Abstract
Introduction:
Fungal infections infect billions of people annually around the world, and invasive types with a high mortality rate are commonly associated with primary immunodeficiencies (PIDs), such as autosomal dominant hyper-immunoglobulin (Ig)E syndrome (AD-HIES), or deficiency of caspase recruitment domain-containing protein 9 (CARD9). Patients with CARD9, which is also associated with invasive fungal infection (such as meningitis) and deep dermatophytosis. The symptoms of CARD9 deficiency usually start at early childhood, and it is essential to diagnose and treat the disease appropriately to minimize infections and prevent mortality. Since CARD9 deficiency is a newly-introduced disease, investigation of the different aspects of this disease has been the focus of several studies.Case Presentation:
We present a case with recurrent fungal infections and abdominal mass, and the result of his gene sequence indicates a CARD9 deficiency. Interestingly, the patient had no serious complications until the age of 14; however, the CARD9 deficiency was a hereditary disorder. Surprisingly, the size of the abdominal mass in the patient was controlled by antifungal treatment.Conclusions:
The present study indicates that a deficiency of CARD9 can be considered one of the possible causes of abdominal mass that can guide physicians toward proper diagnosis and treatment.Keywords
Fungal Infections, Caspase Activation, and Recruitment Domain Immunologic Deficiency Syndromes Abdominal Mass
1. Introduction
Fungal infections, including saprophytic and commensal fungi, infect billions of people annually around the world and are considered a major health problem, as they are associated with great risk of mortality (1). The common fungal infections, causing invasive fungal diseases (IFDs), include candidiasis, aspergillosis, pneumocytosis, and cryptococcosis with a mortality rate of 30 - 50% (2). The most important issue in understanding the pathogenesis of fungal infections and proper diagnosis of patients is to study cellular and molecular mechanisms of anti-fungal immunity (3). Most fungal infections are associated with primary immunodeficiencies (PIDs) (4). Chronic mucocutaneous candidiasis (CMC), characterized by recurrent infections of the mucosa or skin with Candida species, may be a clinical manifestation of PIDs, such as autosomal dominant inborn error of STAT3 in patients with hyper-immunoglobulin-E (IgE) syndrome (HIES), or patients with autosomal recessive inborn deficiency of caspase recruitment domain-containing protein 9 (CARD9), which is also associated with invasive fungal infection (such as meningitis) and deep dermatophytosis (5, 6).
CARD9 is an adaptor molecule expressed in myeloid cells downstream of several C-type lectin receptors, which recognizes fungal pathogen-associated molecular patterns (PAMPs) and couples with B cell lymphoma 10 (BCL-10) and mucosa-associated lymphoid tissue lymphoma translocation gene 1 (MALT-1), leading to secretion of proinflammatory cytokines (7, 8). Susceptibility of CARD9-deficient patients to Candida stimulation is suggested to be due to a selective defect in host defense against fungi, such as impaired helper T-cells, producing interleukin-17 (IL-17), and neutrophils’ killing defect (9, 10). The signs and symptoms of CARD9 deficiency usually start at early childhood, and because it is associated with IFDs that can be life-threatening, appropriate diagnostic, prophylactic, and therapeutic strategies are essential to minimize infections and prevent mortality (10). Since CARD9 deficiency is a newly-introduced disease, different aspects of this disease have been the focus of several studies.
In this report, we present a 14-year-old boy with recurrent fungal infections and abdominal mass whose gene sequencing indicates CARD9 deficiency. Interestingly, the patient did not have any serious complications by the age of 14; however, CARD9 deficiency was an inherited disorder in his family. Owing to the results of a gene sequencing and recurrent fungal infections, he received antifungal therapy. Notably, the size of the abdominal mass in the patient under study was controlled by antifungal therapy.
