Abstract
Background:
Multiple Sclerosis (MS) is a chronic and inflammatory disease that can affect the patients’ quality of life and impose many costs on them. Several types of medicine are used to change the course of the disease, treat disease - related attacks, and treat the symptoms of multiple sclerosis.Objectives:
The aim of this study was to determine and compare the cost-effectiveness and cost - utility of CinnoVex versus ReciGen as the first line treatment in patients with relapsing-remitting multiple sclerosis in Iran, Fars province, in 2016.Methods:
This study was a cost - effectiveness and cost - utility study, in which a Markov model was used. A sample of 178 patients with MS was randomly selected. The costs were summed up from the societal perspective, and the study outcomes were QALY and the mean of relapse was avoided. To collect the required data, the cost data collection form, Kurtzke Expanded Disability Status Scale, and EQ - 5D questionnaire were used. To analyze the data collected, the TreeAge Pro 2011 and Excel 2010 software were used as well.Results:
The results showed that the mean cost for ReciGen and CinnoVex patients were 349.84 and 289.92 USD, respectively. In addition, the QALY means were 0.291 and 0.297 and the means of relapse avoided were 0.309 and 0.239 for ReciGen and CinnoVex patients, respectively. The one - way sensitivity analysis showed that the results of the model were sensitive to effectiveness and utility of both medicines, but had little sensitivity to other parameters.Conclusions:
According to the results, ReciGen was more cost - effective in terms of relapse avoided and CinnoVex was more cost - effective in terms of QALY. Therefore, ReciGen and CinnoVex can be the preferred options for physicians and for health policymakers and managers, respectively.Keywords
Cost - effectiveness Cost - utility Relapsing - Remitting Multiple Sclerosis ReciGen CinnoVex
1. Background
Multiple Sclerosis (MS) is a disease that cripples the brain and the spinal cord (central nervous system) (1), and those with MS have many disabling symptoms, including weakness in movement, changes in mood, pain and sensory problems, visual disturbances, and defecation disorders that have a significant effect on the quality of life of the patients and their families (2). The global prevalence of this disease in 2008 was estimated to be 30 per 100000 people by the World Health Organization (WHO) and the Multiple Sclerosis International Federation (MSIF) in 122 countries (3). Studies conducted in Iran and the Middle East in recent years have shown that MS outbreaks in these areas are increasing, therefore, the incidence rate of the disease in these areas increased from 0.86 per 100000 in 1989 to 2.93 in 2008 (4). In Iran, the incidence rate of MS was lower in the early 1990s, and only 5 people per 1000 were infected by the disease. However, the gradual growth of MS during recent years has caused the incidence rate of over 100 MS patients per 100000 people in some areas, such as Isfahan and Tehran. In addition, the figures are high in regions like Shiraz and some northern cities of the country. Although accurate statistics are not available for MS patients in Iran, estimates by the MS Society of Tehran indicated that there were about 60000 MS patients in Iran in 2013 (5-7). The prevalence of MS was estimated to be 72.1 per 100000 people in the Fars province in 2013 (8).
Furthermore, the results of some studies on the quality of life of MS patients showed that the physical performance of the patients with mild MS reduced by 30% while that of the patients with moderate and severe MS reduced by 40% and 50%, respectively.
Moreover, the social role functioning of the patients with mild and moderate MS reduced by 20% and that of the patients with severe MS reduced by 30% as well. In all MS patients, the mental functioning also decreased regardless of the severity of the disease (9). On the other hand, MS imposes a lot of costs on the community and individuals. According to a study by the US National Institute of Neurological Disorders and Stroke (NINDS) in 1993, the total cost of the disease was over $2.5 million a year (10). A study conducted in Europe within 1996 to 2014 also indicated that the mean annual costs of each MS patient was $24666 to $5167 (11). In addition, the results of various studies conducted in Iran showed that the total annual cost of patients with multiple sclerosis was $9.36 million in 2014 (12). Currently, several types of medicine are prescribed for MS patients, which are mainly used to change the course of the disease or to treat the disease attacks and symptoms .The US Food and Drug Administration (FDA) has approved some medicine for the disease. These types of medicine are used to reduce the development of the disease in many people involved in different types of MS such as relapsing remitting multiple sclerosis (RRMS), secondary progressive multiple sclerosis (SPMS), and progressive relapsing multiple sclerosis (PRMS).
These types of medicine include injectable medicine (Interferon beta - 1awiththe brand of Avonex for intramuscular injection, Interferon beta - 1awith the brand of Rebif for subcutaneous injection, and Glatiramer Acetate) as well as oral and infusion types (slow intravenous injection) (13). As the third largest supplier of MS medication, Iran has offered its manufactured medicine to the market with the names of CinnoVex (since the second half of 2005) and ReciGen (since 2009). CinnoVex is the brand of Interferon beta - 1a with intramuscular injection, and ReciGen is the brand of Interferon beta - 1a with subcutaneous injection (14-16).
