Abstract
Keywords
Stem Cells Myogenesis Human New Born Foreskin Stem Cells PCL Nano Fiber
1. Background
Stem cells have turned to a striking therapeutic instrument for regenerative medicine, immunomodulation, and gene therapy related studies (1, 2), due to their exclusive features, multi-differentiation capabilities, and immune-modulatory purposes. Therefore, a subunit of stem cells called Mesenchymal stem cells (MSCs) may be simply obtained and cultured, and persuaded to turn into various types of cells, such as osteoblasts, adipocytes, chondroblasts, hepatocyte-like cells, and myoblasts (3-5). They may be obtained from a range of foundations such as cartilage, bone marrow, skin, adipose tissue, skeletal muscle, cord blood, placenta, and umbilical cord (4, 6, 7).
Recently, studies have established that the skin might act as an origin of stem cells (8, 9). Human foreskin tissue (hnFST) is the key skin foundation of stem cells due to its abundancy, cheapness, and obtainability over non-invasive techniques that do not bring any ethical apprehensions. A recent paper of the authors of the current study demonstrated cell isolation from prepuce, determination of stem cell properties, and multi-potent and even pluripotent abilities. The results specified that stem cells were positioned among foreskin and had huge capacities for differentiation to endoderm, mesoderm, and ectoderm specific cells. These effects recommended that the storage of hnFSSCs and newborn foreskin tissue might be very beneficial for disease development potentials and treatment actions (10).
Myogenic differentiation is controlled via Myogenic regulatory factors (MRFs), covering MyoD, myogenin, and Acta-2; MyoD is essential for the specification of skeletal myogenic lineages, while myogenin is known to control cell fusion and cell fate (11). Alpha actin 2 (Acta-2) defined as actin, aortic smooth muscle or alpha smooth muscle actin, is a protein, which is programmed via the Acta-2 gene. Acta-2 is a greatly preserved protein, which is important for motility and integrity (12).
Throughout the embryonic stage of life, the satellite cells positioned among basal lamina and muscle fiber sarcolemma are inactive myoblasts. However, they are completely stimulated to myogenic character. Once actuated, they are skilled for final differentiation. The dormant satellite cells are not capable of expressing transcription factors, while the active ones display molecular indicators of MyoD, and, to a reduced level, of myogenin (13). These satellite cells were thought as a perfect basis for muscle renewal and renovation, however, studies showed that they were limited in damaged muscle and that they were drained instantly throughout the recovery period. A recent investigation of an unconventional biological material with comparable myogenic capacity yielded MSCs once the bone marrow-derived MSCs were exposed to develop in vitro, and turned effectively into myoblasts (14).
Tissue-related stem cells participate in the redevelopment and preservation of many mammalian tissues, comprising skeletal muscle, liver, intestine, blood, and the central nervous network. It is mostly thought that tissue-derived stem cells are destined to track precise cellular predestination and differentiate solitary to the tissue from that they had arisen. This hypothesis was tested recently. Different examinations recommended the presence of adult stem cells that are skilled to turn into an extensive range of particular cells. As examples, it was shown that adult neurogenic stem cells obtained from the mouse brain differentiated to blood, skeletal muscle, and endothelial cells (15), human adipogenic cells differentiated to bone, muscle, and cartilage (16), and cells from mammalian dermis differentiated to glial neuron, skeletal muscle, and fat cells (17). Moreover, some revisions suggested that bone marrow-related cells have the capability to differentiate to blood and several cell forms, such as muscle, liver, and brain. It has also been demonstrated that an individual infrequent cell lineage, known as multi-potent adult precursor cells, can discriminate to cell lineages of the entire 3 germ stratums (4). In the meantime, cells that have been isolated and moved with qualified affluence are thought as efficient instruments for somatic cells and gene therapy.
Polycaprolactone (PCL), half crystalline linear absorbable aliphatic polyester, is dependent on impairment related to the defenselessness of its aliphatic ester connection to hydrolysis. The produced crops are either processed by the Tricarboxylic Acid (TCA) cycle or removed through direct renal discharge. Presently, PCL is defined as a lenient and stiff tissue companionable substantial and re-absorbable seal, which can be used for drug transfer arrangement and bone implant alternatives. Therefore, submissions of PCL might be restricted due to the slower resorption kinetics owing to its hydrophobic feature (18). It has been established as a substantial component of skin, cartilage, bone, liver, and protein distribution tools. Scaffolds are manufactured utilizing PCL, which are more resilient to hydrolysis and subsequently they are skilled enough to uphold the practicality, cell growth, and differentiation of seeded cells. The perfunctory and deprivation features of PCL enable longstanding in vitro cell culture earlier to grafting at locations of damage. The PCL scaffolds consequently preserve organizational unity through in vitro culturing, whereas mesenchymal stem cells discriminate and create muscle-like tissue (19).