2. Case Presentation
A 14-year-old boy referred to our center with a history of recurrent oral candidiasis, starting since the age of 6 months, which was resistant to treatment and complicated with fungal mucosal lesions, and onychomycosis. The child was the result of consanguineous marriage, and the parents had no positive history of PIDs. In his past medical history, he was hospitalized three times due to recurrent systemic fungal infection. The first episode occurred at the age of 8 when the patient was hospitalized with fever, abdominal pain, and lack of appetite. In the ultra-sonographic examination, a 54 × 58 mm mass was detected in the left lobe of the liver, a hypodense, and heterogeneous solid mass in the right peritoneum with para-aortic lymphadenopathy. Pathologic examination by biopsy of the lesion had revealed Palisading Necrosis, treated with a slow intravenous injection of ceftriaxone 75 mg/kg and amikacin 10 mg/kg for 10 days. After 10 days of the treatment, the patient recovered and was discharged with a recommendation to visit a surgeon, as follow-up ultrasonography examination showed no change in the size of liver mass.
About one month later, the patient was re-admitted to the hospital with abdominal pain, lethargy, and weight loss, and ultrasonography revealed a heterogeneous 70 × 45 mm hypo- and hyperechoic centered mass in the left lobe of the liver with retroperitoneal lymphadenopathy, suspected of fungal infection. Further investigation by abdominal computed tomography (CT) scan demonstrated a 60 × 60 mm necrotic lesion in the left lobe of the liver with para-aortic lymphadenopathy and the results of pathologic examination reported large necrosis and fungal infection. The patient was further investigated, and aspergillosis was reported in the culture. Thus, he was treated with voriconazole6 mg/kg/dose every 12 h in 2 doses plus 4 mg/kg/dose every 12 h maintenance dose and intravenous slow injection of amphotericin B 5 mg/kg/24 h for 14 days and was discharged with good general conditions and recommendation to use 100 mg oral voriconazole every 12 h for 90 days.
The findings of laboratory and immunologic tests requested during hospitalization are summarized in Tables 1 and 2. For the third time, at the age of 12, the patient was hospitalized with low back pain, anorexia, and weight loss. Ultrasonography revealed a hypo-echoic retroperitoneal mass of 70 × 45 mm with a liquid center in the vicinity of in abdominal aorta, suggestive of retroperitoneal abscess or abdominal aortic aneurysm. In CT angiography, the aorta was not observed in the lower limit of L1 and upper limit of L4 with a 67 × 51 × 27 mm lobulated mass in the prevertebral area, suggestive of fungi. The patient thus received antifungal treatment (amphotericin B and voriconazole), in a protocol similar to the previous time and responded to treatment. After discharge, the patient was recommended to use 100 mg oral itraconazole on a daily basis.
The Results of the Initial Laboratory Examination
| Laboratory Test | First Admission | Third Admission | Normal Range |
|---|---|---|---|
| White blood cells, /mm3 | 12,200 | 7,700 | 4500 - 13500 |
| Neutrophils, % | 51 | 30 | 40 - 75 |
| Lymphocytes | 32.5 | 54.5 | 20 - 45 |
| Monocytes, % | 8.2 | 12 | 2 - 10 |
| Eosinophils, % | 7 | 2.5 | 1 - 6 |
| Hemoglobin, g/dL | 10.6 | 12.9 | 9.4 - 15.6 |
| Platelet, /mm3 | 498,000 | 320,000 | 150000 - 50000 |
| ALT, IU/L | 10 | 9 | 5 - 40 |
| AST, IU/L | 18 | 26 | 5 - 40 |
| ESR | 125 | 69 | < 10 |
| αFP, IU/L | 1.3 | - | Up to 5.5 |
| βHCG, IU/L | 0.8 | - | Up to 5 |
| HIV-Ab | Negative | - | - |
| HIV-PCR × 2 | Negative | - | - |
The Results of Immunologic Assessment of the Patient at His First Admission
| Lab Data | First Admission | Normal Range |
|---|---|---|
