Abstract
Background:
Chlamydia trachomatis is one of the major bacterial agents of the sexually transmitted diseases worldwide, especially among young females. There is no data regarding the prevalence of genital Chlamydia infection among young females in Kashan, Iran.Objectives:
The current study aimed to determine the prevalence of endocervical C. trachomatis infection among females aged 17 - 35 years in Kashan, Iran.Patients and Methods:
In the current descriptive study, 255 endocervical swab samples were collected from the obstetrics and gynecology clinics of Kashan, Iran from December 2012 to July 2013. Cervical swabs were placed in transport media and sent to the laboratory. To identify C. trachomatis in the samples Polymerase Chain Reaction (PCR) was performed to amplify a sequence in the cryptic plasmid, generating a fragment of about 512base pair. Demographic data was collected considering the relevant risk factors by a standard questionnaire.Results:
A total of 255 females were tested. The prevalence of genital C. trachomatis was 2.4% (95% confidence interval [CI] 0.54% - 4.26%); 3.2% of the females in the ≤ 25-year-old group were positive versus 1.8% in the 26 - 35-year-old group. The most general presented symptoms of genital C. trachomatis infection were vaginal discharge (66.6%) and lumbar pain (50%). No significant relationships were found between C. trachomatis infection and the risk factors.Conclusions:
To the authors’ knowledge this is the first study to describe endocervical C. trachomatis infection in this area. The obtained results also emphasized the importance of routine diagnosis of C. trachomatis to control of the infection.Keywords
1. Background
Chlamydia genital infection is the most common bacterial infection sexually transmitted in the world (1). The highest age-specific rate was reported in females aged 15 - 35 years. The majority of the females are asymptomatic (2), thus, providing a continuous reservoir for the infection (3). It is estimated that 5 - 12% of sexually active adults in the age group of 16 - 34 years in the United Kingdom are infected with Chlamydia trachomatis (4). According to the World Health Organization (WHO), 101.5 million people with Chlamydia infection are detected globally each year (5). The infection risk factors include being young or adolescent, having new or frequent sexual contacts, inconsistent use of barrier contraception, history of prior sexual transmitted infection, and low educational and socioeconomic levels (6). Thus, the prevalence of C. trachomatis may differ among racial groups because of differences in sexual risk behavior and cultural backgrounds (7).
Untreated Chlamydial infections may lead to Pelvic Inflammatory Disease (PID), ectopic pregnancy, premature delivery, spontaneous abortion, low birth weight, tubal infertility and subsequent scarring of the fallopian tubes (8). During pregnancy, Chlamydial infection can cause miscarriage, premature rupture of membranes, preterm labor, low birth weight, infant mortality, neonatal Chlamydial infection, and postpartum endometritis (9). Screening, early diagnosis and treatment is considered the main policy to prevent complications and further transmission of the infection (10).
Conventional laboratory diagnosis of C. trachomatis infection is done by cell culture or antigen detection. Recently, Nucleic Acid Amplification Tests (NAATs) are widely used. Commercially available NAATs using methods such as Polymerase Chain Reaction (PCR), and Ligase Chain Reaction (LCR), are now the gold standard tests for genital Chlamydia infection (11). PCR has good sensitivity (100%) and specificity (99.3%) for endocervical samples compared with urethral culture. It is the preferred test and the most commonly used method of diagnosis, which amplifies C. trachomatis DNA sequences (12). There are no accurate data regarding the prevalence of C. trachomatis infection in Kashan and few studies from Iran examined the prevalence of genital Chlamydia infection based on age.
2. Objectives
The current study aimed to assess the prevalence of endocervical C. trachomatis infection in a population of females aged 17 - 35 years in Kashan, Iran.
3. Patients and Methods
3.1. Clinical Specimens
Endocervical swab samples were collected by the gynecologists from 255 married females aged 17 - 35 years, with or without symptoms, referring to the obstetrics and gynecology clinics of Kashan, Iran, from December 2012 to July 2013. The study was approved by the regional Research Ethics Committee of Kashan University of Medical Sciences (P 2331), and all participants signed informed consent forms prior to enrolment in the study. The females were included on the basis of verbal consent. Demographics, sexual behavior, method of contraception, previous history of sexually transmitted infections (STIs) and symptoms were assessed by a questionnaire routinely used in the clinic. Pregnant females and those who used antibiotics in the preceding 14 days were excluded.