In general, considering the above - mentioned issues regarding the incidence of multiple sclerosis and the high costs of the disease for both the patients and the community, on one hand, and as CinnoVex and ReciGenare produced in Iran and the researchers could not find a comparative study on the cost - effectiveness and cost - utility of CinnoVex and ReciGenin Iran. On the other hand, this study was conducted to determine and compare the cost - effectiveness and cost - utility of ReciGen versus CinnoVex, as the first line treatment for patients with RRMS in 2016 in Iran, Fars province, so that the results would help determine the more cost - effective medicine for the treatment of MS patients and also help the health managers, policy - makers, and specialists to prescribe better medicine and also make proper use of the limited available resources.
2. Methods
This cross - sectional study was a full economic evaluation (a cost - effectiveness and cost - utility study) carried out in 2016 on patients with multiple sclerosis, who were living in Iran, Fars province, and were taking CinnoVex and ReciGen for the treatment of their disease.
According to the statistics by the Department of Special Diseases of Shiraz University of Medical Sciences and MS Society of Fars province, and based on the results of the pilot study and assuming α = 0.05, δ = 1.2, and d = 0.25 in each group of patients, the sample size for this study was determined as 89 patients who were selected through simple random sampling method. The inclusion criteria were the use of ReciGen or CinnoVex that was prescribed by neurologists for patients with definite diagnosis or relapsing -remitting multiple sclerosis for at least one year and the willingness to participate in the study.
This study was approved by the Medical Ethics Committee of Iran, Shiraz University of Medical Sciences. In addition, all the patients participating in the study completed the informed consent form. They were free to participate in the study and in case of unwillingness to continue their participation in the project, they could withdraw from the study. Moreover, they were assured that their information would remain confidential. It should be noted that, finally, all the studied patients completed the study.
2.1. Description of the Model
A Markov model was used in this study to evaluate the cost-effectiveness and cost - utility of ReciGen versus CinnoVex and to describe the progression of the disease. Based on the previous studies, one - month Markov cycles as well as a lifetime horizon were used in this study (17, 18). To show the clinical course of RRMS (for example, disease progression and relapse), Kurtzke Expanded Disability Status Scale (EDSS) was applied (19). According to this scale, patients are classified based on their EDSS scores. Different health statuses based on the EDSS score are as follows: EDSS 0 - 2.5 (no limitation or slight limitation in mobility), EDSS 3 - 5.5 (moderate mobility limitation), EDSS 6 - 7.5 (walking with auxiliary equipment or using wheelchairs), EDSS 8 - 9.5 (being limited to bed), death (natural causes or EDSS 10), EDSS 0 - 2.5: relapse (relapse or a change in disability), and EDSS 3 - 5.5: relapse (relapse or a change in disability) (20). Figure 1 shows the schematic diagram of the Markov model. As can be seen, MS patients can move to a higher EDSS or stay in the same health state based on transition probabilities. They can also be transited to the relapse state (in which they are treated as outpatients or inpatients depending on their disease severity) in EDSS 0 - 2.5 or EDSS 3 - 5.5, and remain there for a single cycle. Patients can remain for more than one cycle (one month) in any disease state (one EDSS level). Patients remain in the RRMS state as long as they are not transferred to a level higher than EDSS 3 - 5.5, and when they are transited to the EDSS 6 - 7.5 level or higher, they are considered as SPMS and their remedial medicines are stopped (17).
Schematic Diagram of the Markov Model for MS (21)
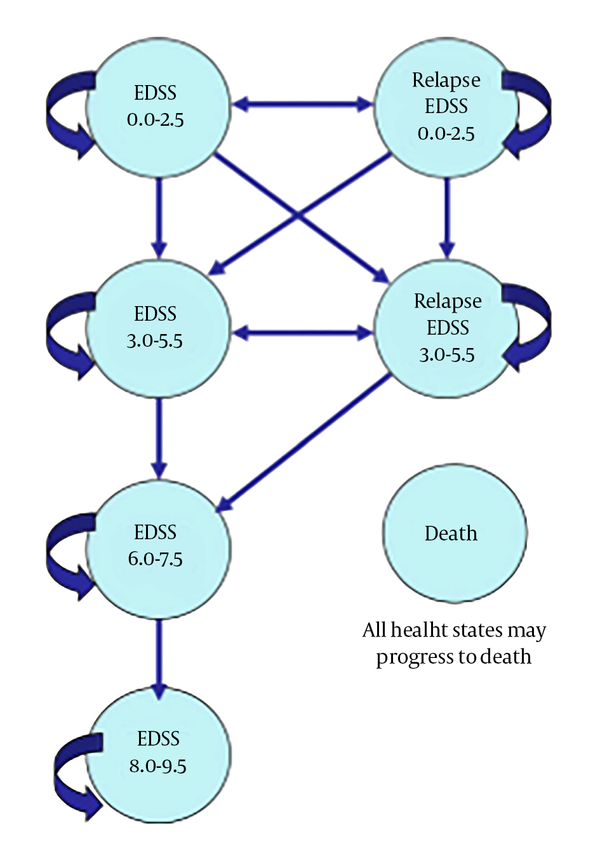
The outcomes used in the model included the mean of relapse avoided as effectiveness and the value of QALY as utility. The cost data and outcomes (utility and effectiveness) of the model were discounted on the basis of the discount rates of 7.2% (22) and 3% (23), respectively. In addition, TreeAge Pro 2011 and Excel 2010 software were used to analyze the collected data.