| C3, mg/dL | 163 | 88 - 150 |
| C4, mg/dL | 23 | 12 - 32 |
| CH50 | 158 | 70 - 150 |
| NBT, % | 100 | 100 |
| DHR, % | 121 | 50 - 200 |
| IgG, IU/mL | 2,843 | 633 - 1280 |
| IgM, IU/mL | 167 | 48 - 207 |
| IgA, IU/mL | 407 | 33 - 202 |
| IgE, IU/mL | > 500 | 1.03 - 161.3 |
| Anti-tetanus antibody, IU/mL | 2.4 | - |
| Anti-diphtheria antibody, IU/mL | > 1 | - |
| PHA,SI | 3.04 | ≥ 3 |
| BCG,SI | 2.5 | ≥ 2.5 |
| Candida,SI | 2.5 | ≥ 2.5 |
| CD3, mg/dL | 72 | 52- 78 |
| CD4, mg/dL | 39 | 25 - 48 |
| CD8, mg/dL | 29 | 9 - 35 |
| CD16, mg/dL | 9 | 10.1 - 20.9 |
| CD56, mg/dL | 10 | 6 - 27 |
| CD19, mg/dL | 14 | 6 - 19 |
| CD11 a monopoly, mg/dL | 89 | - |
| CD11 a lymph, mg/dL | 83 | - |
| CD18 a monopoly, mg/dL | 70 | - |
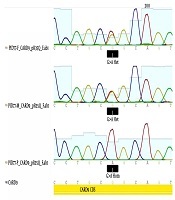
After discharge from the final hospitalization, the patient was referred to an allergy immunology clinic. Recurrent fungal infections and high levels of IgE and eosinophilia suspected us to HIES. Thus, we evaluated the National Institutes of Health (NIH) Scores for the patient, but he scored only 10 points and was not diagnosed with autosomal dominant HIES. He lacked the symptoms of autosomal recessive HIES (Dock8-deficiency), such as eczema and viral/bacterial infections. As a result, whole exome sequencing (WES) was performed for the patient that revealed CARD9 deficiency at cytogenetic location: 9q34.3. Then, both parents were evaluated for this gene mutation. Based on the results, the homozygous mutation was observed in CARD9 gene (NM_052813: exon2: c.G104A) and (CARD9:NM_052814:exon2:c.G104A:p.R35Q), and homozygous mutation in the CD40LG gene (NM_000074: exon5: c.T464C) and parents (father and mother) were carriers heterozygous for this duplication mutation (Figure 1).
In the present study, the result sequence of WES (using Illumine high throughput DNA sequencing technology) was compared to standard references and parental WES and then interpreted by board-certified laboratory clinicians to identify variations in the patient’s DNA sequence related to his medical concerns to discover the cause of the medical disorder. After the diagnosis, the patient was recommended to use two pills of 100 mg itraconazole daily. The parents of the child were explained about the study objectives and gave consent to use their child’s information in the study.
The results of whole exome sequencing test in the patient and his parents based on the obtained data, homozygous mutation was observed in CARD9 gene (NM_052813: exon2: c.G104A) and (CARD9:NM_052814:exon2:c.G104A:p.R35Q), and homozygous mutation in CD40LG gene (NM_000074: exon5: c.T464C) and parents (father and mother) were carrier heterozygous for this duplication mutation.

3. Discussion
Here, we presented a case of CARD9 deficiency with recurrent fungal infections, who have not been diagnosed with a specific PID and had no preventive strategy until the age of 14. Fortunately, the patient had no serious complications when he referred to us; however, he was hospitalized three times with systemic fungal infections and had a history of surgery due to abdominal mass. An abdominal mass is an abnormal growth in the abdomen. An abdominal mass causes visible swelling and may change the shape of the abdomen. A person with an abdominal mass may present with weight gain and symptoms such as abdominal discomfort, pain, and bloating. Abdominal masses are often treatable. However, health complications may arise depending on the cause of the mass (11). Although a list of factors, including an injury, cyst, benign tumor, cancer, or other diseases can cause the abdominal masses (11), it is interesting to note that the patient studied present the manifestation of abdominal mass during the second decade of life, not at birth, and not due to factors mentioned.