Cervical samples were collected from the endocervical region after inserting a sterile speculum into the vagina. Mucus was cleaned with a sterile cotton swab without causing any bleeding or using antiseptics. Dacron swabs were used to collect endocervical specimens. The specimen placed into sterile collection tubes containing 2 mL of transport buffer (NaH2PO4 20 mM, Na2HPO4 20 mM, sucrose 20 mM, glutamin 5 mM, gentamycin 50 µg/ml, vancomycin 100 µg/mL, amphotericin B 25 µg/mL supplemented with 10% of inactivated fetal calf serum) and transported to the laboratory for further processing.
3.2. Bacterial Strains
PCR was set up using positive control DNA from C. trachomatis serovar L2 type strain 434/Bu (ATCC VR-902B). For the negative control, sterile deionized water was used as the PCR template instead of nucleic acid.
3.3. DNA Extraction
The DNA was isolated using a KiaSpin PCR Template Purification Kit, (Kiagen, Iran) according to the manufacturer’s instructions. Briefly, samples were vortexed and centrifuged for 30 minutes at 14000 × g. Then, DNA purification was performed, 200 μL of each specimen was transferred and mixed with 300 μL of buffer LB and 2 μL of DNA/RNA carrier and 5 μL of proteinase K in a 1.5 mL microcentrifuge tube and incubated at 65°C for 10 minutes. After incubation, 100 μL of isopropanol was added to the tubes, mixed and centrifuged for 2 minutes at 5000 × g. The supernatant was applied to the spin column and centrifuged at 12000 × g for 1 minute, after which the filtrate was discarded and 500 μL of buffers IRB and 600 μL of WB were added to each spin column and centrifuged again at 12000 × g for 1 minute. Finally, 100 μL of buffer EB was used to elute the DNA. They were kept frozen at -20°C for further use.
3.4. PCR Assay
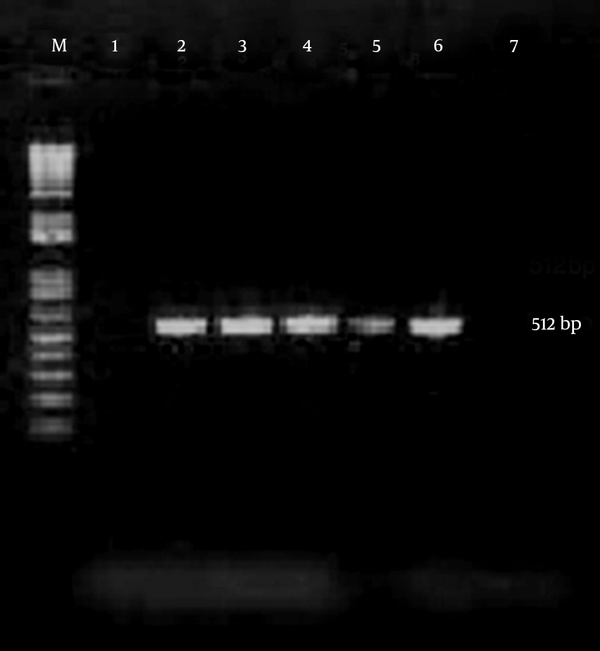
Chlamydia trachomatis was detected by a PCR designed in the laboratory to amplify a sequence in the cryptic plasmid generating a fragment of about 512 base pair. The specificity of the primer set was confirmed by BLAST search in GenBank. Primers were HL1 forward, 5’-TAGAGATAGGAAACCAACTC-3’and HL2 reverse, 5’CTCGGGTTAATGTTGCATGA-3’ (13) (CinnaGen, Tehran, Iran). Amplification reactions were performed in a final volume of 25 µL containing 5 µL of sample, 12.5 µL of the Master mix PCR solution (CinnaGen, Tehran, Iran), and 1 µL of each reverse and forward primers. The reaction mixture was incubated for 5 minutes at 94°C, followed by 40 cycles of 1 minute at 94°C, 1 minute at 45°C, and 1 minute at 72°C, and a final elongation step of 7 minutes at 72°C. The PCR products were analyzed by electrophoresis in the 1% TBE buffer through 1.5% agarose gel, stained with ethidium bromide 0.5 µg/mL and DNA bands were visualized using a UV transilluminator (Figure 1).