2.2. Transition Probabilities
All transition probabilities are reported in Table 1 according to the previous published studies based on the type of medicines and the disability status
Transition Probabilities
| Parameters | ReciGen | CinnoVex | ||
|---|---|---|---|---|
| Probability | Sources | Probability | Sources | |
| Monthly probability of disease progression to next level | ||||
| EDSS 0.0 - 2.5 | 0.0080417 | (24, 25) | 0.009125 | (24, 26) |
| EDSS 3.0 - 5.5 | 0.0047917 | (24, 25) | 0.004583 | (24, 26) |
| Monthly probability of progression to death | ||||
| EDSS 0.0 - 2.5 | 0.001684 | (27) | 0.001684 | (27) |
| EDSS 3.0 - 5.5 | 0.002348 | (27) | 0.002348 | (27) |
| EDSS 6.0 - 7.5 | 0.003121 | (27) | 0.003121 | (27) |
| Monthly relapses | ||||
| For All EDSSs | 0.0755 | (28) | 0.0275 | (21) |
2.3. Cost Data
The societal perspective was used in the present study to extract the costs. The related costs from the societal perspective included direct medical costs, direct non - medical costs, and indirect costs. The direct medical costs of each medicine were retrospectively determined and collected from January 1, 2016 to January 1, 2017 by using a researcher - made checklist and referring to the specialists’ offices and the MS Society of Fars province. The direct non - medical costs included the costs of transportation, meals, and accommodation in other cities and the food used by the patients and their families, as well as the costs of taking care of the patients at home, which were determined by asking the patients under study. To calculate the indirect costs, the human capital approach was used (29-32).
Furthermore, for the purpose of international comparison, the costs were converted to the US dollar, using the exchange rate of each US dollar equaling to 29500 Rials in 2016 (33).
2.4. Health Outcomes
To measure the effectiveness, the mean of relapse avoided was used. In order to obtain the mean of relapse avoided, the patients were first asked by the researcher about the number of relapses at the time of using each medicine, and the relapse rate for each patient was calculated using the following formula:
Relapse rate (RR) = Number of relapses / Number of years of medicine use
Then, the relapse rates of all the patients who used each medicine were summed up and the result was divided into the number of patients who used the same medicine to obtain a mean relapse rate for each medicine (17, 34). Next, according to the previous study, the mean relapse rate before using each medicine was considered as 1.002 (35) and the mean relapse rate in the patients who participated in this study was deduced from it to obtain the mean rate of relapse avoided.
The utility values were calculated using the EQ - 5D questionnaire, and the health outcomes were evaluated based on the quality adjusted life years (QALYs) (36).
2.5. Determining the Incremental Cost - Effectiveness and Cost - Utility Ratio (ICER)
After obtaining the costs as well as utility and effectiveness, the incremental cost - effectiveness and cost - utility ratios were calculated using Equation 1:
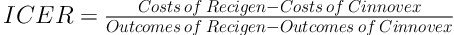
2.6. Sensitivity Analysis
Finally, to determine the effects of the parameters’ uncertainty on the results, a one - way sensitivity analysis was performed. To this end, some of the key parameters of the study, such as cost, utility, and effectiveness were changed by 20% for each medicine strategy, based on which Tornado diagrams were drawn.
3. Results
According to the results of this study, most patients were female (82.1%), housewives (58.99%), with academic degrees (41.01%), and all the patients had the basic insurance coverage. Furthermore, the mean ages of the patients using CinnoVex and ReciGen were 35.1 ± 8.89 and 34.77 ± 7.97, respectively.
Tables 2 and 3 show the means of costs, utility, and relapse avoided in the patients with multiple sclerosis who used CinnoVex and ReciGen.
According to Table 2, the highest mean of direct medical costs and direct non - medical costs for the patients using ReciGen were 569.46 ± 53.65 and 324.67 ± 30.48 dollars, respectively. In addition, the cost of purchasing the main medicine was the highest direct medical cost in both ReciGen and CinnoVex patients ($366.1 for ReciGen and $186.44 for CinnoVex).
In addition, the cost of transportation and the income lost due to outpatient visits were respectively the highest direct non-medical costs and indirect costs in both medicine patients ($195.93 and $93.7 in ReciGen and $171.63 and $90.82 in CinnoVex).
According to Table 3, the highest utility scores obtained from the EQ5D questionnaire for MS patients belonged to those who used CinnoVex and with EDSS 0 - 2.5 (0.71 ± 0.18). Moreover, the highest mean of the relapse avoided was observed in patients using ReciGen and with EDSS 0 - 2.5 (0.732 ± 0.302).
