Abstract
Background:
Methotrexate (MTX), a folate antagonist used to treat cancer and inflammatory diseases, is known to generate reactive oxygen species.Objectives:
The research investigates the impact of dimethyl fumarate (DMF), a nuclear factor erythroid 2-related factor 2 (Nrf2) activator, on an MTX-induced mouse hepatotoxicity model.Methods:
Forty-two mice were divided into 6 groups: Control, MTX, DMF 120, and 3 groups of MTX co-treated with DMF 30, 60, and 120 mg/kg. Dimethyl fumarate was gavaged once daily for 10 days. On the fifth day, the animals received MTX 20 mg/kg intraperitoneally. On the eleventh day, the animals were sacrificed, and serum and liver samples were collected to assess the level of oxidative/anti-oxidative and apoptotic/anti-apoptotic markers.Results:
Dimethyl fumarate prevented the increase of liver function enzymes, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) induced by MTX (P < 0.001). It prevented the increase in AST and ALT levels, indicating liver recovery (P < 0.001). Furthermore, DMF restored antioxidant markers superoxide dismutase, catalase, glutathione peroxidase, and total thiol while reducing the level of thiobarbituric acid reactive substances (P < 0.001). Dimethyl fumarate also downregulated hepatic mRNA expression of caspase 3 and upregulated Bcl-2, heme oxygenase 1, and Nrf2 genes in MTX co-treated DMF groups.Conclusions:
Dimethyl fumarate alleviates oxidative stress and apoptosis, which may be achieved by the Nrf2/HO-1 pathway. Therefore, DMF may be clinically effective in preventing or treating MTX-induced hepatotoxicity.Keywords
Methotrexate Hepatotoxicity Dimethyl Fumarate Anti-oxidative Anti-apoptotic
1. Background
Methotrexate (MTX) is widely used as a chemotherapy and anti-inflammatory drug. However, toxicity will occur following MTX administration. Although clinicians are aware of the hepatotoxic reactions of anticancer drugs at therapeutic doses, in clinical practice, drug-induced liver injury (DILI) is rarely noticed.
Methotrexate prevents the conversion of dihydrofolate to tetrahydrofolate through the inhibition of the dihydrofolate reductase enzyme. Therefore, thymidylate synthetase is inhibited when the folate level is reduced. Methotrexate also inhibits AICAR (5-aminoimidazole-4-carboxamide ribonucleotide) transformylase, which catalyzes the purine nucleotide biosynthesis. The inhibition of these enzymes reduces the synthesis of ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) (1). Moreover, MTX changes the cellular redox state by disrupting the mitochondrial respiratory chain and activating the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. Subsequently, the production of reduced glutathione (GSH) is prevented via glutathione reductase inhibition. Oxidative stress (OS) could trigger inflammatory and apoptotic cascade (2).
Recently, along with acetaminophen and CCl4, MTX has been favored as a potential in vivo model in several studies for DILI, and the protective effects of epicatechin (3), crocin (4), ferulic acid (5), dihydromyricetin (6), and empagliflozin (7) have been confirmed. Although MTX-induced toxicity can be alleviated by supplementation and natural antioxidant administration, the mechanism underlying the effect is not clearly defined. Following MTX administration, various signaling pathways are activated, such as Nrf2 (nuclear factor erythroid 2-related factor 2)-antioxidant response signaling, autophagy, and mitochondrial dysfunction (8).
Fumaric acid is derived from the Fumaria officinalis plant, and its ester, dimethyl fumarate (DMF), has been used for psoriasis treatment for years and recently been approved as a drug for multiple sclerosis (MS). Dimethyl fumarate is rapidly absorbed, excreted, and converted to monomethyl fumarate (MMF) by esterases in the gastrointestinal tract. Dimethyl fumarate (DMF) and MMF trigger the activation of the Nrf2 nuclear factor, leading to the induction of cellular protective heme oxygenase 1 (HO-1) and anti-inflammatory gene expression (9, 10). The protective and beneficial effects of DMF have been shown in many studies, such as the neuroprotection of hippocampal neurons against chemical kindling (11), Nrf2 activation against hepatocarcinogenesis (12), Nrf-2/HO-1 modulation against acetaminophen hepatotoxicity (13), Nrf2 activation and suppression of NLRP3 inflammasome activation against animal model of colitis (14), and protection in combination with curcumin against ischemia/reperfusion injury of liver (15). Thus, targeting the Nrf2/HO-1 pathway as a therapeutic approach can protect liver cells against OS, inflammation, and apoptosis in the MTX model of hepatotoxicity.
