Abstract
Background:
Imaging findings of adhesive capsulitis (AC) have been reported widely with the use of magnetic resonance imaging (MRI) and ultrasonography (US), although diagnosing AC is still based on clinical finding. However, the use of contrast-enhanced ultrasonography (CEUS) has not yet been reported in patients with AC.Objectives:
To validate the application of CEUS in patients with AC, and to compare CEUS findings with those of MRI.Patients and Methods:
Both shoulders of five patients with unilateral AC, who underwent MRI on the affected shoulder, were examined using CEUS (2 men, 3 women; mean age, 54.2 ± 8 years). CEUS was performed after bolus administration of the contrast agent, SonoVue (Bracco, Milan, Italy), followed by a saline flush. Enhancement of the rotator interval was evaluated using a visual enhancement score (0 to 2) and compared with the contrast-enhanced MRI findings. For quantitative analysis, an region of interest was established for each rotator interval, and time-intensity curves were analyzed with parameters including time-to-peak and peak intensity. The difference of peak intensity between the affected and unaffected shoulders was compared.Results:
Contrast enhancement of the rotator interval was notable in all CEUS of affected shoulders, whereas no evident enhancement was detected in all asymptomatic shoulders. The mean visual enhancement score of affected shoulders was 1.4 in CEUS and 2.0 in contrast-enhanced MRI. In quantitative analysis, the mean peak intensity was 5.45 ± 2.80 dB (mean time to peak, 30.6 ± 5.39 seconds) in affected shoulders, and 0.72 ± 0.91 dB in unaffected shoulders (P < 0.05).Conclusion:
CEUS was capable of demonstrating capsular inflammation in patients with AC, and this was comparable to MRI. CEUS could be a feasible imaging tool for evaluating patients with AC.Keywords
Adhesive Capsulitis Shoulder Contrast-Enhanced Ultrasonography Magnetic Resonance Imaging
1. Background
Adhesive capsulitis (AC), also known as frozen shoulder, is a condition of uncertain etiology characterized by pain and significant restriction of both active and passive shoulder motion that occurs in the absence of a known intrinsic shoulder disorder (1-3). Although the etiology is controversial, the underlying pathology of this condition is thought to be inflammation of the capsule subsynovial layer, which produces capsular fibrosis, contracture, and adhesion (3-5). Arthroscopic studies have revealed that these changes frequently involve the subscapularis bursa, rotator interval, and axillary pouch (3, 4). Imaging modalities have also reflected these changes in corresponding locations (6-13).
Diagnosing AC is mainly based on clinical findings, but with the wide use of imaging tools, many magnetic resonance imaging (MRI) and ultrasonography (US) findings have been reported. MRI findings that suggest AC include thickening and T2 hyperintensity in the inferior glenohumeral ligament, subcoracoid fat obliteration around the rotator interval, and contrast enhancement of the joint capsule (6-8). US findings include hypoechoic echotexture and increased vascularity within the rotator interval, and thickening of the coracohumeral or inferior glenohumeral ligaments (9-12). Although US is a more convenient and practical imaging tool, it has some limitations in evaluating the capsular changes of AC. The first is the limited sonic window for the joint capsule, which represents only a small dimension, making it typically difficult to recognize the overall changes. The second is the lower sensitivity of Doppler US for detecting hyperemia of the capsule, which is usually a slow flow in the small vessels (14, 15).
In this study, we used a second-generation ultrasound contrast agent to attempt to evaluate AC, and this agent was expected to depict the joint capsule enhancement more clearly. By using contrast-enhanced ultrasonography (CEUS), microcirculation detection is far more sensitive than with conventional Doppler methods (15). To our knowledge, this is the first study of CEUS application in assessing possible inflammation of the shoulder joint capsule in patients with AC.
2. Objectives
The purpose of this study was to apply CEUS in patients with AC, to evaluate CEUS findings, and compare them with MRI.
3. Patients and Methods
3.1. Subjects
This study was approved by the institutional review board and informed consent was obtained from all participants. From March 2012 to April 2012, five patients with the clinical diagnosis of AC, who underwent MRI on affected shoulders, were prospectively examined using CEUS on both shoulders on the same day.