In this present paper, hnFSSCs derived from human newborn circumcised waste tissue, showing stem cell characters, were reported to be capable of differentiation to cells that express different myogenesis associated genes. The results demonstrated that foreskin is a beneficial biological material of stem cells for dealing with worsening muscular illnesses or muscle injury/forfeiture from trauma, and also a cell foundation for tissue manufacturing studies.
2. Methods
2.1. Insulation and Cultivation of Human Newborn Foreskin Stem Cells
Human newborn foreskin stem cells were isolated from newborn prepuce in Yeditepe University/TURKEY biotechnology laboratories with the same technique used previously (10). Circumcised waste human newborn foreskin tissues were collected and minced physically without using any chemicals in petri dishes. The prepuce tissue was overlaid in 6-well plates (BIOFIL, TCP, Switzerland) and grown in dulbecco’s modified eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) PSA (10.000 units/mL penicillin, 10.000 μg/mL streptomycin, and 25 μg/mL amphotericin B) (Invitrogen, Gibco, UK). After 3 to 4 days, hnFSSCs were viewed under light microscope and the exterior was concealed after 8 days. Later, the cells were trypsinized with 0.25% (v/v) trypsin/EDTA (Invitrogen, Gibco, UK). Medium was added to disconnected cells in order to constrain the activity of trypsin. Once the cells were centrifuged at 300 × g for 5 minutes at room temperature, the pellet was liquefied in fresh medium and seeded on a T-75 flask (Zelkultur Flaschen, Switzerland). The cells were preserved at 37°C and 5% CO2 in a humidified incubator. Cells from passages 3 to 4 were utilized for all trials (10).
2.2. Determination of Stem Cell Properties of Human Newborn Foreskin Stem Cells
Human newborn foreskin stem cells were isolated and characterized, according to the protocol defined formerly by the authors (20). They were trypsinized and raised with primary antibodies that were arranged in PBS. The characterization process was made by the utilization of CD14, CD31, CD34, CD44, CD45, CD73, CD90, CD105, Integrin primary antibodies (SantaCruz Biotechnology Inc., Santa Cruz, CA, USA), and CD29 (Zymed, San Francisco, CA, USA). Human newborn foreskin stem cells were maintained with fluorescein isothiocyanate (FITC) or phycoerythrin (PE) conjugated antibodies at 4°C for 1 hour. The flow cytometry examination of the cells was completed using Becton Dickinson Fluorescent activated cell sorting (FACS) Calibur flow cytometry system (Becton Dickinson, San Jose, CA, USA).
2.3. Myogenic Differentiation of Human Newborn Foreskin Stem Cells
Human newborn foreskin stem cells were persuaded to differentiate to myocytes. The cells were seeded on a 6-well plate for RNA isolation and 48-well plate (BIOFIL, TCP, Switzerland) for immunocytochemistry at a density of 150 × 103 cells/well and 10 × 103 cells/well, individually, for the differentiation process. Differentiation media contents are shown in Table 1. Myogenesis inducing differentiation media was applied to the differentiation groups. The media was renewed every 2 days, for a total of 14 days. Meanwhile, undifferentiated groups were treated with low glucose DMEM media.
Myogenic Differentiation Medium Content
| Differentiation | Content |
|---|---|
| Myogenic differentiation (Prepared in Ham’s F12/DMEM) | 2% Donor Horse Serum |
| 1% Glutamax | |
| 1 ng/mL basic fibroblastic growth factor | |
| 0,1 nM dexamethasone | |
| 1% Penicillin, Streptomycin, Amphotericin |
This preparation protocol was arranged for 5× concentration with respect to Toma C. et al. 2001 (16).