2. Objectives
Dimethyl fumarate, classified as an Nrf2 activator, can upregulate the expression of antioxidant response elements (AREs)-dependent genes and regenerate oxidant-antioxidant balance (16). Therefore, this research was designed to clarify the role of DMF in the hepatotoxicity of MTX in mice.
3. Methods
3.1. Chemicals
Dimethyl fumarate was purchased from Sigma-Aldrich, and MTX was purchased under the brand name Ebetrex (EBEWE Pharma, Austria). All the other chemicals were commercially available, such as glutathione standard (GSH), dithiobis-2-nitrobenzoic acid (DTNB), thiobarbituric acid (TBA), trichloroacetic acid (TCA) and ammonium molybdate (Sigma-Aldrich).
3.2. Animals and Ethics
Forty-two adult male Naval Medical Research Institute (NMRI) mice (aged 6 - 8 weeks, weight range of 25 - 30 g) were used. The mice were kept in the animal house with an ambient temperature of 23 ± 2°C and a 12-hour light/dark cycle with free access to standard food and water in a well-ventilated environment. The protocol was approved by the Animal Ethics Committee of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (ethical approval ID: IR.AJUMS.ABHC.REC.1398.067).
3.3. Induction of Mouse Hepatotoxicity by Methotrexate
Dimethyl fumarate was suspended in distilled water by 1% carboxymethyl cellulose and administered by oral gavage at doses of 30, 60, and 120 mg/kg once a day for 10 days. On the fifth day, MTX was diluted in normal saline and injected intraperitoneally (i.p.) into the mice 1 h after the DMF administration at a single dose of 20 mg/kg. Then, the mice were sacrificed on the eleventh day. The dose of MTX was selected based on the study by Heidari et al. (17). The doses of DMF were within the range of the studies by Vanani et al. (18) and Abdelrahman. R. and Abdel-Rahman. S. (13). The animals were randomly placed in 6 groups (7 mice in each group; Table 1).
Experimental Design and Drug Treatment Timeline
| Groups | Dimethyl Fumarate (DMF) or Vehicle by Gavage (Days 1 - 10) | Methotrexate (MTX) or Saline by i.p. (Day 5) |
|---|---|---|
| Control | Vehicle | Normal saline |
| DMF120 | 120 mg/kg DMF | Normal saline |
| MTX | Vehicle | 20 mg/kg MTX |
| DMF30 + MTX | 30 mg/kg DMF | 20 mg/kg MTX |
| DMF60 + MTX | 60 mg/kg DMF | 20 mg/kg MTX |
| DMF120 + MTX | 120 mg/kg DMF | 20 mg/kg MTX |
3.4. Sample Preparation
On the eleventh day, all the mice were anesthetized with a ketamine/xylazine mixture (100/10 mg/kg, i.p.). Intracardiac blood and liver samples were collected 24 h after the last DMF administration. The blood samples were centrifuged at 4000 × g for 10 min, and the serum samples were stored at - 20°C until measurement of liver enzymes. One part of the hepatic tissue was preserved in 10% formalin for histopathological studies, and the other part was kept at - 80°C until the measurement of OS, apoptosis, and real-time polymerase chain reaction (PCR).
From each mouse, 100 mg of the hepatic tissue was weighed and homogenized in phosphate-buffered saline (PBS, PH = 7.4, 0.1 M) and diluted with 10 µL of antiprotease to 1,000 μL (10 µL in 990 μL). The homogenized tissue was homogenized at 10000 × g at 4°C for 10 min. Then, the supernatant was placed in separate microtubes and stored at - 70°C. Antioxidant and antiapoptotic markers were measured in the liver supernatant (19).
3.5. Determination of Liver Function Biomarkers
The levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) were measured in the serum using Biorex Fars kits/Pars Azmoon kits, Iran.
3.6. Measurement of Antioxidant and Oxidative Biomarkers
Catalase (CAT) activity in the liver supernatant was determined using the Goth method (20). Catalase decomposes H2O2 into water and oxygen. The remaining H2O2 produces a yellow complex with ammonium molybdate. The catalase enzyme activity is inversely proportional to the color intensity, which was measured by reading the absorbance of the dye complex at 405 nm using a microplate reader.