The Means of Costs in RRMS Patients Using ReciGen and CinnoVex
| Costs | CinnoVex | ReciGen | ||
|---|---|---|---|---|
| USD | Percentage | USD | Percentage | |
| Direct medical costs | ||||
| Physicians’ visits | 54.94 | 13.60 | 53.91 | 9.47 |
| Main medicines | 186.44 | 46.16 | 366.10 | 64.29 |
| Supplementary medicines | 16.91 | 4.19 | 13.71 | 2.41 |
| Laboratory tests | 31.38 | 7.77 | 29.86 | 5.24 |
| Mris | 102.99 | 25.50 | 95.22 | 16.72 |
| Physiotherapy & other services costs | 11.27 | 2.79 | 10.66 | 1.87 |
| Hospitalization & surgeries | 0.00 | 0.00 | 0.00 | 0.00 |
| Total | 403.94 | 47.71 | 569.46 | 53.65 |
| Direct non - medical costs | ||||
| Transportation | 171.63 | 61.59 | 195.93 | 60.35 |
| Accommodation | 42.81 | 15.36 | 51.04 | 15.72 |
| Meals | 64.22 | 23.04 | 76.56 | 23.58 |
| Purchasing auxiliary tools | 0.00 | 0.00 | 1.14 | 0.35 |
| Total | 278.66 | 32.91 | 324.67 | 30.48 |
| Indirect costs | ||||
| Income lost due to outpatient visits | 90.82 | 55.33 | 93.70 | 56.02 |
| Income lost due to hospitalization | 0.00 | 0.00 | 0.00 | 0.00 |
| Patient’s family costs | 73.32 | 44.67 | 73.55 | 43.98 |
| Total | 164.14 | 19.38 | 167.25 | 15.86 |
| Total costs | 846.74 | 100.00 | 1061.38 | 100.00 |
The Means of Utility and Relapse Avoided in RRMS Patients Using ReciGen and CinnoVex
| Parameters | CinnoVex | ReciGen | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Utility | ||||
| EDSS 0.0 - 2.5 | 0.71 | 0.18 | 0.69 | 0.19 |
| EDSS 3.0 - 5.5 | 0.41 | 0.23 | 0.44 | 0.24 |
| Relapse avoided | ||||
| EDSS 0.0 - 2.5 | 0.572 | 0.262 | 0.732 | 0.302 |
| EDSS 3.0 - 5.5 | 0.232 | 0.192 | 0.502 | 0.222 |
According to Figure 2 - A and Table 4, the results of the cost-utility analysis using the Markov model showed that the mean cost was $349.84 in the ReciGen arm and the mean QALY was 0.291, however, in the CinnoVex arm, the mean cost and the mean QALY were, respectively, $289.92 and 0.297. Moreover, according to Figure 2 - B and Table 4, the results of the cost - effectiveness analysis using the Markov model showed that the mean cost and the mean relapse avoided were, respectively, $349.84 and 0.309 in the ReciGen arm, however, the mean cost in the CinnoVex arm was $289.92 and the mean relapse avoided was 0.239, as well.
Thus, the cost - effectiveness ratio calculated was $856, which meant that $856 had to be spent for each extra unit of effectiveness (the mean of relapse avoided) caused by ReciGen.
In order to make a decision, the value of incremental cost - effectiveness ratio (ICER) needed to be compared to the threshold. To calculate the threshold, the WHO methodology was used; that is to say, if the ICER value was below one - fold of GDP per capita, the medicine would be highly cost - effective and if it was lower than three times as much as the GDP per capita, the medicine used would be cost - effective (37). The GDP per capita in 2016, according to the Central Bank of Iran, was $5758 (38). In addition, given that the ICER was estimated at $856, which is lower than one - fold of GDP per capita, the treatment with ReciGen was highly cost - effective compared to the treatment with CinnoVex.
Cost - effectiveness and Cost - utility Analyses of ReciGen and CinnoVex Used by the Studied RRMS Patients
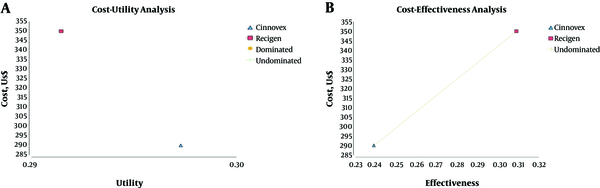
Results of Cost - effectiveness and Cost - utility Analyses of ReciGen and CinnoVex Used by the Studied RRMS Patients
| Strategy | Cost | Util | Eff | incrCost | incrUtil | incrEff | ICER (Cost - utility) | ICER (Cost - effectiveness) |
|---|---|---|---|---|---|---|---|---|
| ReciGen | 349.84 | 0.291 | 0.309 | 0 | 0 | 0 | No need to calculate ICER | 856 |
| CinnoVex | 289.92 | 0.297 | 0.239 | -59.92 | 0.006 | -0.07 | ICER (cost-utility) | ICER (cost-effectiveness) |
3.1. Sensitivity Analysis Results
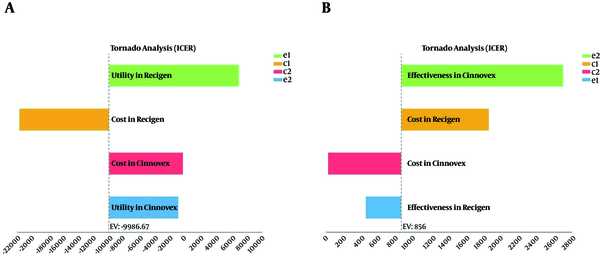
Given that any economic evaluation study is associated with uncertainty, the effects of uncertainty were examined in this study using the one - way sensitivity analysis. In this analysis, the value of each variable increased by 20%, and the Tornado diagrams were drawn (39).