PCR Amplification of Chlamydia trachomatis From Endocervical Samples
3.5. Statistical Analysis
Data were analyzed using SPSS version 16 and the outcome variables including prevalence rates and their 95% Confidence Intervals (CIs) were calculated. The Chi-square or Fisher’s exact test was used to analyze the categorical data. A P value of < 0.05 was considered statistically significant.
4. Results
The age of the 255 subjects ranged 17 - 35 years with a mean of 27.3 ± 4.37 years. Thirty six percent of the participants were ≤ 25 years old. The majority of them were married or living with a partner (98%), had high school education or higher (74%). The prevalence of C. trachomatis was 2.4% (95% confidence interval [CI] 0.54 - 4.26%) with a mean of 25.8 ± 6.17 years; 50% (3/6) of the subjects with a positive Chlamydia test result were under the age of 25. Of the 92 subjects in the ≤ 25-year-old group, 3 (3.3%) were positive for C. trachomatis compared to three out of 163 (1.8%) in the > 25-year-group; 83.3% (5/6) of the subjects with a positive Chlamydia test result were older than 16 at first sexual encounter and all of the infected subjects lived in the city.
The most general symptoms among C. trachomatis infected young subjects were vaginal discharge (66.6%) and lumbar pain (50%). None of the infected subjects had any urinary tract symptoms. No significant relationships were found between C. trachomatis infection and the risk factors, probably reflecting the small number of those who were positive in the test.
5. Discussion
The prevalence of C. trachomatis genital infections among young females in Kashan, Iran was 2.4%. The previous published reports from Iran showed various prevalence of 2% to 11% (14). The study by Chamani-Tabriz et al. on the prevalence of Chlamydia infection in females aged 15 - 42 attending obstetrics and gynecology clinics in Tehran, from May 2003 to October 2003 showed that 12.6% were positive for Chlamydia by PCR (15). In another study on the females < 45 years attending gynecology clinics in Babol, Iran, the infection prevalence was reported 11.6% (16). European Centre for Disease Prevention and Control (ECDC) described the prevalence ranging from 1.4% to 3.0% among females aged 15 - 40 years (17). The prevalence of C. trachomatis infection and the risk factors in individuals < 35 years old attending sexual health clinics in Barcelona province, Spain, in 2006 was 3.8% and the prevalence was significantly higher in those under 25 years (5.8%) compared with the ones aged 25 - 35 (1.6%) (18). Another study in Melbourne, Australia, showed that the Chlamydia prevalence was 3.7% among sexually active females aged 18 - 24 years compared with 0.2% among 25 - 35-year-old ones (19).
A study among the Brazilian females referred to sexually transmitted diseases (STD) Clinic showed that 68% of the infected ones aged 15 - 25 year (13). In the current study, the C. trachomatis prevalence in females ≤ 25 years (3.2%) was similar to those of reported in Australia 4.9% (20), Spain 4% (21), France 3.6% (22) and the United Kingdom 3% (23). The current CDC guidelines recommend annual screening for Chlamydia in sexually active females ≤ 25 years and the ones > 25 years old who are at increased risk (24). Delayed treatment can cause irreparable damage to the fetus. Screening is especially important in pregnant females who are at risk of transmission of their infection to the fetus (25). Chlamydia trachomatis infection is the main causative agent of abortion (26). In the current study, abortions were reported by 16.7% of females with positive test results. Early detection and appropriate treatment of C. trachomatis can prevent the development of the disease after abortion (27).
Prevalence of C. trachomatis was found to be low among females in Kashan, Iran. The reason for C. trachomatis infection low prevalence in the population under study was the scarcity of pre-marriage sexual intercourse frequency in females, having stable sexual intercourse only after marriage. To date it was the first study in Kashan to determine the prevalence of endocervical C. trachomatis infection in young females. There were several limitations to the study, which should be considered in the interpretation of the results. There were no data on specific patient-reported symptoms. Data on sexual behavior was not specifically collected for the current study. Knowing a patient’s number of sexual partners, sexual activity before and after STI diagnosis, and frequency of condom use may help identify the patients who are at increased infection risk. The current study sample size was not large enough.