The total thiol content was evaluated by reacting total thiol with Ellman’s reagent (DTNB), following the method of Khan et al. To this end, 40 μL of DTNB dissolved in absolute methanol was mixed with 40 μL of the supernatant in phosphate-buffered saline (PBS). The yellow absorbance was measured using a microplate reader at 412 nm after 10 min (21).
The activity of superoxide dismutase (SOD) and glutathione peroxidase (Gpx) enzymes was detected in the supernatant based on the manufacturer’s instructions for commercial ZellBio kits (Cat. No. ZB-SOD-96A and Cat. No. ZB-GPX-96A, Germany).
Hepatic levels of Nrf2 and HO-1 proteins were determined using ZellBio ELISA kits in the liver supernatant prepared (Cat. No. ZB-11367C-M9648 and Cat No. ZB-10266C-M9648, Germany). According to the instructions, 40 μL of each supernatant, 10 μL of antibody, and 50 μL of HRP-streptavidin were added to the wells. The microplate was incubated for 1 hr at 37ºC, and after washing the wells, a blue color was created by adding chromogen. The stopper solution was added, and the reading was quantitated as optical density at 450 nm in a microplate reader.
The Uchiyama method was employed to quantify the quantity of thiobarbituric acid reactive substances (TBARS) (22). Briefly, 50 μL of the supernatant was mixed with 150 μL of 10% TCA and 200 μL of 0.67% TBA solution, followed by boiling in a water bath for 45 minutes.
The Bradford protein assay method was adopted to determine the total protein concentration in the liver tissue supernatant (23).
3.7. Quantitative Real-Time PCR for Determination of Nrf2, HO-1, Bcl-2, and Caspase 3 Genes Expression
The expression of Nrf2, HO-1, B-cell lymphoma 2 (Bcl-2), and caspase 3 genes was evaluated in the liver tissue supernatant. Total RNA was extracted using a Trizol reagent (Kiazist Company, Iran). The quality and quantity of the extracted RNA were evaluated by gel electrophoresis and a NanoDrop device (NanoDrop Thermo Scientific), respectively. The cDNA was prepared according to the kit’s instructions (Yekta Tajhiz Azma Company, Iran). The relative expression of Nrf2, HO-1, Bcl-2, and caspase 3 genes was measured in all 6 groups via quantitative real-time PCR. The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was used as a housekeeping gene. These 5 genes’ primers were designed and blasted using the National Center for Biotechnology Information (NCBI) site. The primer sequence is presented in Table 2.
Sequence of Special Primers Used for Real-time PCR Process
| Name of genes | Sequences |
|---|---|
| Nrf2 | |
| Forward | 5′TGTAGATGACCATGAGTCGC |
| Reverse | 5′TCCTGCCAAACTTGCTCCAT |
| HO-1 | |
| Forward | 5′CCCACCAAGTTCAAACAGCTC |
| Reverse | 5′AGGAAGGCGGTCTTAGCCTC |
| Bcl-2 | |
| Forward | 5′CCTGTGGATGACTGAGTACCTG |
| Reverse | 5′AGCCAGGAGAAATCAAACAGAGG |
| Caspase 3 | |
| Forward | 5′CTCGCTCTGGTACGGATGTG |
| Reverse | 5′TCCCATAAATGACCCCTTCATCA |
| GAPDH | |
| Forward | 5′GTTGTCTCCTGCGACTTCA |
| Reverse | 5′GGTGGTCCAGGGTTTCTTA |
The real-time PCR reaction was performed using Mastermix Syber Green (Noavaran Teb Company, Iran) and the ABI device. The PCR reaction was carried out in a volume of 10 μL. The time schedule of the device for amplification included three stages:
1- a cycle of initial denaturation at 95°C for 5 min
2- forty cycles of amplification at 95°C for 15 s, 60°C for 15 - 20 s, and 72°C for 15 - 30 s
3- a melting curve cycle at 95°C for 15 s and 65°C for 60 s
The relative expression of the desired genes (Table 2) was performed using the ΔΔCT method proposed by Livak and Schmittgen (24).
3.8. Histopathological Assessment
The paraffin-embedded hepatic tissue was cut into 5-μm sections and stained with hematoxylin and eosin (H&E). For each animal, 6 slides and 5 microscopic fields per slide were blindly analyzed. Histopathological damages were graded into 4 grades: Intense (+++), moderate (++), weak (+), and normal (-). The percentages of fat deposits were also quantified.
3.9. Statistical Analysis
Analysis of variance (ANOVA), followed by Tukey’s multiple comparisons test, was used to compare the variables. This analysis was done by GraphPad Prism software version 9.5.0 (San Diego, California, USA). The data are presented as mean ± standard error of the mean (SEM). The level of significance was set to P < 0.05.