According to the Tornado diagram in Figure 3 - A, the results showed that ICER had the highest sensitivity to the utility of the patients taking ReciGen, however, it had the lowest sensitivity to the utility of the patients taking CinnoVex. Also, in Figure 3 - B, ICRE had the highest sensitivity to the effectiveness in the patients taking CinnoVex, however, it had the lowest sensitivity to the effectiveness in the patients taking ReciGen.
Tornado Diagrams of Cost - utility and Cost - effectiveness of ReciGen and CinnoVex Used by the Studied RRMS Patients
4. Discussion
The medicines currently used for MS disease are prescribed to control the symptoms during attacks and avoid the relapses of the disease attacks (40). Presently, two Iranian medications, namely ReciGen and CinnoVex, which are of Interferon beta - 1a kind, are used for this purpose (as the first line medicines) (14, 15). The aim of this study was to determine and compare the cost - effectiveness and cost - utility of these two medications (ReciGen and CinnoVex) in patients with relapsing - remitting multiple sclerosis, in order to help the policy makers and healthcare providers select the most cost - effective medicine to reduce the rate and severity of the disease relapses and to slow down the progression of disability in patients with MS.
The findings of the present study indicated that the treatment with ReciGen had the mean costs of $1061.38 per treatment course (one month), while the mean cost was $846.74 for the treatment with CinnoVex. Therefore, the mean cost of a one - month course of treatment for each patient treated with CinnoVex was lower than that of treatment with ReciGen. One of the main reasons for this difference seems to be the higher cost of purchasing ReciGen in comparison with CinnoVex. In this regard, the results of the present study are consistent with those of the study conducted by Imani et al., (2012) and Nikseresht et al. (2011) (14, 41).
The direct medical, direct non - medical, and indirect costs of ReciGen were $569.46 (53.65% of total costs), $324.67 (30.48% of total costs), and $167.25 (15.86% of total costs), respectively, while these costs were $403.94 (47.71% of total costs), $278.66 (32.91% of total costs) and $164.14 (19.38% of total costs) in treatment with CinnoVex. Hence, the highest costs in treatment with both medications were direct medical costs, in which the largest costs were related to the costs of purchasing the main medicine, which were 64.29% and 46.16%, respectively. The results of the present study are in line with those of the studies conducted by Imani et al., (2012) and Nikfar et al., (2013) (24, 41).
The results of this study indicated that the highest utility and also the highest mean relapse avoided in treatment with each medicine were observed in patients with EDSS 0 - 2.5, and as the disability level increased, the quality of life decreased, however, the relapse rate increased. Perhaps the reason is that higher EDSSs usually reduce the effect of medicines and patients shift from the demyelinating and inflammation phase into the irreversible degenerative phase. Hence, it is obvious that with increasing the rate of relapses, the level of quality of life decreases (42).
According to the results of this study, the cost, effectiveness and utility of ReciGen were $349.84, 0.309, and 0.291, respectively, while the cost of CinnoVex was $289.92, its effectiveness was 0.239 and its utility was 0.297.Therefore, the ICER value was obtained $856, indicating that $856 would have to be spent for each extra unit of effectiveness through the use of ReciGen. Regarding the threshold in Iran, which was $5758 in 2016, ReciGen was highly cost - effective in terms of effectiveness due to the below - the - threshold ICER, however, CinnoVex was more cost - effective in terms of QALY due to its lower cost and higher utility. The studies conducted by Newton et al., (2011), Nuijten et al., (2010), and Goldberg et al., (2009), who used the effectiveness index for comparing these two medicines, showed that subcutaneous Interferon beta - 1 (Rebif) was more cost - effective than intramuscular Interferon beta - 1a (Avonex) (43-45). However, the studies conducted by Demkek et al., (2014), Nikfar et al., (2013), Imani et al., (2012), and Bell et al., (2007), using the utility index (QALY) to compare these two medicines, indicated that intramuscular Interferon beta - 1a (Avonex) was more cost - effective than subcutaneous Interferon beta - 1 (Rebif) (21, 24, 28, 46).