2.4. Immunocytochemistry Analysis
Immunocytochemistry (ICC) analysis was finalized regarding the formerly defined protocol (21). In the last part of the differentiation procedure, the cells were incubated with 2% (w/v) paraformaldehyde for 30 minutes at 4°C for fixation and permeabilized with 0.1% (v/v) triton X-100 for 5 minutes along PBS. The hnFSSCs were incubated with 2% (v/v) goat serum (Sigma, USA) for 20 minutes at 4°C for avoiding non-specific binding of primary antibodies. The hnFSSCs were once more washed 3 times with PBS and then incubated overnight with primary antibodies at 4°C. Myogenic differentiation was examined by primary antibodies of myosin heavy chain (MyH) (Santa Cruz Biotechnology, TX, USA, sc-20641), MyoD (Santa Cruz Biotechnology, TX, USA, sc-304), myogenin (Abcam, UK, ab1835), and smooth muscle actin (Abcam, UK, ab5694). Human newborn foreskin stem cells were washed 3 times with PBS to eliminate the leftover antibody after incubating with primary antibodies. Afterwards, the cells were treated with secondary antibodies (Goat anti rabbit IgG Alexa Fluor 488, Goat anti mouse IgG Alexa Fluor 488) and incubated for 1 hour at 4°C, tracked by washing 3 times with PBS. The DAPI (AppliChem, Germany) was utilized to stain the nuclei of the cells through incubating for 20 minutes at 4°C. The hnFSSCs were then washed 3 times with PBS and analyzed under a fluorescence microscope (Nicon Eclipse TE200).
2.5. Quantitative Real Time Polymerase Chain Reaction
Total RNA isolation from differentiated cells was completed via utilizing High Pure RNA Isolation Kit (Roche, Germany), according to the manufacturer’s instructions. The cDNA (complementary DNA) synthesis from isolated RNA samples was achieved by high fidelity cDNA Synthesis Kit (Roche, Germany). Real time polymerase chain reaction (PCR) was implemented via Maxima SYBR Green/ROX (Fermentas, USA) for the examination of expression levels of marker genes after myogenic differentiation. The cDNAs of the differentiated cells were utilized as template and were mixed with primers and Maxima SYBR Green/ROX qPCR Master Mix (2X). Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) was used as the house-keeping gene for the standardization of the data (22). The outcomes of real time PCR data were stabilized by the mRNA level of GAPDH. Primer sequences for marker genes are illustrated in Table 2.
Names and Sequences of Primers Used in Quantitative Real Time-Polymerase Chain Reaction Experiments were Completed by Expending the iCycler RT-PCR Detection System (Bio-Rad, Hercules, CA, USA)
2.6. Determining Cell-Cell Adhesion with Micro-Mold Rods
Micro-mold rods (Sigma-Aldrich, USA) were autoclaved for sterilization. One gram of high quality agarose powder (Invitrogen, USA) was dissolved in 50 mL of sterile saline and boiled via microwave. The molten agarose was allowed to cool down to approximately 60°C to 70°C. In total, 500 µL of molten agarose was place on 16x16 and 9 × 9 micro-mold. Once the agarose was gelled, the micro-mold was flexed. For the equilibration, 2.5 mL/well of cell culture medium was added to the 12-well plate. After 15 minutes of incubation, culture medium was removed and replaced with fresh medium; 1.2 × 106/190 µL cells were seeded in the rods. The cell culture medium was then removed from the surrounding of the micro-mold rods. After 2 days, colony formation was seen under a light microscope. Therefore, cells were seeded in a drop-wise manner and the micro-molds were deported; 2.5 mL/well cell culture medium was added. The images were observed under the light microscope.
2.7. Seeding Differentiated Cells onto Fabricated Polycaprolactone Scaffold in Order to See the Tissue Formation
Polycaprolactone scaffold was ordered from Sigma Aldrich, USA, and the characteristics of ordered scaffold was described with 100% open porosity and organic solvent free feature. The fiber diameter and the pore size were established as 300 u. Cells that were differentiated from human newborn foreskin to myogenic cells were seeded on 12-well plates at a density of 50 × 103 on 3D Biotek 3D Insert PCL scaffold (Sigma Aldrich, USA). Seeded cells were grown in myogenic differentiation medium as mentioned previously. In the meantime, undifferentiated human newborn foreskin cells were seeded as negative controls at a density of 50 × 103. After 21 days the results were examined with scanning electron microscopy (SEM).
2.8. Statistical Analysis
Data were presented by means ± standard deviation (SD). Graphs were drawn by GraphPad Prism 5 software (GraphPad Prism, USA). The numerical examination of the outcomes were completed by One-Way Analysis of Variance (ANOVA) monitored by the multiple-comparison Tukey’s test using and student’s t test with GraphPad Prism 5 software. Statistical significance was set at P < 0.05.