4. Results
4.1. Dimethyl Fumarate Effects on Serum Aminotransferases in the Methotrexate Model of Hepatotoxicity in Mice
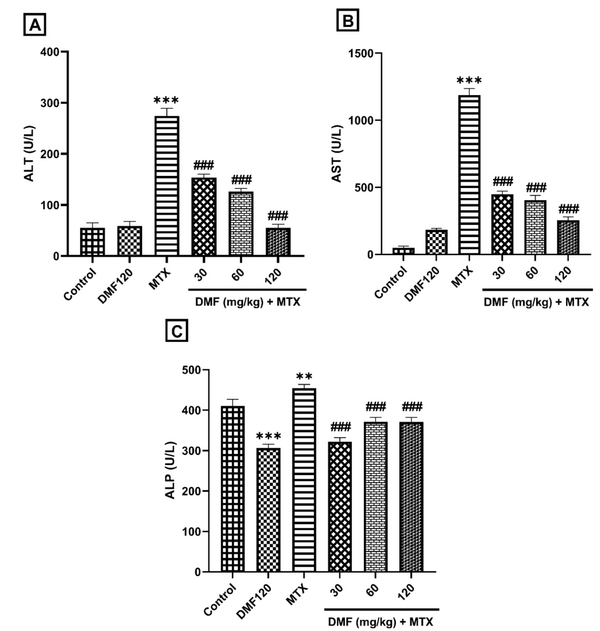
In the MTX group, serum activity of ALT, AST, and ALP enzymes significantly increased compared to the control group (P < 0.001). Dimethyl fumarate in all doses significantly reduced their levels compared to the MTX group (P < 0.001); Figure 1A and B). This indicates the improvement of liver function by DMF. However, a significant decrease in ALP activity was observed between DMF and control groups (Figure 1C).
The effect of DMF (dimethyl fumarate) on the activity of liver enzymes in serum in the mouse model of hepatotoxicity induced by MTX (methotrexate). Serum activities are shown in A, alanine aminotransferase (ALT), B, aspartate aminotransferase (AST), and C, Alkaline phosphatase (ALP). **P < 0.01 and ***P < 0.001: Significant with the control group. ###P < 0.001: Significant with the MTX group
4.2. Dimethyl Fumarate Effects on Antioxidant and Oxidative Biomarkers in Hepatotoxicity of Mice Induced by Methotrexate
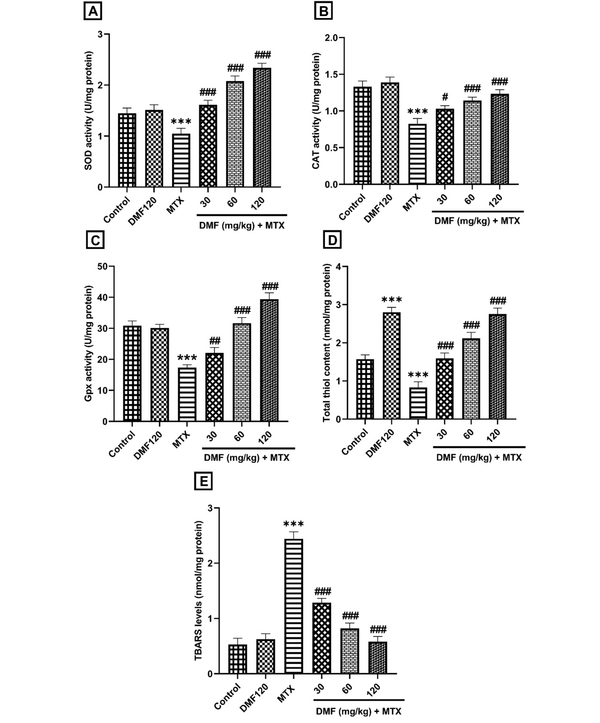
As shown in Figure 2, DMF decreased the MTX-induced OS dose-dependently by increasing the activity of antioxidant biomarkers SOD, CAT, and Gpx and the total thiol level (P < 0.001; (Figure 2A-D). Dimethyl fumarate decreased the OS, thereby increasing the TBARS content in the MTX group (P < 0.001; Figure 2E). Dimethyl fumarate significantly increased the amount of total thiol compared to the control group (Figure 2D).