Chlamydia prevalence was low in young sexually-active females, but there is a significant difference regarding Chlamydia infection in females younger than 25 years. The new information provides evidence for the need to implement active opportunity to screen young sexually active females and the patients in all socioeconomic groups. All sexually active females should be offered a C. trachomatis test when attending a physician.
Acknowledgements
References
-
1.
Kalwij S, Macintosh M, Baraitser P. Screening and treatment of Chlamydia trachomatis infections. BMJ. 2010;340:c1915. [PubMed ID: 20410164]. https://doi.org/10.1136/bmj.c1915.
-
2.
Al-Moushaly A. Recent acquisitions in the medical treatment of infertility caused by Chlamydia Trachomatis. J Med Life. 2013;6(2):168-70. [PubMed ID: 23904877].
-
3.
Cecil JA, Howell MR, Tawes JJ, Gaydos JC, McKee KTJ, Quinn TC, et al. Features of Chlamydia trachomatis and Neisseria gonorrhoeae infection in male Army recruits. J Infect Dis. 2001;184(9):1216-9. [PubMed ID: 11598849]. https://doi.org/10.1086/323662.
-
4.
Sexually transmitted diseases quarterly report: genital infection with Chlamydia trachomatis in England and Wales. Commun Dis Rep CDR Wkly. 1995;5(26):122-3. [PubMed ID: 7655545].
-
5.
Prevalence and incidence of selected sexually transmitted infections, Chlamydia trachomatis, Neisseria gonorrhoeae, syphilis and Trichomonas vaginalis. Methods and results used by WHO to generate 2005 estimates. World Health Organization; 2005. Available from: http://whqlibdoc.who.int/publications/2011/9789241502450_eng.pdf.
-
6.
Su WH, Tsou TS, Chen CS, Ho TY, Lee WL, Yu YY, et al. Diagnosis of Chlamydia infection in women. Taiwan J Obstet Gynecol. 2011;50(3):261-7. [PubMed ID: 22030037]. https://doi.org/10.1016/j.tjog.2011.07.001.
-
7.
Gras MJ, Weide JF, Langendam MW, Coutinho RA, van den Hoek A. HIV prevalence, sexual risk behaviour and sexual mixing patterns among migrants in Amsterdam, The Netherlands. AIDS. 1999;13(14):1953-62. [PubMed ID: 10513655].
-
8.
Mullick S, Watson-Jones D, Beksinska M, Mabey D. Sexually transmitted infections in pregnancy: prevalence, impact on pregnancy outcomes, and approach to treatment in developing countries. Sex Transm Infect. 2005;81(4):294-302. [PubMed ID: 16061534]. https://doi.org/10.1136/sti.2002.004077.
-
9.
Meyers DS, Halvorson H, Luckhaupt S, US Preventive Services Task Force. Screening for Chlamydial infection: an evidence update for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;147(2):135-42. [PubMed ID: 17576995].
-
10.
Low N, Bender N, Nartey L, Shang A, Stephenson JM. Effectiveness of Chlamydia screening: systematic review. Int J Epidemiol. 2009;38(2):435-48. [PubMed ID: 19060033]. https://doi.org/10.1093/ije/dyn222.
-
11.
Van Dyck E, Ieven M, Pattyn S, Van Damme L, Laga M. Detection of Chlamydia trachomatis and Neisseria gonorrhoeae by enzyme immunoassay, culture, and three nucleic acid amplification tests. J Clin Microbiol. 2001;39(5):1751-6. [PubMed ID: 11325985]. https://doi.org/10.1128/JCM.39.5.1751-1756.2001.
-
12.
Masek BJ, Arora N, Quinn N, Aumakhan B, Holden J, Hardick A, et al. Performance of three nucleic acid amplification tests for detection of Chlamydia trachomatis and Neisseria gonorrhoeae by use of self-collected vaginal swabs obtained via an Internet-based screening program. J Clin Microbiol. 2009;47(6):1663-7. [PubMed ID: 19386838]. https://doi.org/10.1128/JCM.02387-08.
-
13.
Lima HE, Oliveira MB, Valente BG, Afonso DA, Darocha WD, Souza MC, et al. Genotyping of Chlamydia trachomatis from endocervical specimens in Brazil. Sex Transm Dis. 2007;34(9):709-17. [PubMed ID: 17414937]. https://doi.org/10.1097/01.olq.0000258399.27873.d9.
