Abstract
Keywords
Basal Cell Nevus Syndrome Lower Extremity Tomography X-Ray Computed
1. Introduction
Gorlin-Goltz syndrome (GGS) is an infrequent autosomal dominant genetic disorder as a distinct entity consisting of ectodermal and mesodermal abnormalities. The gene for GGS is located on chromosome 9 (9q22.3-9q31) and it has been identified as PTCH (Drosophila segment polarity gene patched), the human homolog of the Drosophila "patched" gene PTC (a tumor suppressor gene) (1). The diagnostic criteria of GGS consist of major and minor criteria depending on the clinical and radiological signs and symptoms. The diagnosis is established if two major criteria or one major and two minor criteria are present (2). The patient is most commonly characterized by a broad face, rib malformations, and an extraordinary predisposition to basal cell carcinoma before the age of 15-30. To our knowledge, long cortical lamellar and cystic remodeling of the lower extremity bones have never been described.
2. Case Report
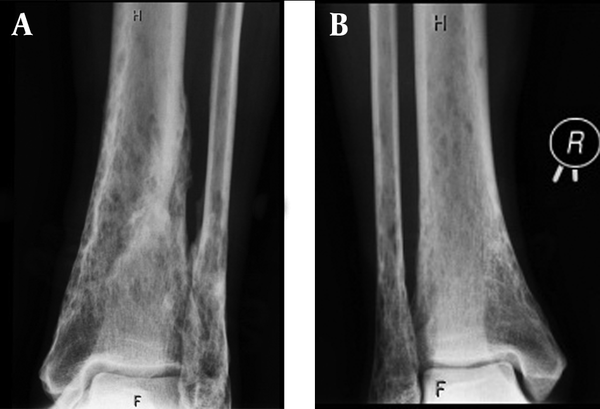
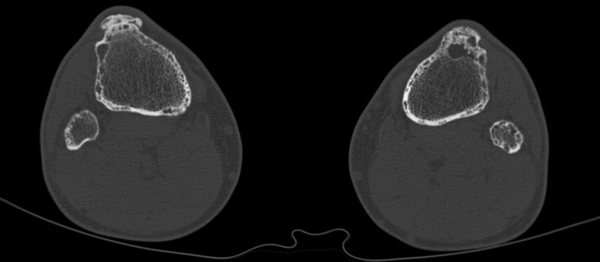
A 52-year-old man was seen at our department with the history of GGS. GGS was determined based on the presence of three major criteria (Table 1) (3-5). Therefore, genetic confirmation was not necessary. The first basal cell cancer were seen on the nape of his neck and below his nose at the age of 17 years. Later in his twenties, more tumors appeared on his trunk and face, which reached 2-6 cm in diameter. The tumors were irradiated several times on the patient’s chest, back and nape of the neck. Surgical excisions were performed on his face, chest and back and intralesional interferon alpha 2b was injected. The patient was given isotretinoin and he was treated by photodynamic therapy and liquid nitrogen cryotherapy. Additionally, dental cyst and vertebrobasilar insufficiency were also present in the patient. The laboratory tests (including serum calcium, phosphate and alkaline phosphatase) were normal. At the age of 48 years, a skull X-ray showed transparent areas with blurred margins and calcification of the cerebral falx. The patient underwent radiographs of the skull, hand, thoracic and lumbar spine recently. These radiographies showed the pathological bone characteristics of GGS: marked hyperostosis (cortical thickening) of the skull and calcification of the cerebral falx; and calcification of the anterior longitudinal ligament on the thoracic part. Hand radiography images showed degenerative changes and cystic lesions of hand bones, and distal parts of the ulna and radius. Skull computed tomography (CT) confirmed the cerebral falx and U-shaped tentorial calcifications without parasellar manifestation and brachycephaly. The tibia and fibula radiographs showed thin cortical and subcortical cystic lesions (Figure 1). CT of the femur, tibia and fibula indicated thin cortical bones mainly on the distal parts of the tibia and fibula, cortical lamellar and cystic remodeling, especially on the left tibia and distal part of the femur (Figure 2).