The effect of DMF (dimethyl fumarate) on antioxidant markers, A, superoxide dismutase (SOD), B, catalase (CAT), C, glutathione peroxidase (Gpx), D, total thiol, and oxidative factor, E, thiobarbituric acid reactive substances (TBARS) in the liver of MTX (methotrexate)-injured mice. ***P < 0.001: Significant with the control group. #P < 0.05, ##P < 0.01, ###P < 0.001: Significant with the MTX group
4.3. Dimethyl Fumarate Effects on the Expression of Caspase 3, Bcl-2, Nrf2, and HO-1 Genes
In comparison to the control group, MTX significantly upregulated caspase 3 mRNA expression (P < 0.001; Figure 3A) and downregulated the mRNA expression of Bcl-2, Nrf2, and HO-1 (P < 0.001; Figure 3B-D). Administration of higher doses of DMF (60 and 120 mg/kg) significantly upregulated Bcl-2, Nrf2, and HO-1 mRNA expression (P < 0.001). Dimethyl fumarate alone significantly decreased the mRNA expression of Bcl-2 and Nrf-2 compared to the control group (Figure 3B and C).
The effect of DMF (dimethyl fumarate) on gene expression of proapoptotic (caspase 3), antiapoptotic B-cell lymphoma 2 (Bcl-2), nuclear factor erythroid 2-related factor 2 (Nrf2), and heme oxygenase 1 (HO-l) in the liver of MTX (methotrexate)-injured mice. ***P < 0.001: Significant with the control group. #P < 0.05, ##P < 0.01, ###P < 0.001: Significant with the MTX group

4.4. Dimethyl Fumarate Effects on Hepatic Expression of Nuclear Factor Erythroid 2-Related Factor 2 and HO-1 Proteins
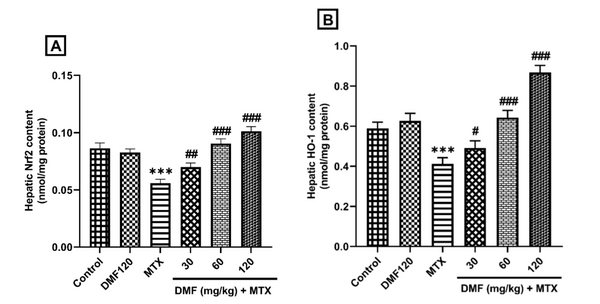
The results of ELISA revealed a decrease in Nrf2 and HO-1 protein expression in the MTX group (P < 0.001). Dimethyl fumarate, in a dose-dependent manner, increased the expression of these proteins compared to the MTX group (Figure 4A and B).
DMF (dimethyl fumarate) effects on the expression of hepatic Nrf2 and HO-1 proteins in MTX (methotrexate)-injured mice. ***P < 0.001: Significant with the control group. #P < 0.05, ##P < 0.01, ###P < 0.001: Significant with the MTX group
4.5. Dimethyl Fumarate Effects on Liver Morphology in MTX-Injured Mice
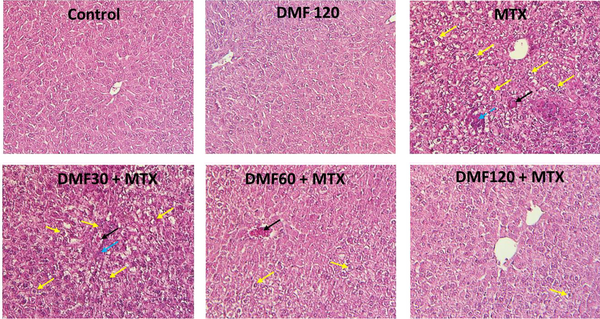
In the control group, the lobular and cellular structure of the liver was normal and healthy. In the tissue section of the liver in the MTX group, significant changes such as fat deposition, the presence of inflammatory cells, and the accumulation of RBC cells were observed in different parts of the liver lobules. In the MTX groups treated with DMF, DMF mitigated tissue damage. In MTX co-treated with DMF 120 mg/kg, no specific pathological changes were observed. The lobular structure of the liver with the central vein and the radial cord towards the center of the hepatocytes seemed completely normal. The accumulation of red blood cells (RBCs) was stopped, and lipid accumulation was significantly reduced with increasing DMF doses (Table 3).