The inclusion criteria were: (a) over 50% loss of movement of the shoulder joint compared with the unaffected side (forward elevation, external rotation, or internal rotation); (b) duration of complaints over one month; (c) radiographs demonstrating no pathologic findings (joint space loss, significant periarticular calcification, unrecognized shoulder subluxation/dislocation); and (d) MRI findings compatible with AC, including T2 hyperintensity and thickening of the axillary capsule, obliteration of the subcoracoid fat triangle, or thickening of the coracohumeral ligament (13). Physical examinations and clinical diagnoses were made by one orthopedic surgeon who had 11 years of experience with the shoulder subspecialty. Exclusion criteria were full-thickness rotator cuff tears that were identified on imaging, previous shoulder surgery, and contraindications for the ultrasound contrast agent.
3.2. MRI Analysis
The MRIs were performed on a 3.0-Tesla scanner TrioTim (Siemens, Erlangen, Germany) or Achieva (Philips, Bothell, Wash) with a dedicated shoulder coil. For the purpose of this study, enhancement was added and given by gadobutrol (Gadovist; Bayer Healthcare, Wayne, NJ) or gadoterate (Dotarem; Guerbet, Roissy, France) injection. The imaging protocol was as follows: oblique coronal and oblique sagittal T2-weighted fat-suppressed (3800 - 5200 ms/53 - 60 ms, repetition time [TR]/echo time [TE]; number of excitations [NEX], 2.0; 3.0 mm, slice thickness; 15 × 15 cm, field of view [FOV]; 390 - 450 × 310-330 matrix) and T1-weighted images, axial proton density-weighted fat-suppressed images (2800 - 3000 ms/30 - 36 ms, TR/TE; NEX, 1.0; 3 mm, slice thickness; 15 × 15 cm, FOV; 330 - 390 × 270 - 310 matrix), and contrast-enhanced fat-suppressed T1-weighted oblique coronal, oblique sagittal, and axial images (580 - 770 ms/13 - 23 ms, TR/TE; NEX, 2.0; 3 mm, slice thickness; 15 × 15 cm, FOV; 390 - 450 × 310 - 330 matrix). The intravenous cannulation was made by a 20-gauge catheter in the antecubital vein of the unaffected arm, considering the subsequent use for CEUS. Two musculoskeletal radiologists reviewed the images and scored the degree of rotator interval enhancement by consensus: 0, no enhancement; 1, partial inhomogeneous enhancement involving less than half of the rotator interval; and 2, diffuse homogeneous enhancement involving more than half of the rotator interval. All scorings were performed in the oblique sagittal image just lateral to the lateral edge of the bony coracoid process. Other MRI findings of AC, including thickening, T2 hyperintensity, and contrast enhancement of the axillary capsule, subcoracoid fat obliteration, and coracohumeral ligament thickening, were recorded (8). A basic review for assessment of supraspinatus tendon and glenoid labrum was also performed (16).
3.3. CEUS Imaging and Analysis
All CEUS examinations were performed by one expert musculoskeletal radiologist, using the same ultrasound machine (Phillips iU22, Phillips Medical System, Bothell, Wash) with a 5-12 MHz linear array transducer. All subjects were examined while seated with elbow flexion and shoulder extension in which the rotator interval was optimally visualized (9). We placed the transducer at the rotator interval with an oblique sagittal plane, in attempt to match the similar imaging section of the MRI, where we scored the degree of enhancement (Figure 1).
All subjects were examined while seated with elbow flexion and shoulder extension with the transducer at the rotator interval with an oblique sagittal plane, in which the rotator interval was optimally visualized.
Contrast administration was performed by injecting a 2.4 mL bolus of SonoVue (Bracco, Milan, Italy) directly through the 20-G cannula without using any extension line, followed by a 5 mL saline flush. Contrast-enhanced images were obtained with a contrast-sensitive low mechanical index (0.08) acquisition mode on a side-by-side screen with a gray-scale image. We captured the dynamic enhancement images during the first three minutes after injection. We examined the affected shoulder first, and the opposite side was examined with the second injection (additional 2.4 mL bolus of SonoVue and 5 ml saline flush) about five minutes after we checked for complete vanishing of enhancing microbubbles. Two radiologists reviewed the cine images and scored the degree of rotator interval enhancement, defined as the visible echo pixels, by consensus: 0, no enhancement; 1, partial inhomogeneous enhancement; and 2, diffuse homogeneous enhancement (Figures 2 - 4). All scorings were performed with reviewers blinded to the MRI scoring, and there was a one-week time interval between MRI scoring and CEUS scoring.