3. Results
3.1. Cell Culturing of Human Newborn Foreskin Stem Cells
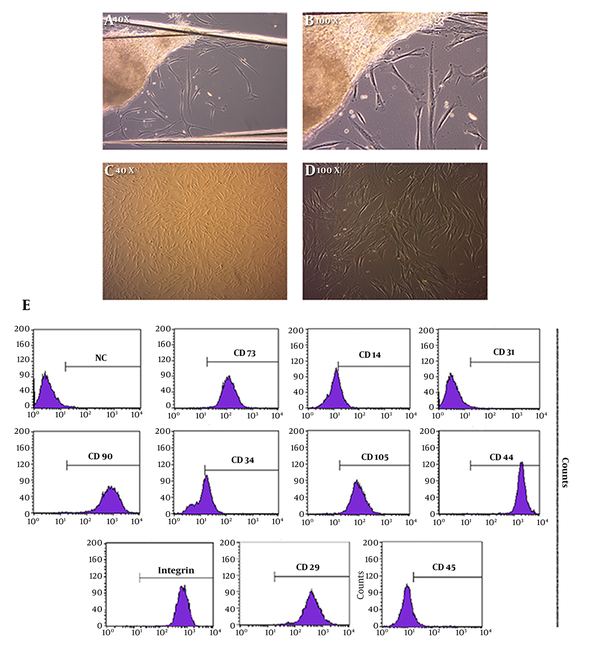
Human newborn Foreskin stem cells were isolated according to regular protocols of mononuclear cell isolation from circumcised hnFST via expending culture procedures. Light microscopy outcomes from cells in passage 0 displayed a fibroblast-like, spindle shaped morphology (Figure 1A and 1B). In passage 3, the rod shaped cells started to display a broadened horizontal phenotype (Figure 1C and 1D).
Isolated Human Newborn Foreskin Cells from Circumcised Waste Tissue. A and B, Primary Cell Culture Image Provided by Explant Culture Technique with Different Magnifications; C and D, Continuous Cell Culture Images of hnFSSCs at Passage 3; E, Characterization of Stem Cell Properties of the Human Newborn Foreskin Cells. Cells Cultured at Passage 3 Were Examined. Major Population of Cells Preserved the Characteristics of Stem Cells. Flow Cytometry Analysis Data Illustrated the Positivity for Both Hematopoietic and Mesenchymal Stem Cell Surface Markers Except from the Endothelial Marker CD31. NC, Negative Control
3.2. Characterization of human newborn Foreskin Stem Cells
Flow cytometry examination established that human newborn foreskin cells expressed mesenchymal markers, such as CD 29, CD, 44, CD 73, and CD 90. They were also acknowledged as positive for the hematopoietic marker CD 14, positive for the hematopoietic marker CD 34 and positive for another hematopoietic marker CD 45. The CD 31 mesenchymal marker, which is an endothelial surface marker, was found negative (Figure 1E).
3.3. Cell-Cell Adhesion with Micro-Mold Rods
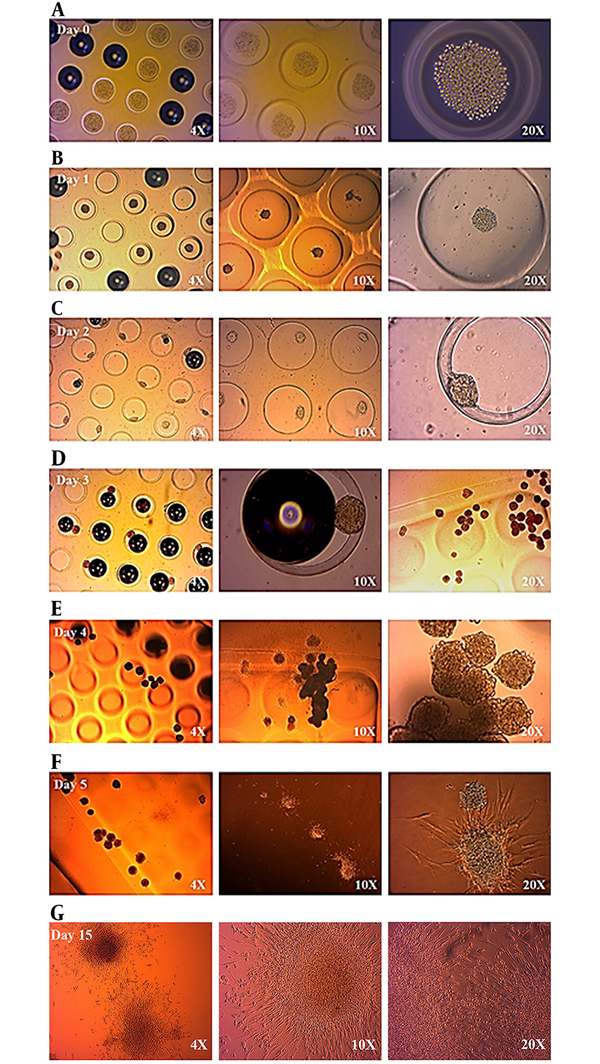
Cell-cell adhesion potentials of myogenically differentiated human newborn foreskin cells were observed via light microscope of cells on micro-mold images. Once seeded into micro-mold rods, human newborn foreskin cells accumulated as three-dimensional (3D) multicellular micro-tissues by the process of cytoskeletal intervened contraction and cell-cell adhesion. On day 3, cell aggregation and clustering were clearly observed. After day 5, cells started to escape from the micro-mold rods and indicated micro-tissue structures. On day 15, even the clustered cells (micro tissues) had started to connect via extracellular extensions. In general, cell-cell adhesion was achieved and micro-tissue developments were indicated with the aid of micro-mold rods (Figure 2).
