Abstract
Background:
Plants are considered as promising sources of new antibacterial agents as well as bioassay guided fractionation.Objectives:
In the present work, the antibacterial properties, especially against methicillin-resistant Staphylococcus aureus (MRSA), of Bromus inermis inflorescence was studied, using the bioassay guided fractionation as well as the bio-autographic method.Materials and Methods:
The plant organic extract was prepared via maceration in methanol, followed by the fractionation using n-hexane. The extracts were subjected for minimum inhibitory concentrations (MICs) against some human pathogenic bacteria via standard broth micro-dilution assay. Thereafter, a bio-autographical method was applied using the high performance thin layer chromatography (HPTLC) coupled with agar overlay assays for the primary characterization and identification of bioactive substance (s).Results:
Through the bioassay guided fractionation method, the greatest antibacterial activities were related to the n-hexane extract. It was also revealed that the effective anti-MRSA agent of the assessed plant was a relatively polar substance with an MIC value of about 8 μg/mL against the tested MRSA strain (in comparison with the MIC value of 32 μg/mL for chloramphenicol).Conclusions:
As a result of the full range UV-Vis scanning of the responsible band in the HPTLC experiments (200-700 nm), the flavonoid was the most imaginable natural compound.Keywords
Chemical Fractionation Methicillin-Resistant Staphylococcus aureus Flavonoid Anti-bacterial Agents
1. Background
The emergence of multidrug-resistant human pathogens is considered as a major concern worldwide. Bacterial pathogens become resistant to the current therapies and transfer the resistance elements to each other via sophisticated systems (1). Plants have somehow known systemic or local defense mechanisms. For this necessity, they produce some family of secondary metabolites, reactive oxygen species, and antimicrobial peptides against bacterial pathogens. Production of these antimicrobials may be constitutive or inducible in different environmental conditions. Moreover, some plant antimicrobials may be reserved or secreted by organisms (2-4).
Weed can be a great source of antibacterial compounds regarding its ability to survive in varied difficult growth conditions (2, 4, 5), including contamination with bacterial pathogens. According to the antibacterial properties of some Iranian weeds, Bromus inermis Leyss extract exhibits a remarkable inhibitory effect on some assessed bacterial strains. This plant is a potent weed and belongs to the Poaceae family (6).
2. Objectives
In the present study, the bioassay-guided fractionation method was used to monitor the inflorescence and potential antibacterial effects of the B. inermis Leyss extract.
3. Materials and Methods
3.1. Microbial Strains
In this study, microbial strains including Staphylococcus aureus ATCC 25923, methicillin-resistant S. aureus (MRSA) and the vancomycin-resistant strain of Enterococcus faecium (kindly dedicated by Prof. M. M. Feizabadi), E. faecalis ATCC 29212, Escherichia coli ATCC 25922, Salmonella enteritidis, Pseudomonas aeruginosa PTCC 1430, P. aeruginosa JH10 and P. aeruginosa JH5 (kindly dedicated by Prof. Jose L. Martínez) were studied.
3.2. Plant and Chemicals Preparation
The inflorescence of B. inermis was obtained from Pakan Bazr Co., Isfahan, Iran. The organic solvents were purchased from Caledon Lab (Ontario, Canada). The reagents and the high performance thin layer chromatography (HPTLC) silica plates were provided by Merck Co., (KGaA, Darmstadt, Germany).
3.3. Plant Extraction
Selected uniform plants were milled and soaked with methanol in a dark glass under constant stirring for 48 hours at room temperature. Thereafter, the mixture was filtered through a filter paper and the filtrate was dried at 40°C using a rotary evaporator instrument (Heidolph Co., UK). Of the remaining, 1 mg was dissolved in 5 mL of water. The extract was further extracted using equal volumes of n-hexane solvent for removing oily and nonpolar ingredients the process was repeated twice more. The organic extract was dried under a reduced pressure at 40°C and was kept at 4°C. In a parallel experiment, extraction was carried out using n-hexane, following methanol to extract the polar compounds.