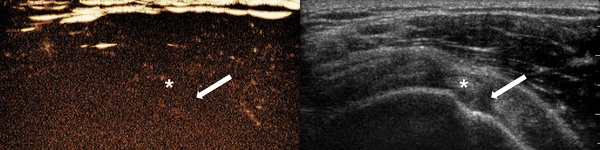
CEUS image of asymptomatic shoulder of 67-year-old woman showed no enhancing portion (score 0) in the rotator interval. Anatomic structures were not clearly demarcated on CEUS image and only distinguishable when referring to the concurrent side-by-side gray-scale image. Arrow = rotator interval, * = biceps long head tendon (Abbreviation: CEUS, contrast-enhanced ultrasonography)
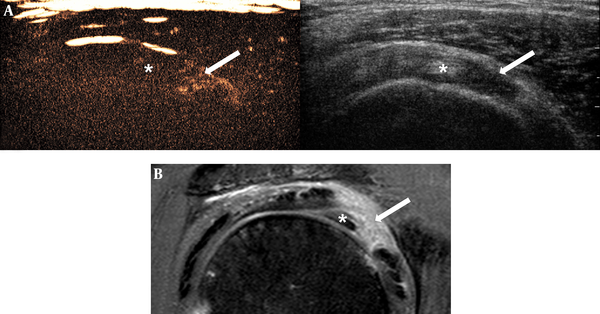
Images of 57-year-old man with clinical diagnosis of adhesive capsulitis. There was partial punctate enhancement (score 1) of the rotator interval in CEUS (A), and diffuse homogeneous enhancement (score 2) in MRI (B). Arrow = rotator interval, * = biceps long head tendon (Abbreviation: CEUS, contrast-enhanced ultrasonography)
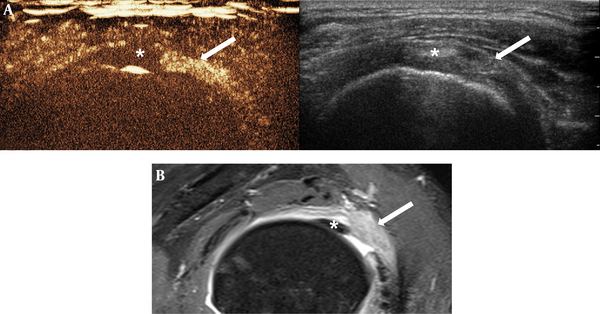
Images of 49-year-old woman with clinical diagnosis of adhesive capsulitis. There was diffuse homogeneous enhancement (score 2) of rotator interval in CEUS (A), and MRI (B). Arrow = rotator interval, * = biceps long head tendon (Abbreviation: CEUS, contrast-enhanced ultrasonography)
We used off-line quantification software (QLAB, Phillips Medical System, Bothell, WA) to analyze the CEUS data. On side-by-side contrast image, we established a free-form region of interest (ROI) in the rotator interval, aiming for the superior glenohumeral ligament, based on the gray-scale image. After activating the motion compensation function provided by the software, we obtained the time-intensity curve (TIC) of the selected ROI. We determined the suitable curve-fitting model and decided the pattern of the TIC; either lognormal or linear. If the TIC showed a lognormal pattern with an identifiable peak, the curve was analyzed for the following parameters: delay time (DT), time-to-peak (TTP), peak intensity (PI), and rate-of-rise (17, 18). The curve was classified as linear when there was no identifiable peak over the baseline noise level (Figure 5).
The ROI positioning (A) and time-intensity curve of the symptomatic shoulder (B) showed lognormal curve fit patterns (thin red line) with an identifiable increased peak intensity. Subsequently, DT, TTP, PI, and rate-of-rise were analyzed (DT: delay time, TTP: time-to-peak, PI: peak intensity, BI: baseline intensity, ROR: rate-of-rise). The time-intensity curve of the asymptomatic shoulder (C) showed a linear pattern without considerable change of echo intensity.

3.4. Statistical Analysis
Statistical analysis was performed with SPSS version 20.0 (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.). The differences of visual enhancement scores of the rotator interval in the affected shoulders between CEUS and MRI were compared using the Wilcoxon signed-rank test. The differences in CEUS scores and the PI of CEUS between the affected and unaffected shoulders were also compared by Wilcoxon signed-rank test. The subgroup parameters, according to the CEUS score in the affected shoulders, were compared using the Mann-Whitney test. A P value < 0.05 was considered statistically significant.