-
14.
Haghighi Hasanabad M, Mohammadzadeh M, Bahador A, Fazel N, Rakhshani H, Majnooni A. Prevalence of Chlamydia trachomatis and Mycoplasma genitalium in pregnant women of Sabzevar-Iran. Iran J Microbiol. 2011;3(3):123-8. [PubMed ID: 22347594].
-
15.
Chamani-Tabriz L, Tehrani MJ, Akhondi MM, Mosavi-Jarrahi A, Zeraati H, Ghasemi J, et al. Chlamydia trachomatis prevalence in Iranian women attending obstetrics and gynaecology clinics. Pak J Biol Sci. 2007;10(24):4490-4. [PubMed ID: 19093517].
-
16.
Bakhtiari A, Firoozjahi A. Chlamydia trachomatis infection in women attending health centres in Babol: prevalence and risk factors. East Mediterr Health J. 2007;13(5):1124-31. [PubMed ID: 18290406].
-
17.
Chlamydia control in Europe. European Centre for Disease Prevention and Control; 2009. Available from: http://ecdc.europa.eu/en/publications/Publications/0906_GUI_Chlamydia_Control_in_Europe.pdf.
-
18.
Corbeto EL, Lugo R, Martro E, Falguera G, Ros R, Avecilla A, et al. [Prevalence and determining factors of acquiring C. trachomatis infection among adolescents and young adults in Catalonia]. Enferm Infecc Microbiol Clin. 2011;29(2):96-101. [PubMed ID: 21324559]. https://doi.org/10.1016/j.eimc.2010.08.011.
-
19.
Hocking JS, Willis J, Tabrizi S, Fairley CK, Garland SM, Hellard M. A Chlamydia prevalence survey of young women living in Melbourne, Victoria. Sex Health. 2006;3(4):235-40. [PubMed ID: 17112433].
-
20.
Walker J, Fairley CK, Bradshaw CS, Tabrizi SN, Chen MY, Twin J, et al. 'The difference in determinants of Chlamydia trachomatis and Mycoplasma genitalium in a sample of young Australian women'. BMC Infect Dis. 2011;11:35. [PubMed ID: 21284887]. https://doi.org/10.1186/1471-2334-11-35.
-
21.
Fernandez-Benitez C, Mejuto-Lopez P, Otero-Guerra L, Margolles-Martins MJ, Suarez-Leiva P, Vazquez F, et al. Prevalence of genital Chlamydia trachomatis infection among young men and women in Spain. BMC Infect Dis. 2013;13:388. [PubMed ID: 23968487]. https://doi.org/10.1186/1471-2334-13-388.
-
22.
Goulet V, de Barbeyrac B, Raherison S, Prudhomme M, Semaille C, Warszawski J, et al. Prevalence of Chlamydia trachomatis: results from the first national population-based survey in France. Sex Transm Infect. 2010;86(4):263-70. [PubMed ID: 20660590]. https://doi.org/10.1136/sti.2009.038752.
-
23.
Fenton KA, Korovessis C, Johnson AM, McCadden A, McManus S, Wellings K, et al. Sexual behaviour in Britain: reported sexually transmitted infections and prevalent genital Chlamydia trachomatis infection. Lancet. 2001;358(9296):1851-4. [PubMed ID: 11741624]. https://doi.org/10.1016/S0140-6736(01)06886-6.
-
24.
Workowski KA, Berman S, Centers for Disease C. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110. [PubMed ID: 21160459].
-
25.
US Preventive Services Task Force. Screening for gonorrhea: recommendation statement. Ann Fam Med. 2005;3(3):263-7. [PubMed ID: 15928231]. https://doi.org/10.1370/afm.337.
-
26.
Wilkowska-Trojniel M, Zdrodowska-Stefanow B, Ostaszewska-Puchalska I, Redzko S, Przepiesc J, Zdrodowski M. The influence of Chlamydia trachomatis infection on spontaneous abortions. Adv Med Sci. 2009;54(1):86-90. [PubMed ID: 19403438]. https://doi.org/10.2478/v10039-009-0008-5.
-
27.
Soper DE. Pelvic inflammatory disease. Obstet Gynecol. 2010;116(2 Pt 1):419-28. [PubMed ID: 20664404]. https://doi.org/10.1097/AOG.0b013e3181e92c54.