| Major Criteria |
|---|
| More than two basal cell carcinomas (BCC) or one BCC in a person < 20 years |
| Odontogenic keratocysts of the jaw |
| Three or more palmar or plantar pits |
| Ectopic (bilamellar) calcifications or early (< 20 years) calcification of the falx cerebri and tentorium |
| Bifid, fused, or markedly splayed ribs |
| First-degree relative with Gorlin-Goltz syndrome |
| Minor Criteria |
| Macrocephaly |
| Congenital malformations (cleft lip or palate, frontal bossing, coarse facies, hypertelorism, eye anomaly such as cataract, coloboma, microphthalmia, nystagmus) |
| Other skeletal abnormalities (Sprengel deformity, pectus deformity, polydactyly, syndactyly) |
| Radiologic abnormalities [bridging of the sella turcica, vertebral anomalies (e.g. hemivertebrae, fusion or elongation of the vertebral bodies) modeling defects, flame-shaped lucencies of the hands and feet] |
| Ovarian and cardiac fibroma or myeloblastoma or medulloblastoma |
| Chest Radiography |
|---|
| Bronchogenic cysts |
| Acute respiratory distress syndrome (ARDS) due to hyaline membrane disease |
| Skeletal Radiography |
| Cystic lesions (polyostotic bone cysts) |
| Skull (frontal, temporal and parietal bossing) |
| Spine (scoliosis, fusion of vertebrae or hemivertebrae, bifid wedges fused vertebrae, bone spur formation, calcification of nuchal ligament) |
| Ribs (bifid, fused, splayed, missing, misshapen) |
| Upper extremity and shoulder (sindactyly, polydactyly, short fourth metacarpus, flame-shaped lucencies of the metacarpi and/or phalanges, modeling deformities of the phalanges, Sprengel shoulder, widened ends of clavicles) |
| Skull Radiography |
| Central Nervous and Auditory System |
| Macrocephaly |
| Ectopic calcification (in falx cerebri, meninx, tentorium cerebelli, sella turcica, atlanto-occipital ligament, petrosellar ligament) |
| Midline shift of calcified choroid plexus due to mass effect (medulloblastoma, multiform glioblastoma, large meningioma) |
| Otosclerosis in the middle ear |
| Abnormal frontal sinus aeration |
| Dental abnormalities |
| Cystic lesions (bone cysts, odontogenic keratocysts, idiopathic pseudocyst) |
| Bone lesions (maxillary fibrosarcoma, cleft palate and lip, high-arched palate, hyperplasia of the mandibular coronoid processes) |
| Teeth lesions (agenesis, ectopy, heterotopy, impactation, odontogenic myxoma) |
| Abdominal Radiography |
| Ovarian calcifications |
| Bone lesions (see above) |
A 52-year-old man with Gorlin-Goltz syndrome.
Tibia and fibula CT images showing thin cortical bones, cortical lamellar and cystic remodeling in the cortical and spongious compartments.