3.4. Antimicrobial Assay
A stock of methanol as well as the n-hexane extract powder were dissolved in dimethyl sulfoxide (DMSO) and stored at 4°C. Antimicrobial susceptibility test was performed using the broth micro-dilution method for determination of the minimum inhibition concentration (MIC) and the minimum bactericidal concentration (MBC), as recommended by the Clinical and Laboratory Standard Institute of the US (CLSI) (7), with some modifications. Briefly, an 18-hour culture of each test microorganism was used for the susceptibility tests. A serial dilution of each extract (from 64 mg/mL to 0.006 mg/mL) was prepared using sterile, Muller Hinton broth (MHB) (Merck & Co., Inc. Germany), containing 0.5% Tween 80. Tween 80 was used as a co-solvent for better solvation of the plant extract in the MHB medium.
Microorganisms were added to each well to final concentrations of 5 × 105 CFU/mL of bacteria. The inoculated 96-well plates were incubated for 20 hours at 37°C for all the bacteria, but at 35°C for the MRSA strain. The results were recorded as the lowest concentration that could inhibit visible growth of microorganisms (MICs). The plant extracts were chromatic, so in some cases it was necessary to use a growth indicator instead of checking the wells turbidity. Therefore, the Resazurin (Sigma-Aldrich, US.) reagent was used as a growth indicator, as illustrated by Sarker et al. (8). MBCs were determined by sub-culturing 100 µL of each negative well on nutrient agar plates. The results were recorded after 24 hours of incubation at appropriate temperatures, as the lowest concentrations that could kill 99.99 % of the initial microorganisms was determined during the quality control step, as recommended by CLSI (5). All the experiments were performed in triplicates.
3.5. High Performance Thin Layer Chromatography
HPTLC was carried out using CAMAG HPTLC instrument (Muttenz, Switzerland). A 1-mg/mL clear solution of n-hexane extract was dissolved in an n-hexane and ethyl acetate (1:1, v/v) solvent mixture and was spotted onto aluminum silica gel plates PF254 under N2, using Linomat 5, under 0.2 µL/sec speed. Each 6-mm band contained about 30 µg of the extract. A variety of mobile phases were used for achieving the highest separation resolution. All the plates were scanned in the range of 180-800 nm, using CAMAG TLC scanner III (9).
3.6. Bio-Autography Experiments
The developed TLC plates with the highest resolutions were assessed against an important antibiotic-resistant bacterium, a clinical strains of MRSA. In brief, a 50°C Mueller Hinton agar (MHA) medium, containing 107 CFU/mL of bacteria, was poured into silica plates together with the resolved bands and then incubated for 18 hours at 37°C (10). An MHA medium supplemented with 2% NaCl was also used. The effective and ineffective bands were chosen through comparison of the normal scanned plates (under 254 nm, 366 nm and visible light) with the bio-autographic plates. Next, the effective and ineffective bands were cut and subjected against the bacteria.
3.7. Semi-Purification of Effective Substance (s) Using Preparative Thin Layer Chromatography
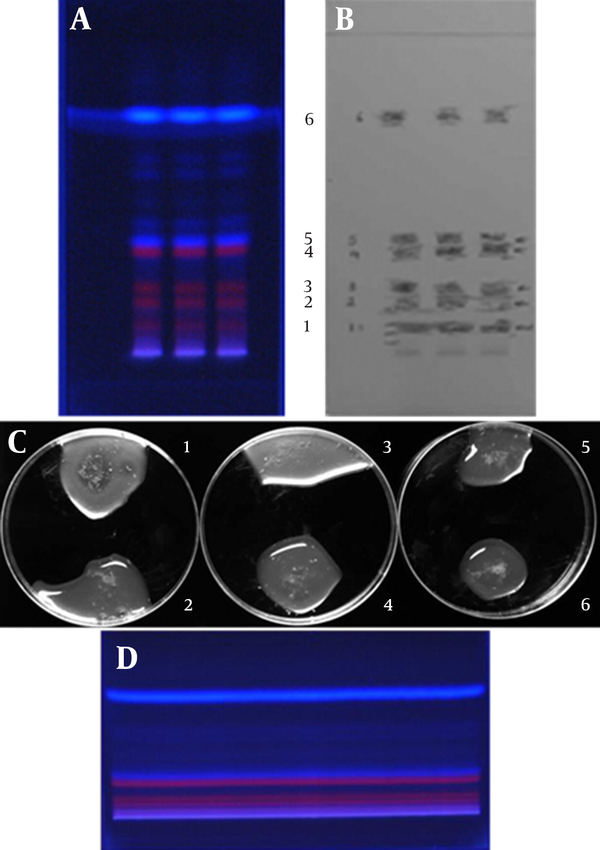
A total of 1500 µg n-hexane extract solution was loaded into aluminum silica gel plates PF254 (Figure 1 D) and the bands were resolved, as mentioned in section 3-5. The effective band (band 1 in Figure 1 A) was scraped from the developed sheet, immersed in methanol, and dissolved using 30 seconds of sonication. MIC of the filtrate was determined against the MRSA strain.