Cell-Cell Adhesion and Micro-Tissue Development Status Between Day 0 and Day 2 (A, B, C). At the End of Day 3, Cell Clusters Can Be Seen Very Clearly Under the Light Microscope (2.d). Cell-Cell Adhesion and Micro-Tissue Development Status Between Day 3 and Day 5 (E, F). At the End of Day 15, 17 Microtissues Have Started to Interact Via Extracellular Extensions (G). Structural Differentiations Can Be Seen Very Clearly Under the Light Microscope
3.4. Polycaprolactone Scaffold Characterization
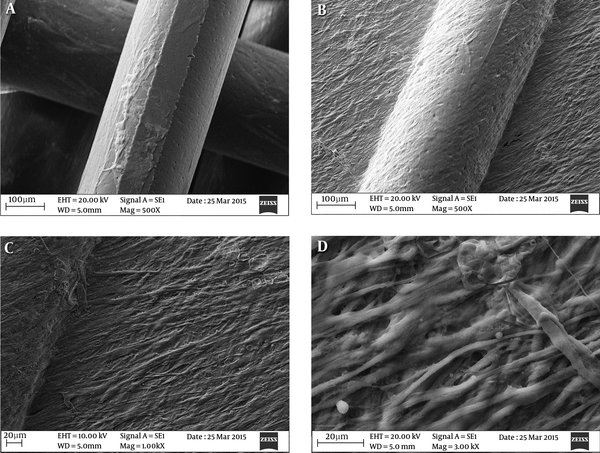
The goal of this experiment was to assess the feasibility of a decomposable, PCL founded nanofibrous construction, as a 3D scaffold, to examine the myogenic potency of human newborn foreskin derivative cells. After a 24-day incubation in culture medium at 37°C, scaffolds preserved their integrity and three-dimensional structure, while showing no obvious alteration in dry weight through the entire culture process (data not shown). Cells were established to have reproduced nearby the micropores all over the scaffold; Figure 3A; negative control, and Figure 3C; positive control. A confluent cell cover was observed on the scaffold (Figure 3B) and cells were viewed within the micropores (Figure 3D).
PCL-based Nanofibers and the Seeding of Human Newborn Foreskin Stem Cells. Human Newborn Foreskin Cells (A) and the Differentiated Cells (B - D) Were Both Apparently Attached To Nanofiber Substrates. PCL Nanofibers Were Still Visible at 24 Days Following the Seeding of Human Newborn Foreskin Stem Cells
3.5. Conformation of Myogenic Differentiation
Quantitative real time experiments were also performed for myogenic differentiation indicator genes such as Acta-2, MyoD, and Myogenin. The genes for the differentiation of human newborn foreskin cells into myocyte were expressed. The MyoD gene expression was almost double in positive control than the negative control (Figure 4A). Meanwhile, Acta-2 and Myogenin gene expressions were found to be almost tripled in the positive control (Figure 4A). Myogenic differentiation of human foreskin cells was successfully achieved via these experiments.