The results of sensitivity analysis showed that in the cost - effectiveness and cost - utility analyses, the highest sensitivities were observed to the utility of ReciGen and the effectiveness of CinnoVex. Thus, due to the fact that the ICER value became positive in the cost - effectiveness and cost - utility analyses, it can be said that, for a short term, CinnoVex was preferable in terms of utility outcome (QALY), and ReciGen was preferable in terms of effectiveness outcome (relapse avoided), however, the changes in the effectiveness, utility, and costs of the dominated option in the future may change the results of this study, and it may not be stated with certainty that the above - mentioned medications would be the dominant option. However, it depends on the new ICER value and comparing it with the threshold.
This study had a few limitations, one of which was that, considering the limited time available, the patients were only examined during one course of treatment with two medicines. Also, in this study, intangible costs were not taken into account due to the inability to accurately measure them.
Regarding the generalizability of the results of this study, it can be said that since ReciGen and CinnoVex are used in Iran for treating MS patients and their prices are the same throughout the country, the results of this study can be generalized to other provinces and the whole country. However, in order to generalize the results of this study to other countries, it is necessary to address some issues, including the epidemiology of the disease and demographic structure, the existence of resources, prices, valuation of the outcomes by individuals, thresholds, and the use of various effectiveness indices in different studies, which may affect the results. Therefore, it is necessary to be cautious in generalizing the results of the present study to other countries.
4.1. Conclusion
The results of this study showed that, of the two first - line medicines studied, CinnoVex was more cost - effective in terms of QALY and ReciGen was more cost - effective in terms of relapse avoided. Therefore, as the effectiveness index is the preferred and more important index for physicians (47), it can be said that ReciGen is the preferred option. However, given the fact that QALY is the ultimate outcome, and health policymakers and managers focus on ultimate outcomes for decision making (47), CinnoVex can be known as the preferred option. Therefore, it is recommended to take steps to improve the patients’ condition by increasing the insurance coverage for these medicines and reducing the out - of - pocket payments of the patients taking such medicines.
Acknowledgements
References
-
1.
Newcombe J, Hawkins CP, Henderson CL, Patel HA, Woodroofe MN, Hayes GM, et al. Histopathology of multiple sclerosis lesions detected by magnetic resonance imaging in unfixed postmortem central nervous system tissue. Brain. 1991;114 ( Pt 2):1013-23. [PubMed ID: 2043938].
-
2.
Boeije HR, Duijnstee MSH, Grypdonck MHF, Pool A. Encountering the downward phase: biographical work in people with multiple sclerosis living at home. Soc Sci Med. 2002;55(6):881-93. https://doi.org/10.1016/s0277-9536(01)00238-6.
-
3.
Dua T, Rompani P; WHO; MSIF. Atlas: Multiple Sclerosis Resources in the World. London: Multiple Sclerosis International Federation; 2008.
-
4.
Elhami SR, Mohammad K, Sahraian MA, Eftekhar H. A 20-year incidence trend (1989-2008) and point prevalence (March 20, 2009) of multiple sclerosis in Tehran, Iran: a population-based study. Neuroepidemiology. 2011;36(3):141-7. [PubMed ID: 21508646]. https://doi.org/10.1159/000324708.
-
5.
Etemadifar M, Abtahi SH. Multiple sclerosis in Isfahan, Iran: Past, Present and Future. Int J Prev Med. 2012;3(5):301-2. [PubMed ID: 22708025]. [PubMed Central ID: PMC3372071].
-
6.
Raiesi R, Baiat A, Karami J, Sarkaregar-Ardakani A, Katorani S, Ramezannezhad P, et al. [Spatial distribution of multiple sclerosis disease]. J Shahrekord Univ Med Sci. 2013;15(4):73-82. Persian.
-
7.
Etemadifar M, Sajjadi S, Nasr Z, Firoozeei TS, Abtahi SH, Akbari M, et al. Epidemiology of multiple sclerosis in Iran: a systematic review. Eur Neurol. 2013;70(5-6):356-63. [PubMed ID: 24192707]. https://doi.org/10.1159/000355140.
-
8.
Izadi S, Nikseresht A, Poursadeghfard M, Borhanihaghighi A, Heydari S. Prevalence and Incidence of Multiple Sclerosis in Fars Province, Southern Iran. Iran J Med Sci. 2015;40(5):390-5. [PubMed ID: 26379344]. [PubMed Central ID: PMC4567597].
-
9.
Trisolini M, Honeycutt M, Wiener J, Lesesne S. Global Economic Impact of Multiple Sclerosis. United Kingdom, London: Multiple Sclerosis International Federation; 2010.
-
10.
The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43(4):655-61. [PubMed ID: 8469318].
-
11.
Ernstsson O, Gyllensten H, Alexanderson K, Tinghog P, Friberg E, Norlund A. Cost of Illness of Multiple Sclerosis - A Systematic Review. PLoS One. 2016;11(7). e0159129. [PubMed ID: 27411042]. [PubMed Central ID: PMC4943600]. https://doi.org/10.1371/journal.pone.0159129.
-
12.