Abdominal masses are usually treated with hormone-based medications, surgery, chemotherapy, and radiotherapy to shrink or even removal of the mass (11), but antifungal medicines have never been recommended for the treatment of this disease. In this study, the patient had an abdominal mass associated with a history of recurrent fungal infections. Although the patient had undergone surgery, the disease recurred after a determined period of the disease. Regarding the patient who had a history of recurrent fungal infections, WES was performed for the patient that revealed CARD9 deficiency at cytogenetic location: 9q34.3. In fact, CARD9 is a protein expressed in myeloid cells that is an adapter molecule in the downstream pathway to identify fungi. Innate responses of C-type lectin receptors (through Dectin-1, Dectin-2, or Mincle stimulation) are activated by CARD9-BCL10 complex in response to fungi, especially Candida (11).
Neutrophil killing capacity and response to fungal infections are impaired in individuals with CARD9 deficiency (10, 12). Accordingly, the impairment of the immune response results in patients with CARD9 deficiency susceptible to recurrent chronic systemic fungal infections associated with a high rate of mortality, in particular invasive fungal infections (2). The most fatal fungal infection in patients with CARD9 deficiency is the involvement of the brain parenchyma, meninges, and central nervous system (CNS) that has a high mortality rate, according to the case reports in the literature (6, 12). Therefore, patients with chronic and recurrent fungal infections with this immunodeficiency need to be further investigated for proper diagnostic, preventive, and treatment strategies (10). Also, CARD9 deficiency is associated with severe fungal infections, especially infection with Candida albicans (13) and CNS-related neurological disorders (12); however, no reports have ever been indicating the association of abdominal mass with CARD9 deficiency.
In the present case, homozygous mutations lead to amino acid change p. R35Q, which is most likely deleterious. Fortunately, the patient was not complicated with CNS fungal infections and had no major adverse effects, except for recurrent/chronic abdominal involvement (liver and peritoneum). In the first admission, it seems that he was not diagnosed and treated appropriately and was recommended to visit a surgeon afterward, but after a short time, about one month, the patient frequently referred to the infectious ward, owing to the repeated Aspergillus infection.
The standard treatment of invasive aspergillosis is combinational therapy with voriconazole and amphotericin B; however, the appropriate preventive measures and improved tools for early detection of these infections have to be further investigated (14). In our patient, the patient was appropriately treated and was recommended to use oral voriconazole, but the patient should refer for further follow-up to investigate the remission of the liver mass. It is of note, the size of the abdominal mass was persistently maintained during a 3-year period of antifungal treatment with voriconazole and amphotericin B.
As CARD9 deficiency is a rare and newly-introduced PID, not all physicians might have visited the patients, and it is essential to increase physicians' awareness on appropriate diagnosis of CARD9 deficiency. In addition, the expensive cost of immunologic and genetic assessments might be a barrier to the proper diagnosis of PIDs, especially in developing countries. Variants that interfere with DNA sequencing and medical procedures, such as bone marrow transplantation and blood transfusion, may result in misleading results, as well; thus, WES is suggested as an efficient newly developed complex test for identification of changes in the patient's DNA. Further investigations are necessary to determine the prophylactic measures required for these patients to prevent further fatal and invasive infections that predispose patients to mortality. Altogether, we suggest that a deficiency of CARD9 can be considered one of the possible causes of abdominal mass that can guide physicians toward proper diagnosis and treatment.
In conclusion, according to the case we presented here, it is essential to consider PIDs in any patient with IFDs, as they might predispose patients with severe immune response impairment that can cause fatal infections and lead to patients’ death. On the other hand, one of the most important PIDs in cases with abdominal mass is CARD9 deficiency, which is a rare genetic disease, and physicians should have a high clinical suspicion to diagnose these patients, as they might have no other symptom, other than recurrent/chronic fungal infections (13). Given that CARD9 deficiency can be treated with antifungal compounds, this genetic disorder should be given more attention in patients with abdominal mass to reach optimized diagnosis and treatment.
Acknowledgements
References
-
1.
Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. 2012;4(165):165rv13. [PubMed ID: 23253612]. https://doi.org/10.1126/scitranslmed.3004404.
-
2.