DMF (dimethyl fumarate) effects on MTX (methotrexate)-induced liver tissue changes (H&E × 250) in mice. Yellow arrows: Deposition of fat; blue arrows: Inflammatory cells’ infiltration; black arrows: Congestion of RBCs. The livers of MTX-injured mice showed inflammatory cells’ infiltration, fat deposition, and RBC accumulation. MTX-induced liver damage in DMF-treated mice was reduced as the dose increased from 30 to 120 mg/kg
| Groups | Accumulation of Red Blood Cells | Infiltration of Inflammatory Cells | Fat Deposit (%) |
|---|---|---|---|
| Control | - | - | 0.00 |
| DMF120 | - | - | 0.00 |
| MTX | + | + | 46 * |
| DMF30+MTX | + | (-) to (+) | 38 * |
| DMF60+MTX | + | (-) to (+) | 17 *# |
| DMF120+MTX | - | - | 6 *# |
5. Discussion
In the presented MTX model of hepatotoxicity, an acute and transient increase in the activities of transaminases was observed. This increase may be related to the excessive production of reactive oxygen species and superoxide anions generation (O2•) in the liver tissue, which damages the hepatocytes, resulting in the release of ALT, AST, and ALP into the circulation (Figure 1). In agreement with this observation, the augmentation of aminotransferases from hepatocytes to the extracellular environment resulted in severe injury in the MTX group (5). The severity of the damage decreased in the treatment groups, indicating that DMF strengthened the antioxidant defense system (Figure 1). These findings are similar to those reported by Ibrahim et al. (15). Alanine aminotransferase (ALT), AST, and ALP biomarkers are reliable factors for the clinical evaluation of hepatotoxicity. However, other markers, such as cytokeratin-18 (CK18), have been suggested in preclinical models of DILI (25). CK18 can diagnose the early stage of DILI and propose the mechanism of hepatocellular injury. Following the induction of liver injury by MTX, the TBARS content as an index of lipid peroxidation was significantly increased, indicating the decreased ability of hepatocytes to scavenge free radicals (Figure 2). Nuclear factor erythroid 2-related factor 2 activators, such as DMF, can restore the cellular level of GSH. Therefore, DMF can be used in both prevention and treatment of diseases (26, 27). However, the beneficial role of GSH is questioned in humans since there is not enough clinical evidence to confirm that increasing the level of GSH has a beneficial role in diseases (27). Methotrexate decreased the antioxidant defense system. Dimethyl fumarate increased the activities of SOD, Gpx, CAT, and levels of total thiol (Figure 2). Dimethyl fumarate decreased the OS state dose-dependently. These findings are in harmony with those of Abdelrahman. R. and Abdel-Rahman. S. (13).
Oxidative stress initiates apoptotic genes’ transcriptional activity. Caspase 3 is a key mediator of the apoptotic signaling pathway, so that the activation of caspase 3 eventually leads to apoptosis. DMF, via its antiapoptotic effects, reversed the effects of MTX on caspase 3, targeting against the activity of hepatic mitochondria. Thus, Bcl-2, Nrf2, and HO-1 mRNA transcript levels were remarkably downregulated by MTX and upregulated after the DMF treatment (Figure 3).
In the groups treated by DMF, the expression of Nrf2 and HO-1 protein increased (Figure 4). This increase indicated that the Nrf2/HO-1 pathway protected the liver from MTX injury (Figure 4). The results showed that the Nrf2/HO-1 antioxidant pathway is necessary for the protection of the liver against MTX-induced injury, which is in line with Gendy et al.’s study (28). Alleviation of hepatic morphological changes (Figure 5 and Table 3) confirmed the biochemical findings of this research.
5.1. Conclusions
Dimethyl fumarate, as an FDA (Food and Drug Administration)-approved drug, has antioxidant and antiapoptotic activities. It managed to suppress apoptosis and improve the antioxidant defense system and targeting of the Nrf2/HO-1 pathway against MTX-induced hepatotoxicity in mice dose-dependently. Therefore, DMF may be clinically effective in the prevention or treatment of hepatotoxicity induced by MTX.
References
-
1.
Brent J, Burkhart K, Dargan P, Hatten B, Megarbane B, Palmer R, et al. Critical Care Toxicology. Cham: Springer International Publishing; 2017. p. 1171-218. https://doi.org/10.1007/978-3-319-17900-1.
-
2.
Abd El-Ghafar OAM, Hassanein EHM, Ali FEM, Omar ZMM, Rashwan EK, Mohammedsaleh ZM, et al. Hepatoprotective effect of acetovanillone against methotrexate hepatotoxicity: Role of Keap-1/Nrf2/ARE, IL6/STAT-3, and NF-kappaB/AP-1 signaling pathways. Phytother Res. 2022;36(1):488-505. [PubMed ID: 34939704]. https://doi.org/10.1002/ptr.7355.