Torabipour A, Asl ZA, Majdinasab N, Ghasemzadeh R, Tabesh H, Arab M. A study on the direct and indirect costs of multiple sclerosis based on expanded disability status scale score in khuzestan, iran. Int J Prev Med. 2014;5(9):1131-8. [PubMed ID: 25317296]. [PubMed Central ID: PMC4192775].
-
13.
National Multiple Sclerosis Society, Medications for Treating MS. 2018. Available from: http://www.nationalmssociety.org/Treating-MS/Medications.
-
14.
Nikseresht A, Izadi S, Rahimi jaberi A. [Usage and Costs of Treatment with Beta Interferon among Patients with Multiple Sclerosis in Fars province]. Hakim Health Sys Res J. 2011;14(3):159-64. Persian.
-
15.
Sharafaddinzadeh N, Majdinasab N, Ghiasian M, Moravej-Aleali A. [Efficacy of interferon β1a (Cinnovex) in relapsing-remitting multiple sclerosis patients]. Zahedan J Res Med Sci. 2011;13(2):3-6. Persian.
-
16.
Foroughipour M, Etemadi M, Nikkhah K, Afzalnia A, Hazrati N, Ebadi F, et al. [Assessment of disability (EDSS) and liver complications in multiple sclerosis patients treated by Recigen in comparison with Betaferon]. Med J Mashhad Univ Med Sci. 2014;57(5):669-75. Persian.
-
17.
Lee S, Baxter DC, Limone B, Roberts MS, Coleman CI. Cost-effectiveness of fingolimod versus interferon beta-1a for relapsing remitting multiple sclerosis in the United States. J Med Econ. 2012;15(6):1088-96. [PubMed ID: 22583065]. https://doi.org/10.3111/13696998.2012.693553.
-
18.
Noyes K, Bajorska A, Chappel A, Schwid SR, Mehta LR, Weinstock-Guttman B, et al. Cost-effectiveness of disease-modifying therapy for multiple sclerosis: a population-based study. Neurology. 2011;77(4):355-63. [PubMed ID: 21775734]. [PubMed Central ID: PMC3140799]. https://doi.org/10.1212/WNL.0b013e3182270402.
-
19.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-52. [PubMed ID: 6685237].
-
20.
Hohol MJ, Orav EJ, Weiner HL. Disease steps in multiple sclerosis: a simple approach to evaluate disease progression. Neurology. 1995;45(2):251-5. [PubMed ID: 7854521].
-
21.
Dembek C, White LA, Quach J, Szkurhan A, Rashid N, Blasco MR. Cost-effectiveness of injectable disease-modifying therapies for the treatment of relapsing forms of multiple sclerosis in Spain. Eur J Health Econ. 2014;15(4):353-62. [PubMed ID: 23615954]. https://doi.org/10.1007/s10198-013-0478-z.
-
22.
Abdoli G. [Estimation Social Discount Rate For Iran]. Econ Res Rev. 2009;10(3):135-56. Persian.
-
23.
Robberstad B. Estimation of private and social time preferences for health in northern Tanzania. Soc Sci Med. 2005;61(7):1597-607. [PubMed ID: 15885866]. https://doi.org/10.1016/j.socscimed.2005.03.013.
-
24.
Nikfar S, Kebriaeezadeh A, Dinarvand R, Abdollahi M, Sahraian MA, Henry D, et al. Cost-effectiveness of different interferon beta products for relapsing-remitting and secondary progressive multiple sclerosis: Decision analysis based on long-term clinical data and switchable treatments. Daru. 2013;21(1):50. [PubMed ID: 23800250]. [PubMed Central ID: PMC3698128]. https://doi.org/10.1186/2008-2231-21-50.
-
25.
Jankovic S, Kostic M, Radosavljevic M, Tesic D, Stefanovic-Stoimenov N, Stevanovic I, et al. Cost-effectiveness of four immunomodulatory therapies for relapsingremitting multiple sclerosis: A Markov model based on data a Balkan country in socioeconomic transition. Vojnosanitetski pregled. 2009;66(7):556-62. https://doi.org/10.2298/vsp0907556j.
-
26.
Kobelt G, Jonsson L, Fredrikson S. Cost-utility of interferon beta1b in the treatment of patients with active relapsing-remitting or secondary progressive multiple sclerosis. Eur J Health Econ. 2003;4(1):50-9. [PubMed ID: 15609169]. https://doi.org/10.1007/s10198-002-0163-0.
-
27.
Hoyert DL, Xu J. Deaths: preliminary data for 2011. Natl Vital Stat Rep. 2012;61(6):1-51.
-
28.
Bell C, Graham J, Earnshaw S, Oleen-Burkey M, Castelli-Haley J, Johnson K. Cost-effectiveness of four immunomodulatory therapies for relapsing-remitting multiple sclerosis: a Markov model based on long-term clinical data. J Manag Care Pharm. 2007;13(3):245-61. [PubMed ID: 17407391]. https://doi.org/10.18553/jmcp.2007.13.3.245.
-
29.
Liljas B. How to calculate indirect costs in economic evaluations. Pharmacoeconomics. 1998;13(1 Pt 1):1-7. [PubMed ID: 10175982].