4. Results
A total of ten shoulders from five subjects (2 men, 3 women; mean age 54.2 ± 8 years) were examined. Among the five patients with AC, one was affected in the right shoulder and four were affected in the left shoulder. The duration of the symptoms was 2 to 12 months (mean: 6.4 months).
4.1. MRI and CEUS: Semi-Quantitative Analysis
All five patients showed diffuse enhancement in the rotator interval along the coracohumeral and superior glenohumeral ligaments in MRI. All subjects showed an enhancement score of two. Other MRI findings that were suggestive of AC included thickening (n = 5), T2 hyperintensity (n = 5) and contrast enhancement of the axillary capsule (n = 5), and fat obliteration (n = 5), which were present in all cases. The mean thickness of the coracohumeral ligament was 3.77 ± 1.1 mm. Other coexisting MRI findings were articular surface partial-thickness, tear of the supraspinatus tendon (n = 3), and superior labral anterior-to-posterior tear (n = 1).
In CEUS examinations, the deltoid muscle was recognizable with its subtle punctate enhancement. In CEUS of the asymptomatic shoulders, the supraspinatus/subscapularis tendon, biceps long head tendon, and superior glenohumeral and coracohumeral ligaments were not clearly demarcated and only distinguishable when referring to concurrent side-by-side gray-scale images (Figure 2). However, in all symptomatic shoulders, CEUS revealed partial or diffuse enhancement of the rotator interval ligament compared with the remaining non-enhancing supraspinatus and biceps tendons (Figures 3 and 4). There was a significant difference in CEUS scores between the symptomatic and asymptomatic shoulders (P = 0.04).
The presence of enhancement in the rotator interval was perfectly matched with MRI findings (5 out of 5). However, the degree of enhancement was less prominent in CEUS (mean score: 1.4) than MRI (mean score: 2; P = 0.08). No participants had adverse reactions to the contrast agent.
4.2. CEUS: Quantitative TIC Analysis
Estimates of TIC revealed a different behavioral trend between the symptomatic and asymptomatic shoulders. We found a lognormal pattern in all affected shoulders (5 out of 5), while a linear pattern was present in unaffected shoulders (5 out of 5). The mean delay time (DT) and time-to-peak (TTP) of affected shoulders were 19.8 ± 8.43 and 30.6 ± 5.39 seconds, respectively. The mean peak intensity (PI) of affected shoulders was 5.45 ± 2.80 decibels, which was significantly higher than that of unaffected shoulders (0.72 ± 0.91) (P = 0.04). The rate-of-rise of affected shoulders was 0.49 ± 0.15.
In cases of a CEUS score of 1, the mean DT (24.6 ± 7.45) was longer than cases of a CEUS score of 2 (12.6 ± 0.08) (P=0.08), and the mean TTP (33.5 ± 5.24) was slower compared with cases of a CEUS score of 2 (26.4 ± 0.81) (P = 0.08). The mean PI of CEUS scores of 1 (3.69 ± 0.52) was less than the cases with a CEUS score of 2 (8.11 ± 2.72) (P = 0.08). The mean rate-of-rise was 0.43 ± 0.13 and 0.59 ± 0.15 for CEUS scores 1 and 2, respectively (P = 0.25).
Table 1 summarizes the subject demographics, CE MRI and US enhancement scores, as well as CEUS quantitative parameters.
| No. | Age | Sex | Side | Duration (m) | CE Score a | TIC Pattern | DT (sec) | TTP (sec) | PI (dB/mm2) b | Rate of Rise | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MRI | CEUS c | Symptomatic | Asymptomatic | |||||||||
| 1 | 49 | F | L | 11 | 2 | 2 | Lognormal | 12.56 | 26.95 | 10.03 | 0.70 | |
| R | 0 | Linear | 2.28 | |||||||||
| 2 | 50 | M | L | 2 | 2 | 1 | Lognormal | 18.72 | 29.91 | 4.15 | 0.37 | |
| R | 0 | Linear | 0.24 | |||||||||
| 3 | 48 | F | L | 12 | 2 | 2 | Lognormal | 12.67 | 25.80 | 6.18 | 0.47 | |
| R | 0 | Linear | 0.76 | |||||||||
| 4 | 57 | M | R | 5 | 2 | 1 | Lognormal | 22.15 | 31.06 | 3.12 | 0.35 | |
| L | 0 | Linear | 0.22 | |||||||||
| 5 | 67 | F | L | 2 | 2 | 1 | Lognormal | 33.00 | 39.50 | 3.79 | 0.58 | |
| R | 0 | Linear | 0.10 | |||||||||
| Mean (SD) | 54.2 (8.0) | 6.4 (4.8) | 19.8 (8.43) | 30.6 (5.39) | 5.45 (2.80) | 0.72 (0.91) | 0.49 (0.15) | |||||
5. Discussion
AC is characterized by painful, gradual loss of active and passive shoulder motion, resulting from fibrosis and contracture of the joint capsule. Estimated incidence of AC is 2% to 5% in the general population (1). Physical examination does not reveal a specific point of tenderness and rotator cuff strength is usually normal. A mechanical restraint to passive motion is the hallmark of AC. AC progresses from capsular inflammation to fibrosis according to the stage. Especially in the pre-adhesive stage, symptoms are nonspecific and misdiagnosis is common (5). In this regard, although diagnosis of AC remains a clinical entity, imaging findings are useful in excluding other conditions that may cause similar symptoms. Furthermore, with the increasingly broad use of imaging modalities, they can play a greater role than simply excluding other conditions (6-11).