-
3.
Azadnasab R, Kalantar H, Khorsandi L, Kalantari H, Khodayar MJ. Epicatechin ameliorative effects on methotrexate-induced hepatotoxicity in mice. Hum Exp Toxicol. 2021;40(12_suppl):S603-10. [PubMed ID: 34802285]. https://doi.org/10.1177/09603271211047924.
-
4.
Kalantar M, Kalantari H, Goudarzi M, Khorsandi L, Bakhit S, Kalantar H. Crocin ameliorates methotrexate-induced liver injury via inhibition of oxidative stress and inflammation in rats. Pharmacol Rep. 2019;71(4):746-52. [PubMed ID: 31220735]. https://doi.org/10.1016/j.pharep.2019.04.004.
-
5.
Roghani M, Kalantari H, Khodayar MJ, Khorsandi L, Kalantar M, Goudarzi M, et al. Alleviation of liver dysfunction, oxidative stress and inflammation underlies the protective effect of ferulic acid in methotrexate-induced hepatotoxicity. Drug Des Devel Ther. 2020;14:1933-41. [PubMed ID: 32546960]. [PubMed Central ID: PMC7250701]. https://doi.org/10.2147/DDDT.S237107.
-
6.
Matouk AI, Awad EM, El-Tahawy NFG, El-Sheikh AAK, Waz S. Dihydromyricetin alleviates methotrexate-induced hepatotoxicity via suppressing the TLR4/NF-kappaB pathway and NLRP3 inflammasome/caspase 1 axis. Biomed Pharmacother. 2022;155:113752. [PubMed ID: 36182732]. https://doi.org/10.1016/j.biopha.2022.113752.
-
7.
El-Kashef DH, Sewilam HM. Empagliflozin mitigates methotrexate-induced hepatotoxicity: Targeting ASK-1/JNK/Caspase-3 pathway. Int Immunopharmacol. 2023;114:109494. [PubMed ID: 36462340]. https://doi.org/10.1016/j.intimp.2022.109494.
-
8.
Dufour J, Clavien P. Signaling pathways in liver diseases. 3rd ed. Switzerland: John Wiley & Sons; 2015. p. 469-78. https://doi.org/10.1002/9781118663387.
-
9.
Linker RA, Haghikia A. Dimethyl fumarate in multiple sclerosis: Latest developments, evidence and place in therapy. Ther Adv Chronic Dis. 2016;7(4):198-207. [PubMed ID: 27433310]. [PubMed Central ID: PMC4935836]. https://doi.org/10.1177/2040622316653307.
-
10.
Bruck J, Dringen R, Amasuno A, Pau-Charles I, Ghoreschi K. A review of the mechanisms of action of dimethylfumarate in the treatment of psoriasis. Exp Dermatol. 2018;27(6):611-24. [PubMed ID: 29603404]. https://doi.org/10.1111/exd.13548.
-
11.
Singh N, Saha L, Kumari P, Singh J, Bhatia A, Banerjee D, et al. Effect of dimethyl fumarate on neuroinflammation and apoptosis in pentylenetetrazol kindling model in rats. Brain Res Bull. 2019;144:233-45. [PubMed ID: 30472152]. https://doi.org/10.1016/j.brainresbull.2018.11.013.
-
12.
Dwivedi DK, Jena GB. Diethylnitrosamine and thioacetamide-induced hepatic damage and early carcinogenesis in rats: Role of Nrf2 activator dimethyl fumarate and NLRP3 inhibitor glibenclamide. Biochem Biophys Res Commun. 2020;522(2):381-7. [PubMed ID: 31761320]. https://doi.org/10.1016/j.bbrc.2019.11.100.
-
13.
Abdelrahman RS, Abdel-Rahman N. Dimethyl fumarate ameliorates acetaminophen-induced hepatic injury in mice dependent of Nrf-2/HO-1 pathway. Life Sci. 2019;217:251-60. [PubMed ID: 30550888]. https://doi.org/10.1016/j.lfs.2018.12.013.
-
14.
Liu X, Zhou W, Zhang X, Lu P, Du Q, Tao L, et al. Dimethyl fumarate ameliorates dextran sulfate sodium-induced murine experimental colitis by activating Nrf2 and suppressing NLRP3 inflammasome activation. Biochem Pharmacol. 2016;112:37-49. [PubMed ID: 27184504]. https://doi.org/10.1016/j.bcp.2016.05.002.
