroon. BMC Med Educ. 2013;13:148. [PubMed ID: 24200149]. [PubMed Central ID: PMC3874660]. https://doi.org/10.1186/1472-6920-13-148.
-
29.
Paunio M, Hedman K, Davidkin I, Valle M, Heinonen OP, Leinikki P, et al. Secondary measles vaccine failures identified by measurement of IgG avidity: High occurrence among teenagers vaccinated at a young age. Epidemiol Infect. 2000;124(2):263-71. [PubMed ID: 10813152]. [PubMed Central ID: PMC2810910]. https://doi.org/10.1017/s0950268899003222.
-
30.
Keshavarz M, Nicknam MH, Tebyanian M, Shahkarami MK, Izad M. Anti-rubella, mumps and measles IgG antibodies in medical students of Tehran University. Iran J Allergy Asthma Immunol. 2016;15(3):244-50. [PubMed ID: 27424140].
-
31.
Azami M, Hafezi Ahmadi MR, Sayehmiri K. Hepatitis B vaccination efficacy in Iranian healthcare workers: A meta-analysis study. Hepat Mon. 2016;17(1). https://doi.org/10.5812/hepatmon.37781.
-
32.
Poorolajal J, Mahmoodi M, Majdzadeh R, Nasseri-Moghaddam S, Haghdoost A, Fotouhi A. Long-term protection provided by hepatitis B vaccine and need for booster dose: A meta-analysis. Vaccine. 2010;28(3):623-31. [PubMed ID: 19887132]. https://doi.org/10.1016/j.vaccine.2009.10.068.
-
33.
Zaffina S, Marcellini V, Santoro AP, Scarsella M, Camisa V, Vinci MR, et al. Repeated vaccinations do not improve specific immune defenses against Hepatitis B in non-responder health care workers. Vaccine. 2014;32(51):6902-10. [PubMed ID: 25444815]. https://doi.org/10.1016/j.vaccine.2014.10.066.
-
34.
World Health Organization. Hepatitis B vaccines: WHO position paper, July 2017 - recommendations. Vaccine. 2019;37(2):223-5. [PubMed ID: 28743487]. https://doi.org/10.1016/j.vaccine.2017.07.046.