Various MRI findings of AC have been reported and include thickening and signal changes in the inferior glenohumeral ligament, subcoracoid fat obliteration around the rotator interval, and contrast enhancement of the joint capsule (6-8). Contrast enhancement is not essential for diagnosing AC, but is fairly helpful in MRI (7, 8). Tamai et al. postulated that contrast enhancement of the joint capsule seems to be associated with increased vascularity in the synovium and subsynovial layer in AC (19).
Contrary to MRI, US findings of AC are not as prevalent in the literature (9-12). This could be related to the limited approach of US for visualizing the shoulder joint capsule, although US has the advantages of lower cost and easy accessibility. Michelin et al. reported inferior glenohumeral thickening in shoulders with AC in a maximally abducted position (12). They suggested that assessment of axillary recess is more useful, because the rotator interval could be influenced by other conditions, such as biceps pulley lesion or biceps/subscapularis tendinopathy, and it is difficult to obtain a reliable thickness measurement of the coracohumeral ligament. However, in our preliminary trial, a patient with AC, who mostly suffered from pain and limited range of motion, had difficulty in maintaining an abducted shoulder position to evaluate the axillary recess during our CEUS exam. Other studies using US for AC have mainly focused on the rotator interval. Homsi et al. reported that a thickened coracohumeral ligament was a finding suggestive of AC on oblique axial and sagittal planes over a neutrally positioned arm (10). In the present study, we did not evaluate the coracohumeral ligament thickness, because it was not easily measurable, as noted by Michelin et al. Instead, we could see the obvious enhancement of the coracohumeral ligament together with the superior glenohumeral ligament on CEUS in the rotator interval of the symptomatic shoulders.
Lee et al. described hypoechoic change and increased vascularity in the rotator interval as a finding of AC, with 87% sensitivity and 100% specificity (9). Walmsley et al. also reported increased vascularity in the rotator interval in their Doppler study, however, this finding was only observed in 29% of patients with early-stage AC (11). Although Doppler US can reveal increased vascularity in the rotator interval in patients with AC, this method is limited because it can only detect larger vessels with signals above the noise level and with flow velocities above the threshold of the wall filter (14, 15). We hypothesized that CEUS could overcome this limitation and increase the detectability of vascular flow in an inflamed joint capsule.
Various applications of CEUS have been reported in assessing the vascularity of joints, and most studies agree that CEUS effectively visualizes the synovial vascularity, especially in rheumatoid arthritis (20-22). CEUS was more sensitive than Doppler US and helpful in differentiating active and inactive rheumatoid arthritis, so it is clinically useful in early detection and in monitoring therapeutic response. To our knowledge, this is the first study of CEUS applied to the shoulders of patients with AC, and we have concluded that CEUS can directly detect capsular enhancement, possibly due to inflammation.