A, Quantification of Cells Expressing Muscle-Specific Markers Regarding to the Quantitative Real Time PCR Results. MyoD; Myoblast Determination Gene, Myogenin, ACTA2; Alpha-Actin- 2 Gene, GAPDH Glyceraldehyde3-Phosphatedehydrogenase, qPCR Quantitative Real-Time Polymerase Chain Reaction; *P < 0.05 Stands for DF Versus NC; B, Mesoderm Derived Myogenic Differentiation of Human Newborn Foreskin Cells. SMA; Smooth Mucle Actin, MYH; Myosin Heavy Chain. Immunocytochemistry Results Demonstrate Positive Immunofluorescent Results for Each and Every Antibody that Have Been Utilized. NC, Negative Control; DF, Differentiated
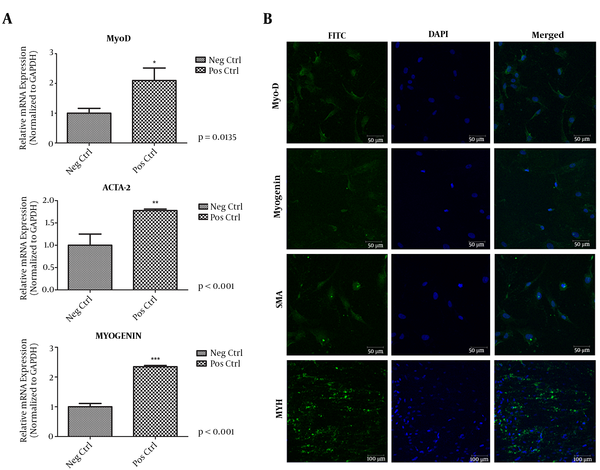
The capability of hnFSSCs differentiation to myogenic cell lineages was inspected in the current work. After 21 days of culture through a myogenic inducing medium, ICC trial results for myogenic distinction were illustrated for 4 myocyte precise antibodies. Differentiated cell cultures established positive fluorescent results for MyoD (key protein in myogenic differentiation), myogenin (muscle specific transcription factor), and actin smooth muscle (predominate isoform of the thin microfilament that forms the contractile machinery) and MYH (base excision repair protein) antibodies (Figure 4B).
4. Discussion
The waste tissue coming from the surgical operation, known as circumcision, has demonstrated treatment potentials for numerous diseases. Different studies have indicated that human foreskin tissue development continues after birth (26). Pluripotent stem cells are maintained in several organs after birth and at receiving extracellular signals they start to proliferate and differentiate (23). Therefore, examination and characterization of the human newborn foreskin cells was recently performed by the authors of the current study (10).
This study revealed that hnFSSCs carries different differentiation capabilities for both mesenchymal and hematopoietic lineages. In other words, they turn into all 3 (ectodermal, endodermal, and mesodermal) lineages and also show hematopoietic properties. Therefore, these cells managed to successfully differentiate to cells such as osteoblasts, chondrocytes, neurogenic cells, epithelial cells, and adipocytes (10).
Production of a muscle tissue is a challenging field, but also a promising area for new approaches to eliminate damaged muscular tissues. Trauma, tumor or diseases can be listed as the reasons for the loss of muscle tissue (24). Therefore, hnFSSCs may be a precious cell source regarding their ability to differentiate to the myogenic lineage in vitro, capability of allogenic transplantation, and their immunomodulatory effects.
Myogenesis is an evolving cascade that covers the regulatory protein MyoD, which controls the differentiation of multi-potent SCs into myogenic cell lines. The MyoD expression arises at the initial phase of myogenic differentiation while myogenin is articulated later, associated with cell merging and differentiation (25). In this study, the myogenic differentiation potentials of hnFSSCs were investigated. The flow cytometry results showed that these cells are both positive for mesenchymal and hematopoietic surface markers, similar to the authors’ previous study. At the same time, immunocytochemistry results revealed fluorescently positive outcomes of the myogenesis-specific antibodies such as MYH, Myogenin, Myo D, and actin smooth muscle. With respect to myogenesis-specific gene expression analysis with quantitative real time PCR, positive control MyoD gene expression was almost doubled. In the meantime, ACTA-2 and Myogenin gene expressions were found to be almost tripled in comparison with the negative control. Myogenic differentiation of human foreskin cells was achieved by these tests.