Lortholary O, Gangneux JP, Sitbon K, Lebeau B, de Monbrison F, Le Strat Y, et al. Epidemiological trends in invasive aspergillosis in France: the SAIF network (2005-2007). Clin Microbiol Infect. 2011;17(12):1882-9. [PubMed ID: 21668573]. https://doi.org/10.1111/j.1469-0691.2011.03548.x.
-
3.
LeibundGut-Landmann S, Wuthrich M, Hohl TM. Immunity to fungi. Curr Opin Immunol. 2012;24(4):449-58. eng. [PubMed ID: 22613091]. [PubMed Central ID: PMCPmc3538869]. https://doi.org/10.1016/j.coi.2012.04.007.
-
4.
Lanternier F, Cypowyj S, Picard C, Bustamante J, Lortholary O, Casanova JL, et al. Primary immunodeficiencies underlying fungal infections. Curr Opin Pediatr. 2013;25(6):736-47. [PubMed ID: 24240293]. [PubMed Central ID: PMCPmc4098727]. https://doi.org/10.1097/mop.0000000000000031.
-
5.
Puel A, Picard C, Cypowyj S, Lilic D, Abel L, Casanova JL. Inborn errors of mucocutaneous immunity to Candida albicans in humans: a role for IL-17 cytokines? Curr Opin Immunol. 2010;22(4):467-74. [PubMed ID: 20674321]. [PubMed Central ID: PMCPmc3770911]. https://doi.org/10.1016/j.coi.2010.06.009.
-
6.
Glocker EO, Hennigs A, Nabavi M, Schaffer AA, Woellner C, Salzer U, et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361(18):1727-35. [PubMed ID: 19864672]. [PubMed Central ID: PMCPmc2793117]. https://doi.org/10.1056/NEJMoa0810719.
-
7.
Vautier S, MacCallum DM, Brown GD. C-type lectin receptors and cytokines in fungal immunity. Cytokine. 2012;58(1):89-99. [PubMed ID: 21924922]. https://doi.org/10.1016/j.cyto.2011.08.031.
-
8.
Hara H, Ishihara C, Takeuchi A, Imanishi T, Xue L, Morris SW, et al. The adaptor protein CARD9 is essential for the activation of myeloid cells through ITAM-associated and Toll-like receptors. Nat Immunol. 2007;8(6):619-29. [PubMed ID: 17486093]. https://doi.org/10.1038/ni1466.
-
9.
Okada S, Puel A, Casanova J, Kobayashi M. Chronic mucocutaneous candidiasis disease associated with inborn errors of IL-17 immunity. Clin Transl Immunology. 2016;5(12). e114. [PubMed ID: 28090315]. https://doi.org/10.1038/cti.2016.71.
-
10.
Drewniak A, Gazendam RP, Tool AT, van Houdt M, Jansen MH, van Hamme JL, et al. Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood. 2013;121(13):2385-92. [PubMed ID: 23335372]. https://doi.org/10.1182/blood-2012-08-450551.
-
11.
Eisen LK, Cunningham JD, Aufses A. Intussusception in adults: institutional review. J Am Coll Surg. 1999;188(4):390-5.
-
12.
Gavino C, Cotter A, Lichtenstein D, Lejtenyi D, Fortin C, Legault C, et al. CARD9 deficiency and spontaneous central nervous system candidiasis: complete clinical remission with GM-CSF therapy. Clin Infect Dis. 2014;59(1):81-4. [PubMed ID: 24704721]. https://doi.org/10.1093/cid/ciu215.
-
13.
Lanternier F, Mahdaviani SA, Barbati E, Chaussade H, Koumar Y, Levy R, et al. Inherited CARD9 deficiency in otherwise healthy children and adults with Candida species–induced meningoencephalitis, colitis, or both. J Allergy Clin Immunol. 2015;135(6):1558-6800. [PubMed ID: 25702837]. [PubMed Central ID: PMC4831587]. https://doi.org/10.1016/j.jaci.2014.12.1930.
-
14.
Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clinical infectious diseases. 2008;46(3):327-60. [PubMed ID: 18177225].