-
15.
Ibrahim SG, El-Emam SZ, Mohamed EA, Abd Ellah MF. Dimethyl fumarate and curcumin attenuate hepatic ischemia/reperfusion injury via Nrf2/HO-1 activation and anti-inflammatory properties. Int Immunopharmacol. 2020;80:106131. [PubMed ID: 31981960]. https://doi.org/10.1016/j.intimp.2019.106131.
-
16.
Rochette L, Zeller M, Cottin Y, Vergely C. Redox functions of heme oxygenase-1 and biliverdin reductase in diabetes. Trends Endocrinol Metab. 2018;29(2):74-85. [PubMed ID: 29249571]. https://doi.org/10.1016/j.tem.2017.11.005.
-
17.
Heidari R, Ahmadi A, Mohammadi H, Ommati MM, Azarpira N, Niknahad H. Mitochondrial dysfunction and oxidative stress are involved in the mechanism of methotrexate-induced renal injury and electrolytes imbalance. Biomed Pharmacother. 2018;107:834-40. [PubMed ID: 30142545]. https://doi.org/10.1016/j.biopha.2018.08.050.
-
18.
Vanani AR, Kalantari H, Mahdavinia M, Rashno M, Khorsandi L, Khodayar MJ. Dimethyl fumarate reduces oxidative stress, inflammation and fat deposition by modulation of Nrf2, SREBP-1c and NF-kappaB signaling in HFD fed mice. Life Sci. 2021;283:119852. [PubMed ID: 34332979]. https://doi.org/10.1016/j.lfs.2021.119852.
-
19.
Acedo SC, Caria CR, Gotardo EM, Pereira JA, Pedrazzoli J, Ribeiro ML, et al. Role of pentoxifylline in non-alcoholic fatty liver disease in high-fat diet-induced obesity in mice. World J Hepatol. 2015;7(24):2551-8. [PubMed ID: 26523207]. [PubMed Central ID: PMC4621469]. https://doi.org/10.4254/wjh.v7.i24.2551.
-
20.
Goth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta. 1991;196(2-3):143-51. [PubMed ID: 2029780]. https://doi.org/10.1016/0009-8981(91)90067-m.
-
21.
Khan H, Khan MF, Jan SU, Khan KA, Shah SU. Study of the chemical and metabolic changes in plasma glutathione (Gsh) of human blood after lithium introduction. Journal of Applied Pharmacy. 2011;3:201-11. https://doi.org/10.21065/19204159.3.201.
-
22.
Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86(1):271-8. [PubMed ID: 655387]. https://doi.org/10.1016/0003-2697(78)90342-1.
-
23.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-54. [PubMed ID: 942051]. https://doi.org/10.1006/abio.1976.9999.
-
24.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402-8. [PubMed ID: 11846609]. https://doi.org/10.1006/meth.2001.1262.
-
25.
Korver S, Bowen J, Pearson K, Gonzalez RJ, French N, Park K, et al. The application of cytokeratin-18 as a biomarker for drug-induced liver injury. Arch Toxicol. 2021;95(11):3435-48. [PubMed ID: 34322741]. [PubMed Central ID: PMC8492595]. https://doi.org/10.1007/s00204-021-03121-0.
-
26.
Jing X, Shi H, Zhang C, Ren M, Han M, Wei X, et al. Dimethyl fumarate attenuates 6-OHDA-induced neurotoxicity in SH-SY5Y cells and in animal model of Parkinson's disease by enhancing Nrf2 activity. Neuroscience. 2015;286:131-40. [PubMed ID: 25449120]. https://doi.org/10.1016/j.neuroscience.2014.11.047.
-
27.
Giustarini D, Milzani A, Dalle-Donne I, Rossi R. How to increase cellular glutathione. Antioxidants (Basel). 2023;12(5). [PubMed ID: 37237960]. [PubMed Central ID: PMC10215789]. https://doi.org/10.3390/antiox12051094.
-
28.
Gendy A, Soubh A, Al-Mokaddem A, Kotb El-Sayed M. Dimethyl fumarate protects against intestinal ischemia/reperfusion lesion: Participation of Nrf2/HO-1, GSK-3beta and Wnt/beta-catenin pathway. Biomed Pharmacother. 2021;134:111130. [PubMed ID: 33348309]. https://doi.org/10.1016/j.biopha.2020.111130.