The 3D microwells were utilized to investigate the effects of internment short-term culture of anchorage-dependent cells. Cells that attached to micro-wells, exhibited a regular extended morphology. Small colonies of myogenically differentiated hnFSSCs tissues, started to interact by day 3. At the end of day 5, small interacted tissues maintained viable. This data suggests that cell-cell interactions were strong and the differentiated cells may be an effective source for tissue engineering.
By day 4 following human newborn foreskin stem cell seeding, the attached cells were seemingly stuck to a nanofiber surface and entered PCL nanofiber scaffold pores. The seeded cells were then treated with myogenic differentiation medium for 21 days. The SEM electron microscopy results suggested that even the differentiated cells attached to the scaffold quite well. The cell-cell integration and the cell-scaffold interactions were found more visible than the undifferentiated hnFSSCs. These results suggest the efficient differentiation capabilities of hnFSSCs on a scaffold, which could be utilized for tissue engineering.
There is certainly no study, which investigates the influence of fibers and exterior structure concomitantly on myogenic differentiation of hnFSSCs. For that reason, in this study an assessment was performed with scaffolds and hnFSSCs. Meanwhile, there has been several studies examining the adipose derived stem cells seeded on PCL scaffolds, indicating the potential utilizations of stem cells on PCL scaffolds (19). The results indicated that hnFSSCs carry higher differentiation potentials than adipose derived stem cells, and so it is expected to carry better tissue engineering capacities.
In summary, if they are isolated from hnFST right after birth, hnFSSCs can be used as an effective and valuable stem cell source for repairing the damage caused by diseases and for tissue engineering. In this study, hnFSSCs proved its ability to differentiate to a myogenic lineage. The ability of the prepared tissue, such as the structure to adapt to in vivo conditions will be examined in future studies.
4.1. Conclusion
In this study, myogenesis of hnFSSCs seeded onto PCL was examined to define the possible submission of such a scaffold. Given that the PCL may be manufactured in different forms and dimensions, and that it delivers mechanical stability, it could be suggested that the 3D PCL is a contender bioactive transporter for stem cell replacement in tissue engineering related to myogenic damages in the future. On the other hand, hnFSSCs may be distinguished as active and valuable stem cell foundation for numerous diseases, once it is obtained from a newborn prepuce. Regarding this situation, utilizing hnFSSCs for the stem cell source to be seeded on scaffolds may be very beneficial for future complications.
Acknowledgements
References
-
1.
Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815-22. [PubMed ID: 15494428]. https://doi.org/10.1182/blood-2004-04-1559.
-
2.
Wulf GG, Chapuy B, Trumper L. [Mesenchymal stem cells from bone marrow. Phenotype, aspects of biology, and clinical perspectives]. Med Klin (Munich). 2006;101(5):408-13. [PubMed ID: 16685488]. https://doi.org/10.1007/s00063-006-1052-6.
-
3.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143-7. [PubMed ID: 10102814]. https://doi.org/10.1126/science.284.5411.143.
-
4.
Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41-9. [PubMed ID: 12077603]. https://doi.org/10.1038/nature00870.
-
5.
Xu W, Zhang X, Qian H, Zhu W, Sun X, Hu J, et al. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp Biol Med (Maywood). 2004;229(7):623-31. [PubMed ID: 15229356].
-
6.
Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000;28(8):875-84. [PubMed ID: 10989188]. https://doi.org/10.1016/S0301-472X(00)00482-3.
-
7.
Qiao C, Xu W, Zhu W, Hu J, Qian H, Yin Q, et al. Human mesenchymal stem cells isolated from the umbilical cord. Cell Biol Int. 2008;32(1):8-15. [PubMed ID: 17904875]. https://doi.org/10.1016/j.cellbi.2007.08.002.
-
8.
Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22:339-73. [PubMed ID: 16824012]. https://doi.org/10.1146/annurev.cellbio.22.010305.104357.
-
9.
Fernandes KJ, McKenzie IA, Mill P, Smith KM, Akhavan M, Barnabe-Heider F, et al. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol. 2004;6(11):1082-93. [PubMed ID: 15517002]. https://doi.org/10.1038/ncb1181.
-
10.
Somuncu OS, Tasli PN, Sisli HB, Somuncu S, Sahin F. Characterization and Differentiation of Stem Cells Isolated from Human Newborn Foreskin Tissue. Appl Biochem Biotechnol. 2015;177(5):1040-54. [PubMed ID: 26304127]. https://doi.org/10.1007/s12010-015-1795-8.