3.8. Total Flavonoid Content Determination
Total flavonoid content of the semi-purified sample was determined by the aluminum chloride colorimetric method and the total flavonoid content was expressed as mg quercetin equivalents (QE) (11).
Developed Thin Layer Chromatography Plate and Primary Bio-Autography of Bands Against Methicillin-Resistant Staphylococcus aureus Strain
4. Results and Discussion
The primary screening of 15 different methanol and n-hexane plant extracts against 12 different microorganisms (a collection of bacteria and yeast, data are not shown), indicated the inflorescence of B. inermis Leyss. Those results demonstrated a remarkable inhibition of Gram-positive bacteria, but a relatively slight activity against Gram-negative ones. The antibacterial effects of plant extracts are presented in Table 1. It is apparent that not only the overall antibacterial effects of the n-hexane extract were better than the methanol extract, but also it had better inhibitory effects against Gram-positives rather than Gram-negative bacteria. This extract inhibited the growth of S. aureus and E. faecalis at concentrations of 0.06 and 0.125 mg/mL, respectively. However, the best results against the Gram-negative strains were achieved by E. coli and S. enteritidis strains (1 and 2 mg/mL, respectively).
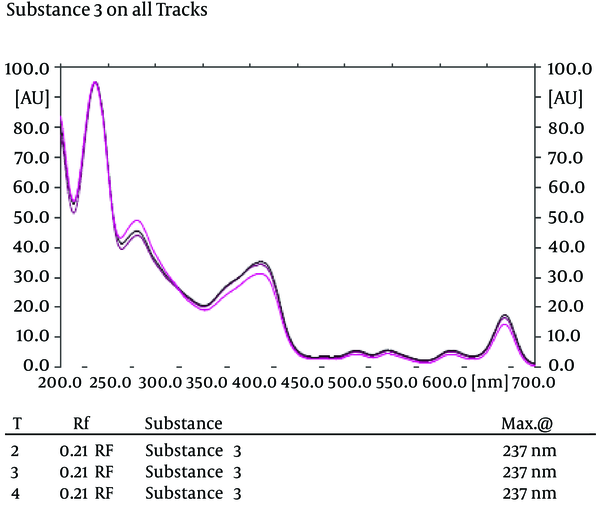
In the HPTLC experiments, the best resolution of bands was gained using n-hexane: ethyl acetate (4:1 v/v) developing solvent system (the chromatography was repeated twice) (Figure 1 A). According to the Bio-autography experiments, there were clear zones of inhibition around the bottom of the developed silica plate, which for the MRSA strain, they were repeated using MHA supplemented by 2% NaCl (Figure 2). As shown in Figure 1 (A, B and C), for identification of the bands containing effective antibacterial substances, different bands were cut from the developed plates and assessed against the MRSA strain. The results revealed that band 1 contained anti-MRSA substance (s). Full range scanning of this band showed that it may be a member of flavonoids. As shown in Figure 3, the maximum absorbance of the substance was in the range of 250-254 nm, which seems to be the maximum absorbance of flavonoids (12).
The semi-purified antibacterial substance was subjected to more study of its total flavonoid content and antibacterial activity. Its total flavonoid content was about 86 mg QE/g. The anti-bacterial effects as well as the total flavonoid content results may confirm the flavonoid identity of the chosen substance (s). While the MRSA strain could be inhibited by 32 μg/mL of chloramphenicol, MIC of semi-purified substance was about 8 μg/mL against the tested bacterium.