We analyzed the CEUS-derived TIC curves for vascular flow and perfusion of the joint capsule. The TIC of affected shoulders showed a lognormal fitting curve, which is the usual pattern of tissue perfusion after bolus injection of a contrast agent. However, in asymptomatic shoulders, there was a trend of no remarkable increase between baseline and thereafter intensities. This is likely the result of slow, low-volume blood flow of the normal joint capsule, which can hardly be detected, even when using a contrast agent. Among the parameters of TIC, TTP is related to vascular flow of the analyzed region, while PI and rate-of-rise represent vascular volume and perfusion, respectively (18, 23). In a study by Platzgummer et al., which evaluated the TIC in patients with hand and wrist synovitis due to rheumatoid arthritis, the TTP ranged from 8.13 to 36.2 (mean: 18.6) and PI from 1.61 to 15.7 (mean: 7.51) (17). Their results with rheumatoid arthritis appear to be comparable to ours with AC, where TTP ranged from 25.8 to 39.5 (mean: 30.6) and PI from 3.12 to 10.03 (mean: 5.45). In comparing CEUS scores of 1 and 2, cases with score 1 showed slower TTP, smaller PI, and similar rate-of-rise, which represents relatively slower vascular flow, lower vascular volume, and similar perfusion in the ROI.
Our study has several limitations. The major limitation is the small size of the study population that might be biased because we only included the AC patients who underwent MRI. Due to the small sample size, we could not consider the effect of the laterality of the IV route, gender, age, and symptom duration, all of which could possibly influence the enhancing pattern and quantification parameters. In addition, the borderline p-values in our results could be derived from the small sample size. Considering clinically different numeric profiles, a larger sample study may yield better results. A larger population study is required to validate our results.
Another limitation is the absence of reliability testing on the CEUS exam and quantification analysis. Due to the short time window of CEUS, we could not include an observer variability test in our study. In our study, the existence of contrast enhancement was evident in all cases and slight imaging plane inconsistency was not a significant problem. However, our quantification method has limitations, particularly in ROI selection. Since the ROI did not cover the overall enhancing capsule, the parameters derived from our ROI may contain limited information regarding vascular volume or perfusion. Due to the paucity of available quantification data regarding CEUS of the shoulder joint capsule, we could not compare our results with other references.
In conclusion, our preliminary results revealed that CEUS was capable of demonstrating capsular enhancement, possibly from inflammation, of the rotator interval in patients with AC, and the findings of CEUS were comparable to those of MRI.
Acknowledgements
References
-
1.
Hsu JE, Anakwenze OA, Warrender WJ, Abboud JA. Current review of adhesive capsulitis. J Shoulder Elbow Surg. 2011;20(3):502-14. [PubMed ID: 21167743]. https://doi.org/10.1016/j.jse.2010.08.023.
-
2.
Codman EA. The shoulder: rupture of the supraspinatus tendon and other lesions in or about the subacromial bursa. Boston, Mass.: T. Todd company; 1934.
-
3.
Neviaser JS. Adhesive capsulitis of the shoulder. J Bone Joint Surg Am. 1945;27(2):211-22.
-
4.
Bunker TD, Anthony PP. The pathology of frozen shoulder. A Dupuytren-like disease. J Bone Joint Surg Br. 1995;77(5):677-83. [PubMed ID: 7559688].
-
5.
Neviaser AS, Neviaser RJ. Adhesive capsulitis of the shoulder. J Am Acad Orthop Surg. 2011;19(9):536-42. [PubMed ID: 21885699].
-
6.
Emig EW, Schweitzer ME, Karasick D, Lubowitz J. Adhesive capsulitis of the shoulder: MR diagnosis. AJR Am J Roentgenol. 1995;164(6):1457-9. [PubMed ID: 7754892]. https://doi.org/10.2214/ajr.164.6.7754892.
-
7.
Connell D, Padmanabhan R, Buchbinder R. Adhesive capsulitis: role of MR imaging in differential diagnosis. Eur Radiol. 2002;12(8):2100-6. [PubMed ID: 12136330]. https://doi.org/10.1007/s00330-002-1349-7.
-
8.
Gondim Teixeira PA, Balaj C, Chanson A, Lecocq S, Louis M, Blum A. Adhesive capsulitis of the shoulder: value of inferior glenohumeral ligament signal changes on T2-weighted fat-saturated images. AJR Am J Roentgenol. 2012;198(6):W589-96. [PubMed ID: 22623575]. https://doi.org/10.2214/AJR.11.7453.
-
9.
Lee JC, Sykes C, Saifuddin A, Connell D. Adhesive capsulitis: sonographic changes in the rotator cuff interval with arthroscopic correlation. Skeletal Radiol. 2005;34(9):522-7. [PubMed ID: 15999280]. https://doi.org/10.1007/s00256-005-0957-0.
-
10.