3. Discussion
The diagnosis of the patient was established on the presence of bone characteristics and the previous history of multiple basal cell carcinomas. The large number of basal cell carcinomas, the marked hyperostosis (cortical thickening) of the skull, calcification of the cerebral falx and tentorium, cystic lesions of extremity bones confirmed by radiography and CT match the major criteria of GGS (2, 3). Radiology has an important role in the diagnosis and management of the disease in order to make an early diagnosis of serious complications like tumors. Ultrasound scans during pregnancy also play a role in the early detection (4). Most of the bone lesions can be detected by CT scan. Magnetic resonance imaging is useful in the detection of soft tissue manifestations. Skeletal manifestations (including the calcification of the atlanto-occipital ligament) found on the radiography are also present and they are helpful in making the diagnosis (4). Chest, skeletal, skull and abdominal radiographies are useful in the detection of different manifestations of the disease (Table 2) (3). The patient was given isotretinoin, which is a retinoid, a vitamin A derivative. Retinoids have some anti-tumor effects other than their usual role of reducing sebum production. It has been known to cause premature epiphyseal closure in humans as an unwanted side effect of chronic treatment (6) and hyperostotic changes especially on the tibia (7). Risk of bone fractures was not changed with increasing doses or durations of treatment with isotretinoin in a nationwide study (8). In our particular case, the uncommon tibial and fibular osteal changes (cortical lamellar and cystic remodeling) do not show a clear image of a well-defined cyst in any location. Instead, they rather show a ”mouse eaten” like appearance of osteopenic reaction that is most probably due to medication, which the patient had been taking chronically. Therefore, these changes might be rather related to retinoid therapy than the presentation of GGS. Accordingly, these findings are not included in the criteria of GGS. Various skeletal abnormalities such as periosteal thickening, hyperostosis of the vertebral column, disk degeneration, osteoporosis, calcification of the spinal ligaments, and slender long bones were associated with retinoid therapy (9). In contrast, another study has not observed any association between retinoid therapy (chronic hypervitaminosis A) and bone mineral density (5). First-degree relatives should be examined for GGS in order to establish an early diagnosis. Radiographic examination protocol should include a skull radiography (large head, frontal and temporal bossing, and cleft palate) and chest radiography as well (bifid ribs and vertebral anomalies). Calcification of ovarian fibromas is also characteristic. Sometimes, if the major criteria such as basal cell carcinomas, jaw cysts, or cerebral falx calcification are absent until the teen years, other radiological manifestations of the disorder can permit early diagnosis in childhood (4). Patients suffering from GGS are recommended to undergo follow-up examinations in order to avoid the associated malignant tumors and other related pathologies (Table 2). However, the carcinogenic effect of radiation exposure must be taken into account. In conclusion, radiography is a useful method for detecting the characteristic findings of GGS. Specific cortical bone lesions of the lower extremity (thin cortical and subcortical cystic lesions) may occur and these abnormalities can be detected by plain radiography or CT, which may rather be attributed to retinoid treatment than GGS.
Acknowledgements
References
-
1.
Gorlin RJ. 2004 ASHG Award for Excellence in Human Genetics Education. And the band played on. Am J Hum Genet. 2005;76(2):216-8. [PubMed ID: 15714687].
-
2.
Gorlin RJ, Goltz RW. Multiple nevoid basal-cell epithelioma, jaw cysts and bifid rib. A syndrome. N Engl J Med. 1960;262:908-12. [PubMed ID: 13851319]. https://doi.org/10.1056/NEJM196005052621803.
-
3.
Lo Muzio L. Nevoid basal cell carcinoma syndrome (Gorlin syndrome). Orphanet J Rare Dis. 2008;3:32. [PubMed ID: 19032739]. https://doi.org/10.1186/1750-1172-3-32.
-
4.
Kimonis VE, Mehta SG, Digiovanna JJ, Bale SJ, Pastakia B. Radiological features in 82 patients with nevoid basal cell carcinoma (NBCC or Gorlin) syndrome. Genet Med. 2004;6(6):495-502. [PubMed ID: 15545745].
-
5.
McMullen EA, McCarron P, Irvine AD, Dolan OM, Allen GE. Association between long-term acitretin therapy and osteoporosis: no evidence of increased risk. Clin Exp Dermatol. 2003;28(3):307-9. [PubMed ID: 12780720].
-
6.
Standeven AM, Davies PJ, Chandraratna RA, Mader DR, Johnson AT, Thomazy VA. Retinoid-induced epiphyseal plate closure in guinea pigs. Fundam Appl Toxicol. 1996;34(1):91-8. [PubMed ID: 8937896].
-
7.
DiGiovanna JJ. Isotretinoin effects on bone. J Am Acad Dermatol. 2001;45(5):S176-82. [PubMed ID: 11606950]. https://doi.org/10.1067/mjd.2001.113721.
-
8.
Vestergaard P, Rejnmark L, Mosekilde L. High-dose treatment with vitamin A analogues and risk of fractures. Arch Dermatol. 2010;146(5):478-82. [PubMed ID: 20479294]. https://doi.org/10.1001/archdermatol.2010.59.
-
9.
Halkier-Sorensen L, Andresen J. A retrospective study of bone changes in adults treated with etretinate. J Am Acad Dermatol. 1989;20(1):83-7. [PubMed ID: 2913084].