| Methanol Extract | n-Hexane Extract | |
|---|---|---|
| S. aureus | ||
| MIC | 4 | 0.06 |
| MBC | 16 | 0.5 |
| MRSA strain | ||
| MIC | 16 | 0.125 |
| MBC | 32 | 2 |
| VRE strain | ||
| MIC | 32 | 0.5 |
| MBC | 32 | 8 |
| E. faecium | ||
| MIC | 16 | 32 |
| MBC | 0.5 | 4 |
| E. faecalis | ||
| MIC | 8 | 16 |
| MBC | 0.125 | 0.5 |
| E. coli | ||
| MIC | 16 | 1 |
| MBC | 16 | 4 |
| S. enteritidis | ||
| MIC | 18 | 2 |
| MBC | 16 | 4 |
| P. aeruginosa JH5c | ||
| MIC | 64 | 16 |
| MBC | > 64 | 64 |
| P. aeruginosa JH10d | ||
| MIC | 32 | 8 |
| MBC | > 64 | 64 |
| P. aeruginosae | ||
| MIC | 16 | 4 |
| MBC | 64 | 16 |
Bio-Autography of n-Hexane Extract Resolved in Silica Plate Against Methicillin-Resistant Staphylococcus aureus

Scan of the Effective Band in 200-700 nm in Three Repeats
Weeds can be the promising sources of finding new and more effective antibacterial substances because of their characteristics related to their dominancy in severe growth and living conditions. To the best of our knowledge, it was the first report on such great antibacterial activity regarding the inflorescence of B. inermis weed plant. It can hypothesize that further investigations with HPLC fractionation and subsequent nuclear magnetic resonance (NMR) analysis could reveal the identity of the effective substances in our continuous research. Such antibacterial substances can also be considered as the lead compounds for semi-synthesis of new effective antibiotics.
Acknowledgements
References
-
1.
Wenzel RP, Edmond MB. The impact of hospital-acquired bloodstream infections. Emerg Infect Dis. 2001;7(2):174-7. [PubMed ID: 11294700]. https://doi.org/10.3201/eid0702.700174.
-
2.
Maroti G, Kereszt A, Kondorosi E, Mergaert P. Natural roles of antimicrobial peptides in microbes, plants and animals. Res Microbiol. 2011;162(4):363-74. [PubMed ID: 21320593]. https://doi.org/10.1016/j.resmic.2011.02.005.
-
3.
Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12(4):564-82. [PubMed ID: 10515903].
-
4.
Thevissen K, Kristensen HH, Thomma BP, Cammue BP, Francois IE. Therapeutic potential of antifungal plant and insect defensins. Drug Discov Today. 2007;12(21-22):966-71. [PubMed ID: 17993416]. https://doi.org/10.1016/j.drudis.2007.07.016.
-
5.
Odintsova TI, Rogozhin EA, Baranov Y, Musolyamov A, Yalpani N, Egorov TA, et al. Seed defensins of barnyard grass Echinochloa crusgalli (L.) Beauv. Biochimie. 2008;90(11-12):1667-73. [PubMed ID: 18625284]. https://doi.org/10.1016/j.biochi.2008.06.007.
-
6.
Watson L, Dallwitz MJ. The grass genera of the world: descriptions, illustrations, identification, and information retrieval; including synonyms, morphology, anatomy, physiology, phytochemistry, cytology, classification, pathogens, world and local distribution, and references. 1992, [updated 5th February 2014]. Available from: http://delta-intkey.com.
-
7.
Jorgensen JH, Turnidge JD. Susceptibility test methods: Dilution and disk diffusion methods. In: Murray PR, Baron EJ, Jorgensen JH, Landry MR, Pfaller MA, editors. Manual of Clinical Microbiology. 9 ed. Washington DC: ASM PRESS; 2007. p. 1152-72.
-
8.
Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods. 2007;42(4):321-4. [PubMed ID: 17560319]. https://doi.org/10.1016/j.ymeth.2007.01.006.
-
9.
Waksmundzka-Hajnos M, Sherma J, Kowalska T. Thin Layer Chromatography in Phytochemistry. Taylor & Francis; 2008.
-
10.
Mirhosseini M, Nahvi I, Emtiazi G, Tavassoli M. Characterisation of anti-Listeria monocytogenesbacteriocins fromEnterococcus faeciumstrains isolated from dairy products. Int J Dairy Tech. 2010;63(1):55-61. https://doi.org/10.1111/j.1471-0307.2009.00543.x.
-
11.
Huck CW, Buchmeiser MR, Bonn GK. Fast analysis of flavonoids in plant extracts by liquid chromatography-ultraviolet absorbance detection on poly(carboxylic acid)-coated silica and electrospray ionization tandem mass spectrometric detection. J Chromatogr A. 2002;943(1):33-8. [PubMed ID: 11820278].
-
12.
Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64(4):555-9. https://doi.org/10.1016/s0308-8146(98)00102-2.