Homsi C, Bordalo-Rodrigues M, da Silva JJ, Stump XM. Ultrasound in adhesive capsulitis of the shoulder: is assessment of the coracohumeral ligament a valuable diagnostic tool? Skeletal Radiol. 2006;35(9):673-8. [PubMed ID: 16724200]. https://doi.org/10.1007/s00256-006-0136-y.
-
11.
Walmsley S, Osmotherly PG, Walker CJ, Rivett DA. Power Doppler ultrasonography in the early diagnosis of primary/idiopathic adhesive capsulitis: an exploratory study. J Manipulative Physiol Ther. 2013;36(7):428-35. [PubMed ID: 23830711]. https://doi.org/10.1016/j.jmpt.2013.05.024.
-
12.
Michelin P, Delarue Y, Duparc F, Dacher JN. Thickening of the inferior glenohumeral capsule: an ultrasound sign for shoulder capsular contracture. Eur Radiol. 2013;23(10):2802-6. [PubMed ID: 23652851]. https://doi.org/10.1007/s00330-013-2874-2.
-
13.
Ahn KS, Kang CH, Oh YW, Jeong WK. Correlation between magnetic resonance imaging and clinical impairment in patients with adhesive capsulitis. Skeletal Radiol. 2012;41(10):1301-8. [PubMed ID: 22430562]. https://doi.org/10.1007/s00256-012-1391-8.
-
14.
Quaia E. Assessment of tissue perfusion by contrast-enhanced ultrasound. Eur Radiol. 2011;21(3):604-15. [PubMed ID: 20927527]. https://doi.org/10.1007/s00330-010-1965-6.
-
15.
Greis C. Technology overview: SonoVue (Bracco, Milan). Eur Radiol. 2004;14 Suppl 8:P11-5. [PubMed ID: 15700328].
-
16.
Magee T. 3-T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192(1):86-92. [PubMed ID: 19098184]. https://doi.org/10.2214/AJR.08.1097.
-
17.
Platzgummer H, Schueller G, Grisar J, Weber M, Schueller-Weidekamm C. Quantification of synovitis in rheumatoid arthritis: do we really need quantitative measurement of contrast-enhanced ultrasound? Eur J Radiol. 2009;71(2):237-41. [PubMed ID: 19410408]. https://doi.org/10.1016/j.ejrad.2009.03.044.
-
18.
Gamradt SC, Gallo RA, Adler RS, Maderazo A, Altchek DW, Warren RF, et al. Vascularity of the supraspinatus tendon three months after repair: characterization using contrast-enhanced ultrasound. J Shoulder Elbow Surg. 2010;19(1):73-80. [PubMed ID: 19525129]. https://doi.org/10.1016/j.jse.2009.04.004.
-
19.
Tamai K, Yamato M. Abnormal synovium in the frozen shoulder: A preliminary report with dynamic magnetic resonance imaging. J Shoulder Elbow Surg. 1997;6(6):534-43.
-
20.
Klauser A, Demharter J, De Marchi A, Sureda D, Barile A, Masciocchi C, et al. Contrast enhanced gray-scale sonography in assessment of joint vascularity in rheumatoid arthritis: results from the IACUS study group. Eur Radiol. 2005;15(12):2404-10. [PubMed ID: 16132921]. https://doi.org/10.1007/s00330-005-2884-9.
-
21.
Magarelli N, Guglielmi G, Di Matteo L, Tartaro A, Mattei PA, Bonomo L. Diagnostic utility of an echo-contrast agent in patients with synovitis using power Doppler ultrasound: a preliminary study with comparison to contrast-enhanced MRI. Eur Radiol. 2001;11(6):1039-46. [PubMed ID: 11419150]. https://doi.org/10.1007/s003300000650.
-
22.
Klauser A, Frauscher F, Schirmer M, Halpern E, Pallwein L, Herold M, et al. The value of contrast-enhanced color Doppler ultrasound in the detection of vascularization of finger joints in patients with rheumatoid arthritis. Arthritis Rheum. 2002;46(3):647-53. [PubMed ID: 11920400]. https://doi.org/10.1002/art.10136.
-
23.
Dietrich CF, Averkiou MA, Correas JM, Lassau N, Leen E, Piscaglia F. An EFSUMB introduction into Dynamic Contrast-Enhanced Ultrasound (DCE-US) for quantification of tumour perfusion. Ultraschall Med. 2012;33(4):344-51. [PubMed ID: 22843433]. https://doi.org/10.1055/s-0032-1313026.