-
30.
Hasoumi M, Nasehi M, Khakian M, Mohseni M, Ziaiifar H, Keykale MS. Cost of illness of tuberculosis in tehran in the year 2011. Mater Sociomed. 2014;26(5):339-42. [PubMed ID: 25568635]. [PubMed Central ID: PMC4272848]. https://doi.org/10.5455/msm.2014.26.339-342.
-
31.
Lopez-Bastida J, Oliva-Moreno J, Perestelo-Perez L, Serrano-Aguilar P. The economic costs and health-related quality of life of people with HIV/AIDS in the Canary Islands, Spain. BMC Health Serv Res. 2009;9:55. [PubMed ID: 19331682]. [PubMed Central ID: PMC2670289]. https://doi.org/10.1186/1472-6963-9-55.
-
32.
Ravangard R, Mirzaei Z, Keshavarz Kh, Haghpanah S, Karimi M. Blood transfusion versus hydroxyurea in betathalassemia in Iran: a cost-effectiveness study. Hematology. 2018;23(7):417-22. [PubMed ID: 29157136]. https://doi.org/10.1080/10245332.2017.1404262.
-
33.
Exchange rate. 2018. Available from: http://www.cbi.ir/default_en.aspx.
-
34.
Roskell NS, Zimovetz EA, Rycroft CE, Eckert BJ, Tyas DA. Annualized relapse rate of first-line treatments for multiple sclerosis: a meta-analysis, including indirect comparisons versus fingolimod. Curr Med Res Opin. 2012;28(5):767-80. https://doi.org/10.1185/03007995.2012.681637.
-
35.
Inusah S, Sormani MP, Cofield SS, Aban IB, Musani SK, Srinivasasainagendra V, et al. Assessing changes in relapse rates in multiple sclerosis. Mult Scler. 2010;16(12):1414-21. [PubMed ID: 20810517]. https://doi.org/10.1177/1352458510379246.
-
36.
Touboul C, Amate P, Ballester M, Bazot M, Fauconnier A, Darai E. Quality of Life Assessment Using EuroQOL EQ-5D Questionnaire in Patients with Deep Infiltrating Endometriosis: The Relation with Symptoms and Locations. Int J Chronic Dis. 2013;2013:452134. [PubMed ID: 26464845]. [PubMed Central ID: PMC4590926]. https://doi.org/10.1155/2013/452134.
-
37.
Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93(2):118-24. [PubMed ID: 25883405]. [PubMed Central ID: PMC4339959]. https://doi.org/10.2471/BLT.14.138206.
-
38.
Iran - GDP per capita. 2018. Available from: https://fa.tradingeconomics.com/iran/gdp-per-capita.
-
39.
Briggs A, Sculpher M, Buxton M. Uncertainty in the economic evaluation of health care technologies: the role of sensitivity analysis. Health Econ. 1994;3(2):95-104. [PubMed ID: 8044216].
-
40.
Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol. 1996;39(3):285-94. [PubMed ID: 8602746]. https://doi.org/10.1002/ana.410390304.
-
41.
Imani A, Rasekh HR, Asefzadeh S, Salamzadeh J, Haghdoost AA, Golestani M. Cost Analysis of Disease-Modifying Drugs Therapy for Patients with Multiple Sclerosis in Iran. Am J Sci Res. 2012;(67):95-102.
-
42.
Achiron A, Gabbay U, Gilad R, Hassin-Baer S, Barak Y, Gornish M, et al. Intravenous immunoglobulin treatment in multiple sclerosis. Effect on relapses. Neurology. 1998;50(2):398-402. [PubMed ID: 9484361].
-
43.
Nuijten M, Mittendorf T. A health-economic evaluation of disease-modifying drugs for the treatment of relapsing-remitting multiple sclerosis from the German societal perspective. Clin Ther. 2010;32(4):717-28. [PubMed ID: 20435242]. https://doi.org/10.1016/j.clinthera.2010.03.019.
-
44.
Goldberg LD, Edwards NC, Fincher C, Doan QV, Al-Sabbagh A, Meletiche DM. Comparing the cost-effectiveness of disease-modifying drugs for the first-line treatment of relapsing-remitting multiple sclerosis. J Manag Care Pharm. 2009;15(7):543-55. [PubMed ID: 19739877]. https://doi.org/10.18553/jmcp.2009.15.7.543.
-
45.
Newton AN, Stica CM. A comprehensive cost-effectiveness analysis of treatments for multiple sclerosis. Int J MS Care. 2011;13(3):128-35. [PubMed ID: 24453716]. [PubMed Central ID: PMC3882969]. https://doi.org/10.7224/1537-2073-13.3.128.
-
46.
Imani A, Golestani M. Cost-utility analysis of disease-modifying drugs in relapsing-remitting multiple sclerosis in Iran. Iran J Neurol. 2012;11(3):87-90. [PubMed ID: 24250871]. [PubMed Central ID: PMC3829258].
-
47.
Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. 3rd ed. New York: Oxford University Press; 2005.