-
11.
Aurade F, Pinset C, Chafey P, Gros F, Montarras D. Myf5, MyoD, myogenin and MRF4 myogenic derivatives of the embryonic mesenchymal cell line C3H10T1/2 exhibit the same adult muscle phenotype. Differentiation. 1994;55(3):185-92. [PubMed ID: 8187980]. https://doi.org/10.1046/j.1432-0436.1994.5530185.x.
-
12.
Huang NF, Chu J, Lee RJ, Li S. Biophysical and chemical effects of fibrin on mesenchymal stromal cell gene expression. Acta Biomater. 2010;6(10):3947-56. [PubMed ID: 20678460]. https://doi.org/10.1016/j.actbio.2010.05.020.
-
13.
Valdez MR, Richardson JA, Klein WH, Olson EN. Failure of Myf5 to support myogenic differentiation without myogenin, MyoD, and MRF4. Dev Biol. 2000;219(2):287-98. [PubMed ID: 10694423]. https://doi.org/10.1006/dbio.2000.9621.
-
14.
De Angelis L, Berghella L, Coletta M, Lattanzi L, Zanchi M, Cusella-De Angelis MG, et al. Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. J Cell Biol. 1999;147(4):869-78. [PubMed ID: 10562287]. https://doi.org/10.1083/jcb.147.4.869.
-
15.
Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283(5401):534-7. [PubMed ID: 9915700]. https://doi.org/10.1126/science.283.5401.534.
-
16.
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211-28. [PubMed ID: 11304456]. https://doi.org/10.1089/107632701300062859.
-
17.
Toma JG, Akhavan M, Fernandes KJ, Barnabe-Heider F, Sadikot A, Kaplan DR, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3(9):778-84. [PubMed ID: 11533656]. https://doi.org/10.1038/ncb0901-778.
-
18.
Oh SH, Park IK, Kim JM, Lee JH. In vitro and in vivo characteristics of PCL scaffolds with pore size gradient fabricated by a centrifugation method. Biomaterials. 2007;28(9):1664-71. [PubMed ID: 17196648]. https://doi.org/10.1016/j.biomaterials.2006.11.024.
-
19.
Kim TH, Oh SH, Kwon EB, Lee JY, Lee JH. In vitro evaluation of osteogenesis and myogenesis from adipose-derived stem cells in a pore size gradient scaffold. Macromol Res. 2013;21(8):878-85. https://doi.org/10.1007/s13233-013-1099-1.
-
20.
Tasli PN, Dogan A, Demirci S, Sahin F. Boron enhances odontogenic and osteogenic differentiation of human tooth germ stem cells (hTGSCs) in vitro. Biol Trace Elem Res. 2013;153(1-3):419-27. [PubMed ID: 23575901]. https://doi.org/10.1007/s12011-013-9657-0.
-
21.
Tasli PN, Dogan A, Demirci S, Sahin F. Myogenic and neurogenic differentiation of human tooth germ stem cells (hTGSCs) are regulated by pluronic block copolymers. Cytotechnology. 2016;68(2):319-29. [PubMed ID: 25698158]. https://doi.org/10.1007/s10616-014-9784-2.
-
22.
Tasli PN, Sahin F. Effect of lactoferrin on odontogenic differentiation of stem cells derived from human 3rd molar tooth germ. Appl Biochem Biotechnol. 2014;174(6):2257-66. [PubMed ID: 25173676]. https://doi.org/10.1007/s12010-014-1204-8.
-
23.
Cold CJ, Taylor JR. The prepuce. BJU Int. 1999;83 Suppl 1:34-44. [PubMed ID: 10349413].
-
24.
Bitto FF, Klumpp D, Lange C, Boos AM, Arkudas A, Bleiziffer O, et al. Myogenic differentiation of mesenchymal stem cells in a newly developed neurotised AV-loop model. Biomed Res Int. 2013;2013:935046. [PubMed ID: 24106724]. https://doi.org/10.1155/2013/935046.
-
25.
Berkes CA, Tapscott SJ. MyoD and the transcriptional control of myogenesis. Semin Cell Dev Biol. 2005;16(4-5):585-95. [PubMed ID: 16099183]. https://doi.org/10.1016/j.semcdb.2005.07.006.
-
26.
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917-20. [PubMed ID: 18029452]. https://doi.org/10.1126/science.1151526.